Effect of Long-Term Set-Aside Management System on Soil Health by Evaluation of Biodiversity Indicators
Abstract
1. Introduction
2. Materials and Methods
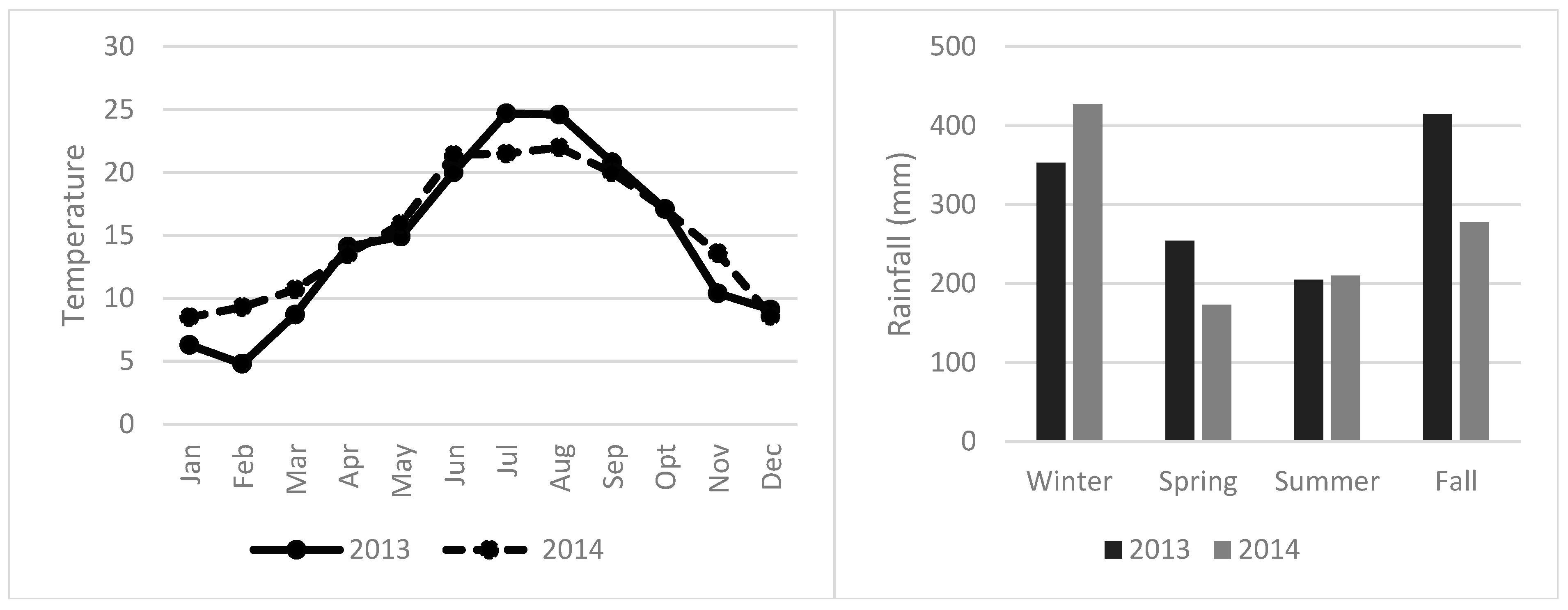
2.1. Field Site and Treatments Description
2.2. Soil Sampling Design
2.3. Soil Chemical Analysis
2.4. Soil Microbial Community Analysis
2.5. Soil Nematode Community Analysis
2.6. Soil Microarthropod Community Analysis
2.7. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
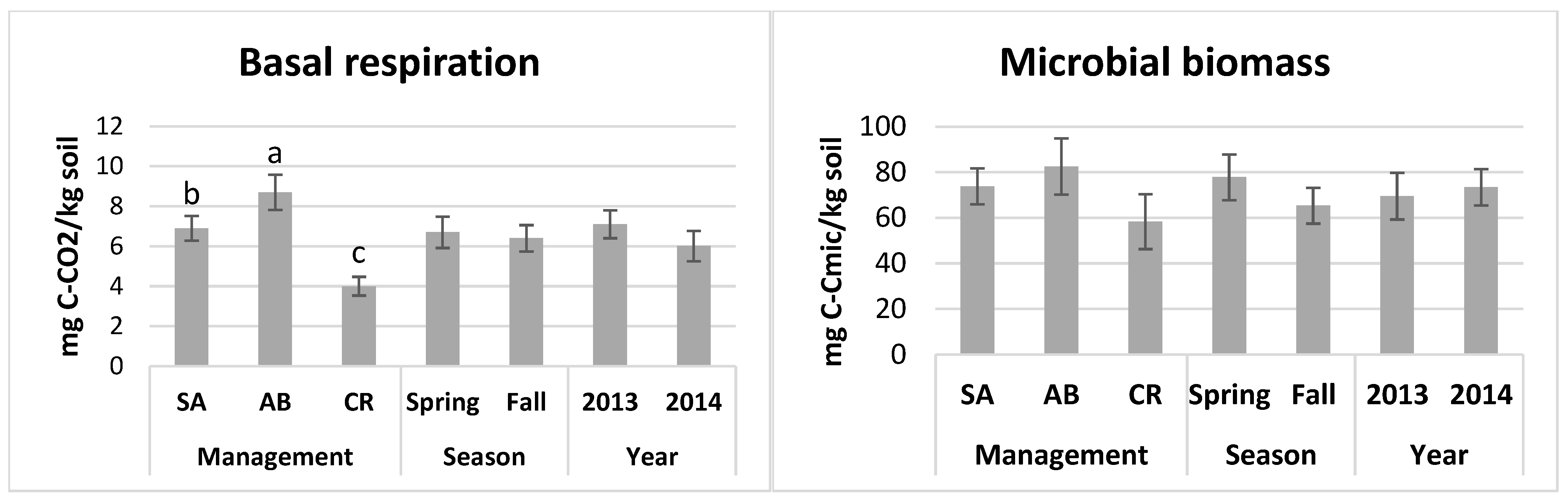
3.2. Soil Biological Activity
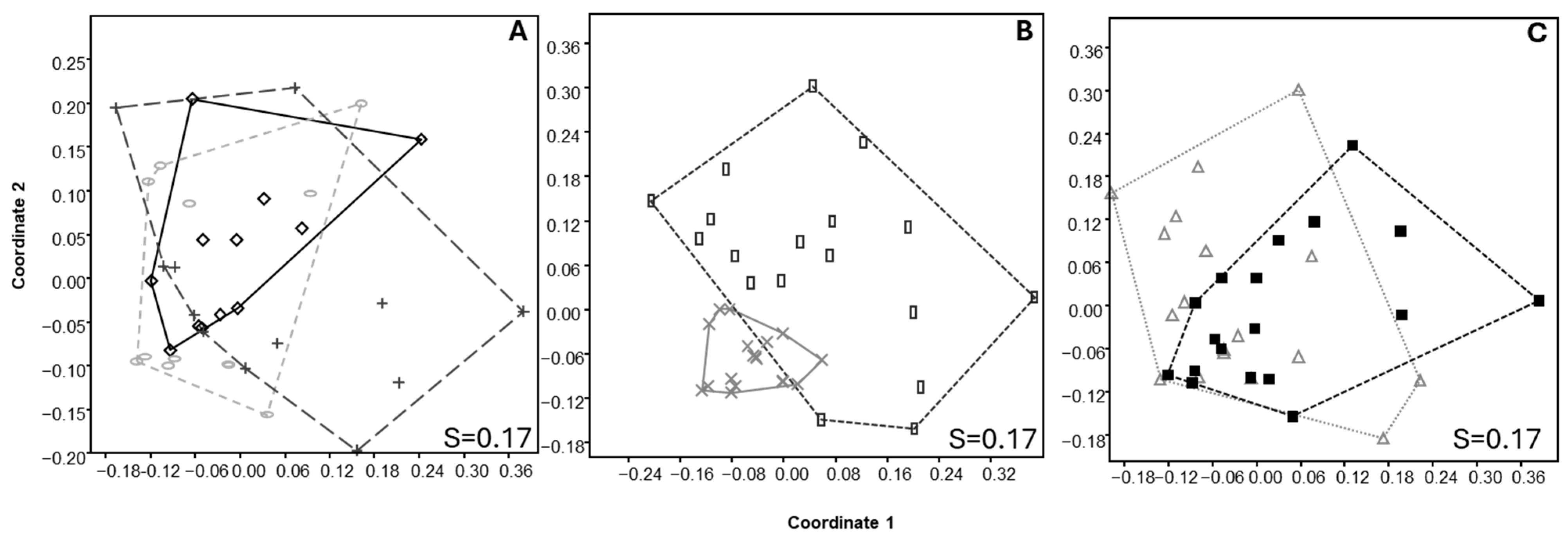
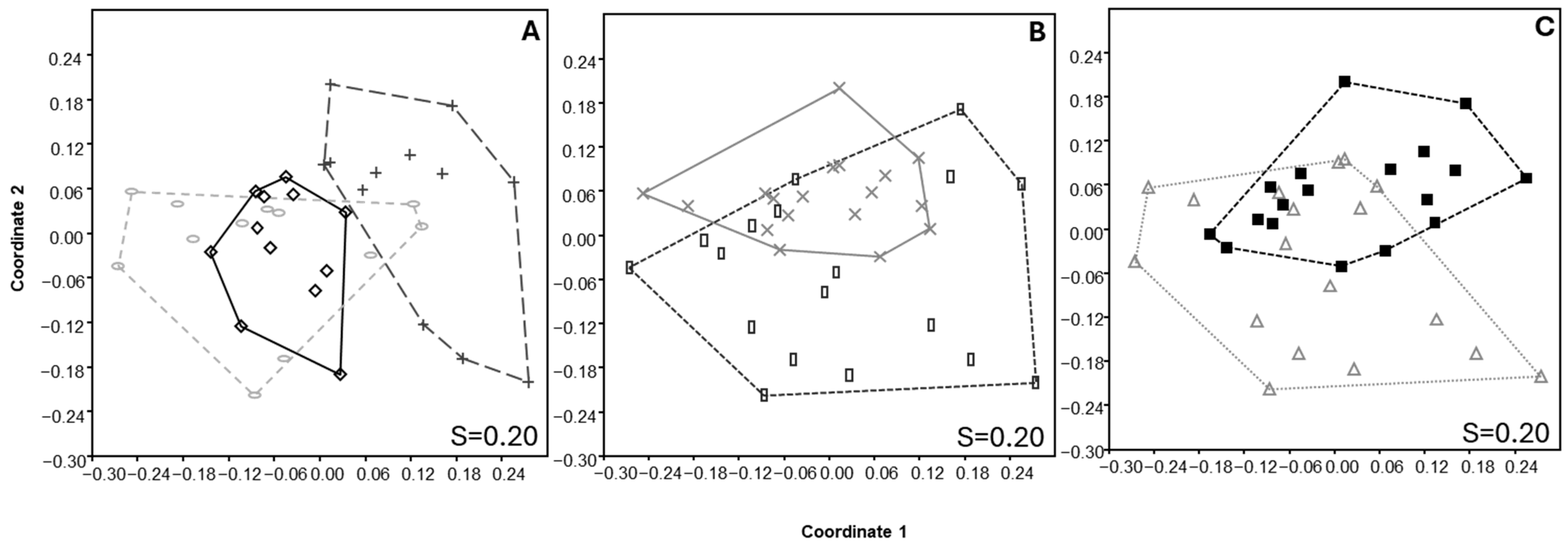
3.3. Soil Nematode Structure
3.4. Soil Microarthropod Structure
3.5. Soil Biological Indicators
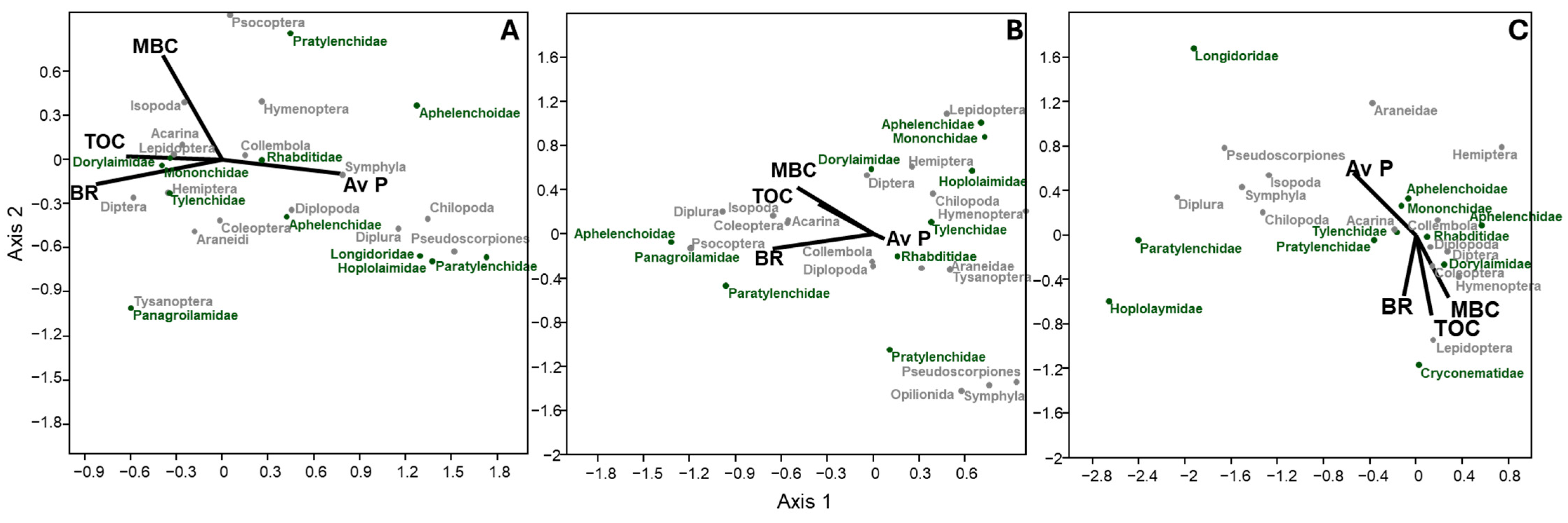
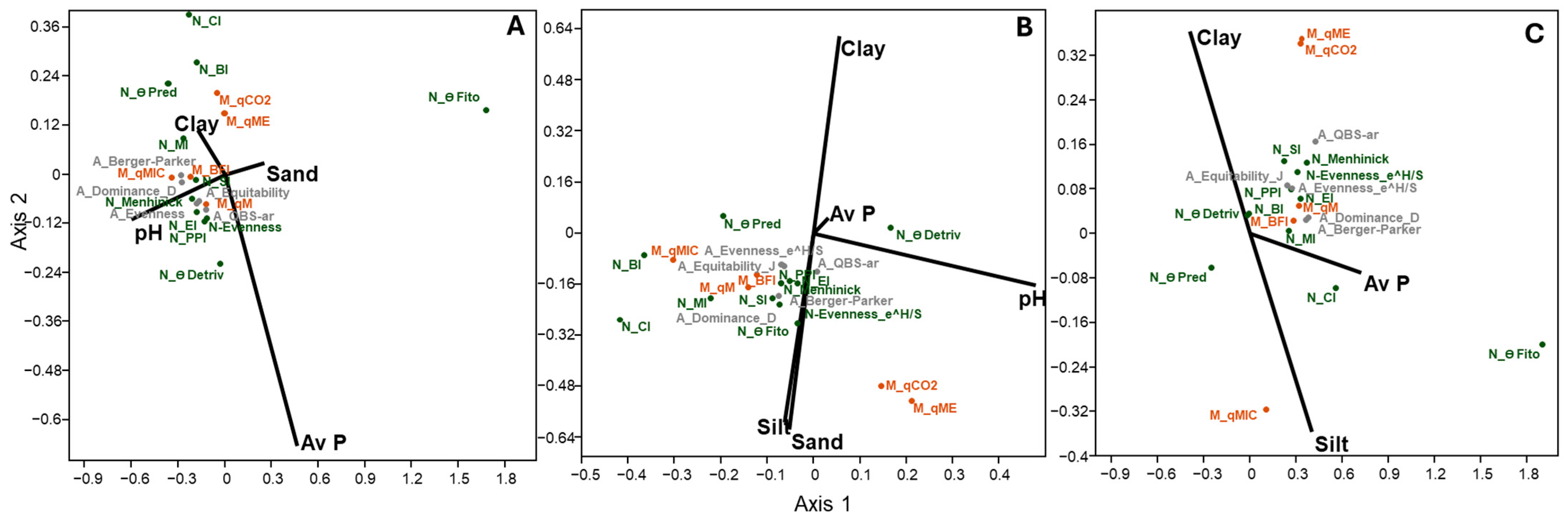
3.6. Relationship Between Environmental Variables and Community Structure of Nematodes and Microarthropods
4. Discussion
4.1. Effect of Set-Aside and Abandonment Management Systems on Soil Fertility
4.2. Effects of Set-Aside and Abandonment on Soil Nematode and Microarthropod Community Structure
4.3. Soil Factors and Microbial Parameters Influencing Soil Nematode and Microarthropod Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero-Díaz, A.; Pérez-Morales, A.; Marín-Sanleandro, P. Prevalence, causes, and consequences of agricultural land abandonment: A case study in the Region of Murcia, Spain. Catena 2024, 241, 108071. [Google Scholar] [CrossRef]
- Lieskovský, J.; Kenderessy, P.; Petlušová, V.; Peter Petlǔs, P. Effect of grass cover and abandonment on soil surface changes and soil properties in traditional vineyards in Vrable viticultural region in southwestern Slovakia. Catena 2024, 235, 107702. [Google Scholar] [CrossRef]
- Van Buskirk, J.; Willi, Y. Enhancement of farmland biodiversity within set-aside land. Conserv. Biol. 2004, 18, 987–994. [Google Scholar]
- Masciandaro, G.; Ceccanti, B.; Gallardo-Lancho, J.F. Organic matter properties in cultivated versus set-aside arable soils. Agric. Ecosyst. Environ. 1998, 67, 267–274. [Google Scholar]
- Smith, P. Carbon sequestration in croplands: The potential in Europe and the global context. Europ. J. Agron. 2004, 20, 229–236. [Google Scholar]
- Landi, S.; Papini, R.; d’Errico, G.; Brandi, G.; Rocchini, A.; Roversi, P.F.; Bazzoffi, P.; Mocali, M. Effect of different set-aside management systems on soil nematode community and soil fertility in North, Central and South Italy Agriculture, Ecosystems and Environment. Agric. Ecosyst. Environ. 2018, 261, 251–260. [Google Scholar] [CrossRef]
- Mocali, S.; Landi, S.; Fabiani, A.; Piccolo, R.; Agnelli, A.; d’Errico, G.; Mazza, G.; Fedrizzi, M.; Sperandio, G.; Guerrieri, M.; et al. Environmental effectiveness of GAEC cross-compliance Standard 4.2 on biodiversity in set-aside management and economic evaluation of the competitiveness gap for farmers, part I. Ital. J. Agron. 2015, 10 (Suppl. S1), 711. [Google Scholar] [CrossRef]
- Hamer, U.; Makeschin, F.; Stadler, J.; Klotz, S. Soil organic matter and microbial community structure in set-aside and intensively managed arable soils in NE-Saxony, Germany. Appl. Soil Ecol. 2008, 40, 465–475. [Google Scholar]
- Freckman, D.W.; Ettema, C.H. Assessing nematode communities in agroecosystems of varying human intervention. Agric. Ecosyst. Environ. 1993, 45, 239–261. [Google Scholar] [CrossRef]
- Landi, S.; d’Errico, G.; Manachini, B. Nematode Communities in Soils of the Same Volcanic Origin across a Gradient of Naturalization: From Intensive Agriculture to Forest. Sustainability 2024, 16, 6307. [Google Scholar] [CrossRef]
- Boag, B.; Hebden, P.M.; Neilson, R.; Rodger, S.J. Observations on the effect of different management regimes of set-aside land on nematode community structure. Appl. Soil Ecol. 1998, 9, 339–343. [Google Scholar] [CrossRef]
- Biaggini, M.; Bazzoffi, P.; Gentile, R.; Corti, C. Effectiveness of the GAEC cross-compliance Standard Rational management of set-aside, Grass strips to control soil erosion and Vegetation buffers along watercourses on surface animal diversity and biological quality of soil. Ital. J. Agron. 2011, 6 (Suppl. S1), e14. [Google Scholar] [CrossRef]
- Raglione, M.; Lorenzoni, P.; Bonifazi, A.; Nannelli, R.; Gagnarli, E.; Bazzoffi, P.; Gentile, R. Effectiveness of the GAEC cross-compliance Standard Rational management of set-aside (weed control through mowing) in assuring a minimum level of habitats maintenance. Ital. J. Agron. 2011, 6 (Suppl. S1), e13. [Google Scholar] [CrossRef]
- Maharning, A.R.; Mills, A.A.S.; Adl, S.M. Soil community changes during secondary succession to naturalized grasslands. Appl. Soil Ecol. 2009, 41, 137–147. [Google Scholar] [CrossRef]
- Pastorelli, R.; Landi, S.; Trabelsi, D.; Piccolo, R.; Mengoni, A.; Bazzicalupo, M.; Pagliai, M. Effects of soil management on structure and activity of denitrifying bacterial communities. Appl. Soil Ecol. 2011, 49, 46–58. [Google Scholar] [CrossRef]
- Papini, R.; Valboa, G.; Favilli, F.; L’Abate, G. Influence of land use on organic carbon pool and chemical properties of Vertic Cambisols in central and southern Italy. Agric. Ecosyst. Environ. 2011, 140, 68–79. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; De Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Wu, L.; Ren, C.; Jiang, H.; Zhang, W.; Chen, N.; Zhao, X.; Wei, G. Land abandonment transforms soil microbiome stability and functional profiles in apple orchards of the Chinese Losses Plateau. Sci. Total Environ. 2024, 906, 167556. [Google Scholar] [CrossRef]
- You, Y.; Li, W.; Chen, Y.; Zhang, Q.; Zhang, K. Soil carbon and nitrogen accumulation during long-term natural vegetation restoration following agricultural abandonment in Qingling Mountains. Ecol. Eng. 2024, 201, 107212. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed.; Buxton, R.H., Miller Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Isermeyer, H. Eine einfache Methode zur Bestimmung der Bodenatmung und der Karbonate im Boden. Z. Pflanzenernaehr. Bodenkd. 1952, 56, 26–38. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar]
- Mocali, S.; Paffetti, D.; Emiliani, G.; Benedetti, A.; Fani, R. Diversity of heterotrophic aerobic cultivable microbial communities of soils treated with fumigants and dynamics of metabolic, microbial, and mineralization quotients. Biol. Fertil. Soils 2008, 44, 557–569. [Google Scholar] [CrossRef]
- Dilly, O.; Winter, K.; Lang, A.; Munch, J.C. Energetic eco-physiology of the soil microbiota in two landscapes of southern and northern Germany. J. Plant Nutr. Soil Sci. 2001, 164, 407–413. [Google Scholar]
- Renzi, G.; Canfora, L.; Salvati, L.; Benedetti, A. Validation of the soil Biological Fertility Index (BFI) using a multidimensional statistical approach: A country-scale exercise. Catena 2017, 149, 294–299. [Google Scholar]
- Mai, W.F.; Lyon, H.H. Pictorial Key to Genera of Plant Parasitic Nematodes; New York State College of Agriculture, Cornell University: Ithaca, NY, USA, 1962. [Google Scholar]
- Bongers, T. De Nematoden van Nederland; K.N.N.V.: Utrecht, The Nederlands, 1988. [Google Scholar]
- Marinari-Palmisano, A.; Vinciguerra, M. Classificazione dei Nematodi. In Nematologia Agraria Generale e Applicate; Ambrogioni, L., d’Errico, F.P., Greco, N.A., Marinari-Palmisano, A., Roversi, P.F., Eds.; Società Italiana di Nematologia: Bari, Italia, 2014; pp. 23–42. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.; Freckman, D.; Georgieva, S. Feeding habits in soil nematode families and genera in outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar]
- Okada, H.; Harada, H.; Kadota, I. Fungal-feeding habits of six nematode isolates in the genus Filenchus. Soil Biol. Biochem. 2005, 37, 1113–1120. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; De Goede, R. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar]
- Ferris, H.; Tuomisto, H. Unearthing the role of biological diversity in soil health. Soil Biol. Biochem. 2015, 85, 101–109. [Google Scholar] [CrossRef]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agr. Ecosys. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.; Ryan, P. PAST—Palaeontological statistics, ver. 1. 89. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Petersson, T.; Antoniella, G.; Perugini, L.; Chiriacò, M.V.; Chiti, T. Carbon farming practices for European cropland: A review on the effect on soil organic carbon. Soil Tillage Res. 2025, 247, 106353. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Isermann, K. Soil Organic Matter Dynamics. In Handbook of Processes and Modeling in the Soil-Plant System; CRC Press: Boca Raton, FL, USA, 2024; pp. 345–408. [Google Scholar]
- Francaviglia, R.; Renzi, G.; Ledda, L.; Benedetti, A. Organic carbon pools and soil biological fertility are affected by land use intensity in Mediterranean ecosystems of Sardinia, Italy. Sci. Total Environ. 2017, 599, 789–796. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Cucci, G.; Lacolla, G.; Lanzellotti, L.; Crecchio, C. Soil fertility and bacterial community composition in a semiarid Mediterranean agricultural soil under long-term tillage management. Soil Use Manag. 2020, 36, 604–615. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Pettersson, M.; Bååth, E. Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol. Ecol. 2005, 52, 49–58. [Google Scholar] [CrossRef]
- Tóth, Z.; Hornung, E.; Báldi, A. Effects of set-aside management on certain elements of soil biota and early stage organic matter decomposition in a High Nature Value Area, Hungary. Nat. Conserv. 2018, 29, 1–26. [Google Scholar] [CrossRef]
- Bhat, N.A.; Riar, A.; Ramesh, A.; Iqbal, S.; Sharma, M.P.; Sharma, S.K.; Bhullar, G.S. Soil biological activity contributing to phosphorus availability in vertisols under long-term organic and conventional agricultural management. Front. Plant Sci. 2017, 8, 1523. [Google Scholar] [CrossRef] [PubMed]
- Xomphoutheb, T.; Jiao, S.; Guo, X.; Mabagala, F.S.; Sui, B.; Wang, H.; Zhao, L.; Zhao, X. The effect of tillage systems on phosphorus distribution and forms in rhizosphere and non-rhizosphere soil under maize (Zea mays L.) in Northeast China. Sci. Rep. 2020, 10, 6574. [Google Scholar]
- Cerevková, A. Diversity and Distribution of Nematode Communities in Grassland in Relation to Its Establishment Age and Utilization. In Nematodes Morphology, Functions and Management Strategies; Nova Science Publishers: Hauppauge, NY, USA, 2011; Volume 9, pp. 1–16. [Google Scholar]
- Barker, K.R.; Koenning, S.R. Developing sustainable systems for nematode management. Annu. Rev. Phytopathol. 1998, 36, 165–205. [Google Scholar]
- Bird, G. Nematodes and Soil Ecology. In Michigan Field Crop Ecology; E-2704; Michigan State University Extension: East Lansing, MI, USA, 2000; pp. 84–94. [Google Scholar]
- Menta, C.; Leoni, A.; Bardini, M.; Gardi, C.; Gatti, F. Nematode and microarthropod communities: Comparative use of soil quality bioindicators in covered dump and natural soils. Environ. Bioind. 2008, 3, 35–46. [Google Scholar]
- Ugarte, C.M.; Zaborski, E.R.; Wander, M.M. Nematode indicators as integrative measures of soil condition in organic cropping systems. Soil Biol. Biochem. 2013, 64, 103–113. [Google Scholar]
- Ferrazzi, P.; Berger, F.; Verti, F. Biomonitoraggio della Qualità dei Suoli nei Comuni di Agenda 21 Laghi. In Proceedings of the 4° Forum di Agenda 21 Laghi, Torino, Italy, 16 June 2007. [Google Scholar]
- Menta, C.; Conti, F.D.; Pinto, S.P.; Bodini, A. Soil Biological Quality index (QBS-ar): 15 years of application at global scale. Ecol. Indic. 2018, 85, 773–780. [Google Scholar]
- Del Duca, S.; Mocali, S.; Vitali, F.; Fabiani, A.; Cucu, M.A.; Valboa, G.; d’Errico, G.; Binazzi, F.; Storchi, P.; Landi, S.; et al. Impacts of Soil Management and Sustainable Plant Protection Strategies on Soil Biodiversity in a Sangiovese Vineyard. Land 2024, 13, 599. [Google Scholar] [CrossRef]
- Landi, S.; Pennacchio, F.; Papini, R.; d’Errico, G.; Torrini, G.; Strangi, A.; Barabaschi, D.; Roversi, P.F. Evaluation of sheep grazing effects on nematode community, insect infestation and soil fertility in sweet chestnut orchards: A case of study. REDIA 2016, 99, 117–126. [Google Scholar]
- Lazarovits, G.; Tenuta, M.; Conn, K.L. Organic amendments as a disease control strategy for soilborne diseases of high-value agricultural crops. Australas. Plant Pathol. 2001, 30, 111–117. [Google Scholar]
- Freitas, J.R.B.; Moitinho, M.R.; De Bortoli Teixeira, D.; da Silva Bicalho, E.; da Silva, J.F.; Siqueira, D.S.; Barbosa, B.F.F.; Soares, P.L.M.; Pereira, G.T. Soil factors influencing nematode spatial variability in soybean. Agron. J. 2017, 109, 610–619. [Google Scholar]
- Landi, S.; Valboa, G.; Vignozzi, V.; d’Errico, G.; Pellegrini, S.; Simoncini, S.; Torrini, G.; Roversi, P.F.; Priori, S. Response of nematode community structure to different restoration practices in two vineyard soils in Tuscany (Italy). Biol. Agric. Hortic. 2023, 39, 149–169. [Google Scholar] [CrossRef]
- Adejuyigbe, C.O.; Kodaolu, E.O. Faunal population as parameter for soil fertility assessment: A case of soil microarthropod study in South-Western Nigeria. Arch. Agron. Soil Sci. 2012, 58 (Suppl. S1), 116–121. [Google Scholar] [CrossRef]
- Barbato, D.; Perini, C.; Mocali, S.; Bacaro, G.; Tordoni, E.; Maccherini, S.; Marchi, M.; Cantiani, P.; De Meo, I.; Bianchetto, E.; et al. Teamwork makes the dream work: Disentangling cross-taxon congruence across soil biota in black pine plantations. Sci. Total Environ. 2019, 656, 659–669. [Google Scholar] [CrossRef] [PubMed]

| Taxa | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Main Effect | Interaction | ||||||
| M | S | Y | M + S | M + Y | S + Y | M + S + Y | |
| Soil pH | 0.05 | 0.07 | 0.02 | 0.06 | 0.72 | 0.98 | 0.01 |
| TOC (g kg−1) | 0.00001 | 0.0003 | 0.85 | 0.39 | 0.002 | 0.0003 | 0.0008 |
| Olsen P (mg kg−1) | 0.12 | 0.00001 | 0.07 | 0.43 | 0.005 | 0.00001 | 0.67 |
| Taxa | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Main Effect | Interaction | ||||||
| M | S | Y | M + S | M + Y | S + Y | M + S + Y | |
| BR (mg C-CO2 kg−1 d−1) | 0.00001 | 0.57 | 0.11 | 0.47 | 0.44 | 0.002 | 0.15 |
| MBC (mg C kg−1) | 0.22 | 0.28 | 0.71 | 0.51 | 0.21 | 0.004 | 0.35 |
| Taxa | Management | Season | Year | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | AB | CR | Spring | Autumn | 2013 | 2014 | M | S | Y | M + S | M + Y | S + Y | M + S + Y | |
| Microorganisms | ||||||||||||||
| qCO2 | 4.5 ± 0.76 | 6.3 ± 1.82 | 4.4 ± 1.17 | 4.8 ± 0.87 | 5.3 ± 1.26 | 5.9 ± 1.27 | 4.3 ± 0.81 | 0.53 | 0.75 | 0.30 | 0.14 | 0.39 | 0.23 | 0.57 |
| qM | 3.5 ± 0.08 a | 3.6 ± 0.12 a | 2.5 ± 0.15 b | 2.9 ± 0.16 b | 3.4 ± 0.13 a | 3.2 ± 0.12 | 3.1 ± 0.18 | 0.00001 | 0.0001 | 0.67 | 0.43 | 0.10 | 0.04 | 0.22 |
| qMIC | 0.6 ± 0.05 | 0.5 ± 0.07 | 0.5 ± 0.09 | 0.5 ± 0.07 | 0.5 ± 0.05 | 0.5 ± 0.06 | 0.5 ± 0.05 | 0.69 | 0.59 | 0.64 | 0.59 | 0.37 | 0.02 | 0.79 |
| qME | 3.5 ± 0.55 | 4.4 ± 1.39 | 3.6 ± 0.97 | 3.6 ± 0.71 | 4.1 ± 3.96 | 4.4 ± 0.95 | 3.3 ± 0.67 | 0.77 | 0.63 | 0.32 | 0.16 | 0.35 | 0.14 | 0.53 |
| BFI | 15.6 ± 0.26 b | 17.1 ± 0.51 a | 12.9 ± 0.47 c | 15.2 ± 0.58 | 15.2 ± 0.49 | 15.6 ± 0.47 | 14.8 ± 0.59 | 0.00001 | 0.89 | 0.08 | 0.92 | 0.02 | 0.002 | 0.23 |
| Nematodes | ||||||||||||||
| Dominance_D | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.006 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.008 | 0.68 | 0.62 | 0.23 | 0.35 | 0.98 | 0.05 | 0.85 |
| Shannon_H | 1.3 ± 0.04 | 1.2 ± 0.04 | 1.3 ± 0.03 | 1.2 ± 0.02 | 1.3 ± 0.03 | 1.3 ± 0.03 | 1.3 ± 0.03 | 0.44 | 0.23 | 0.53 | 0.47 | 0.60 | 0.25 | 0.92 |
| Evenness_e^H/S | 0.6 ± 0.02 b | 0.7 ± 0.03 a | 0.7 ± 0.03 a | 0.6 ± 0.02 b | 0.7 ± 0.02 a | 0.6 ± 0.02 | 0.7 ± 0.02 | 0.05 | 0.01 | 0.21 | 0.28 | 0.08 | 0.07 | 0.22 |
| Brillouin | 1.3 ± 0.04 | 1.2 ± 0.04 | 1.2 ± 0.03 | 1.2 ± 0.02 | 1.3 ± 0.04 a | 1.2 ± 0.03 b | 1.3 ± 0.03 a | 0.41 | 0.33 | 0.57 | 0.39 | 0.63 | 0.27 | 0.92 |
| Menhinick | 0.2 ± 0.01 b | 0.2 ± 0.01 b | 0.3 ± 0.03 a | 0.2 ± 0.009 a | 0.3 ± 0.02 b | 0.2 ± 0.01 b | 0.3 ± 0.02 a | 0.008 | 0.03 | 0.55 | 0.01 | 0.33 | 0.43 | 0.59 |
| Margalef | 0.8 ± 0.66 | 0.7 ± 0.05 | 0.7 ± 0.04 | 0.7 ± 0.03 a | 0.7 ± 0.04 b | 0.7 ± 0.04 | 0.7 ± 0.03 | 0.22 | 0.92 | 0.87 | 0.31 | 0.73 | 0.79 | 0.79 |
| Equitability_J | 0.7 ± 0.02 | 0.8 ± 0.02 | 0.8 ± 0.02 | 0.7 ± 0.01 b | 0.8 ± 0.02 a | 0.7 ± 0.02 | 0.8 ± 0.02 | 0.07 | 0.005 | 0.12 | 0.14 | 0.04 | 0.02 | 0.76 |
| Fisher_alpha | 1.0 ± 0.05 | 0.8 ± 0.05 | 0.9 ± 0.05 | 0.9 ± 0.04 b | 0.9 ± 0.05 a | 0.9 ± 0.04 | 0.9 ± 0.04 | 0.22 | 0.74 | 0.93 | 0.58 | 0.29 | 0.82 | 0.38 |
| Berger–Parker | 0.4 ± 0.02 | 0.4 ± 0.02 | 0.4 ± 0.02 | 0.4 ± 0.01 b | 0.5 ± 0.02 a | 0.4 ± 0.02 | 0.4 ± 0.01 | 0.67 | 0.03 | 0.32 | 0.57 | 0.52 | 0.04 | 0.41 |
| MI | 2.1 ± 0.12 | 2.1 ± 0.15 | 2.1 ± 0.11 | 2.4 ± 0.04 a | 1.8 ± 0.10 b | 2.0 ± 0.11 b | 2.2 ± 0.08 a | 0.88 | 0.00001 | 0.009 | 0.81 | 0.001 | 0.003 | 0.002 |
| PPI | 2.5 ± 0.12 | 2.2 ± 0.05 | 2.3 ± 0.07 | 2.2 ± 0.04 b | 2.5 ± 0.08 a | 2.3 ± 0.08 | 2.3 ± 0.07 | 0.08 | 0.0006 | 0.87 | 0.04 | 0.98 | 1.00 | 0.79 |
| BI | 65.13 ± 9.6 | 66.7 ± 11.1 | 53.2 ± 11.19 | 86.4 ± 7.78 a | 37.0 ± 4.37 b | 60.9 ± 8.51 | 62.5 ± 8.88 | 0.51 | 0.0001 | 0.88 | 0.88 | 0.89 | 0.94 | 0.99 |
| EI | 89.8 ± 1.65 | 89.8 ± 1.65 | 87.0 ± 2.41 | 87.5 ± 0.94 b | 91.7 ± 1.90 a | 89.5 ± 1.84 | 89.7 ± 1.28 | 0.15 | 0.08 | 0.90 | 0.08 | 0.46 | 0.92 | 0.62 |
| SI | 92.0 ± 1.39 a | 80.54 ± 3.87 b | 76.5 ± 6.02 b | 87.4 ± 0.88 a | 74.8 ± 4.68 b | 78.7 ± 3.21 | 83.5 ± 4.05 | 0.16 | 0.005 | 0.25 | 0.19 | 0.07 | 0.19 | 0.06 |
| CI | 8.0 ± 1.39 | 10.2 ± 1.65 | 13.1 ± 2.43 | 12.6 ± 0.94 a | 8.3 ± 1.92 b | 10.5 ± 1.84 | 10.3 ± 1.31 | 0.15 | 0.05 | 0.93 | 0.08 | 0.49 | 0.90 | 0.65 |
| Detritivores θ | 1398.5 ± 133.3 a | 1226.9 ± 182.7 a | 626.2 ± 103.1 b | 1007.13 ± 58.1 | 1160.6 188.3 | 1205.6 ± 144.1 | 962.1 ± 87.02 | 0.00001 | 0.20 | 0.05 | 0.0005 | 0.08 | 0.02 | 0.22 |
| Predators θ | 1130.6 ± 144.9 | 782.9 ± 161.2 | 725.6 ± 221.5 | 1287.0 ± 116.4 a | 472.4 ± 110.2 b | 729.04 ± 115.3 | 1030.3 ± 136.42 | 0.05 | 0.00001 | 0.04 | 0.12 | 0.19 | 0.18 | 0.48 |
| Plant parasitics θ | 314.2 ± 294.14 | 23.0 ± 3.52 | 55.5 ± 41.46 | 19.3 ± 2.6 | 242.5 ± 196.5 | 244.3 ± 170.03 | 17.6 ± 2.03 | 0.43 | 0.27 | 0.26 | 0.46 | 0.43 | 0.26 | 0.44 |
| Microarthropods | ||||||||||||||
| Dominance_D | 0.4 ± 0.03 a | 0.4 ± 0.04 a | 0.3 ± 0.02 b | 0.4 ± 0.03 | 0.4 ± 0.03 | 0.4 ± 0.02 | 0.4 ± 0.03 | 0.05 | 0.10 | 0.59 | 0.009 | 0.002 | 0.20 | 0.76 |
| Shannon_H | 1.3 ± 0.08 | 1.2 ± 0.09 | 1.3 ± 0.04 | 1.3 ± 0.05 | 1.2 ± 0.07 | 1.3 ± 0.06 | 1.2 ± 0.06 | 0.27 | 0.29 | 0.44 | 0.03 | 0.003 | 0.03 | 0.92 |
| Evenness_e^H/S | 0.4 ± 0.03 b | 0.4 ± 0.04 b | 0.5 ± 0.03 a | 0.4 ± 0.03 | 0.4 ± 0.03 | 0.4 ± 0.02 | 0.4 ± 0.04 | 0.0001 | 0.06 | 0.06 | 0.02 | 0.002 | 0.34 | 0.34 |
| Brillouin | 1.2 ± 0.07 | 1.1 ± 0.08 | 1.2 ± 0.04 | 1.2 ± 0.05 | 1.1 ± 0.06 | 1.2 ± 0.06 | 1.2 ± 0.05 | 0.47 | 0.14 | 0.47 | 0.02 | 0.002 | 0.05 | 0.94 |
| Menhinick | 0.6 ± 0.05 | 0.5 ± 0.06 | 0.7 ± 0.07 | 0.5 ± 0.03 b | 0.7 ± 0.06 a | 0.7 ± 0.06 | 0.6 ± 0.04 | 0.12 | 0.02 | 0.18 | 0.09 | 0.09 | 0.02 | 0.44 |
| Margalef | 1.6 ± 0.12 | 1.5 ± 0.11 | 1.4 ± 0.10 | 1.4 ± 0.07 | 1.6 ± 0.10 | 1.6 ± 0.10 | 1.4 ± 0.08 | 0.18 | 0.07 | 0.07 | 0.96 | 0.38 | 0.05 | 0.77 |
| Equitability_J | 0.5 ± 0.03 b | 0.5 ± 0.04 b | 0.7 ± 0.02 a | 0.6 ± 0.03 | 0.6 ± 0.03 | 0.6 ± 0.03 | 0.6 ± 0.03 | 0.0003 | 0.07 | 0.37 | 0.008 | 0.0009 | 0.15 | 0.52 |
| Fisher_alpha | 2.1 ± 0.18 | 1.9 ± 0.17 | 1.8 ± 0.17 | 1.8 ± 0.10 b | 2.2 ± 0.16 a | 2.1 ± 0.15 | 1.8 ± 0.11 | 0.39 | 0.04 | 0.06 | 0.79 | 0.31 | 0.03 | 0.66 |
| Berger–Parker | 0.6 ± 0.04 a | 0.6 ± 0.04 a | 0.5 ± 0.03 b | 0.5 ± 0.03 | 0.6 ± 0.03 | 0.6 ± 0.03 | 0.6 ± 0.04 | 0.05 | 0.09 | 0.90 | 0.003 | 0.001 | 0.12 | 0.93 |
| QBS-ar | 116.8 ± 7.51 a | 104.8 ± 7.44 a | 80.3 ± 7.95 b | 91.3 ± 4.59 b | 109.9 ± 8.46 a | 110.2 ± 6.91 a | 91.1 ± 6.67 b | 0.88 | 0.002 | 0.02 | 0.39 | 0.86 | 0.04 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landi, S.; Binazzi, F.; Papini, R.; d’Errico, G.; Del Duca, S.; Fabiani, A.; Mocali, S. Effect of Long-Term Set-Aside Management System on Soil Health by Evaluation of Biodiversity Indicators. Diversity 2025, 17, 240. https://doi.org/10.3390/d17040240
Landi S, Binazzi F, Papini R, d’Errico G, Del Duca S, Fabiani A, Mocali S. Effect of Long-Term Set-Aside Management System on Soil Health by Evaluation of Biodiversity Indicators. Diversity. 2025; 17(4):240. https://doi.org/10.3390/d17040240
Chicago/Turabian StyleLandi, Silvia, Francesco Binazzi, Rossella Papini, Giada d’Errico, Sara Del Duca, Arturo Fabiani, and Stefano Mocali. 2025. "Effect of Long-Term Set-Aside Management System on Soil Health by Evaluation of Biodiversity Indicators" Diversity 17, no. 4: 240. https://doi.org/10.3390/d17040240
APA StyleLandi, S., Binazzi, F., Papini, R., d’Errico, G., Del Duca, S., Fabiani, A., & Mocali, S. (2025). Effect of Long-Term Set-Aside Management System on Soil Health by Evaluation of Biodiversity Indicators. Diversity, 17(4), 240. https://doi.org/10.3390/d17040240








