Wildflower Strips Increase Aculeate Pollinator Diversity but Not Abundance in Agricultural Landscapes with Rapeseed in Crop Rotations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Data Collection
2.3. Statistical Analysis
3. Results
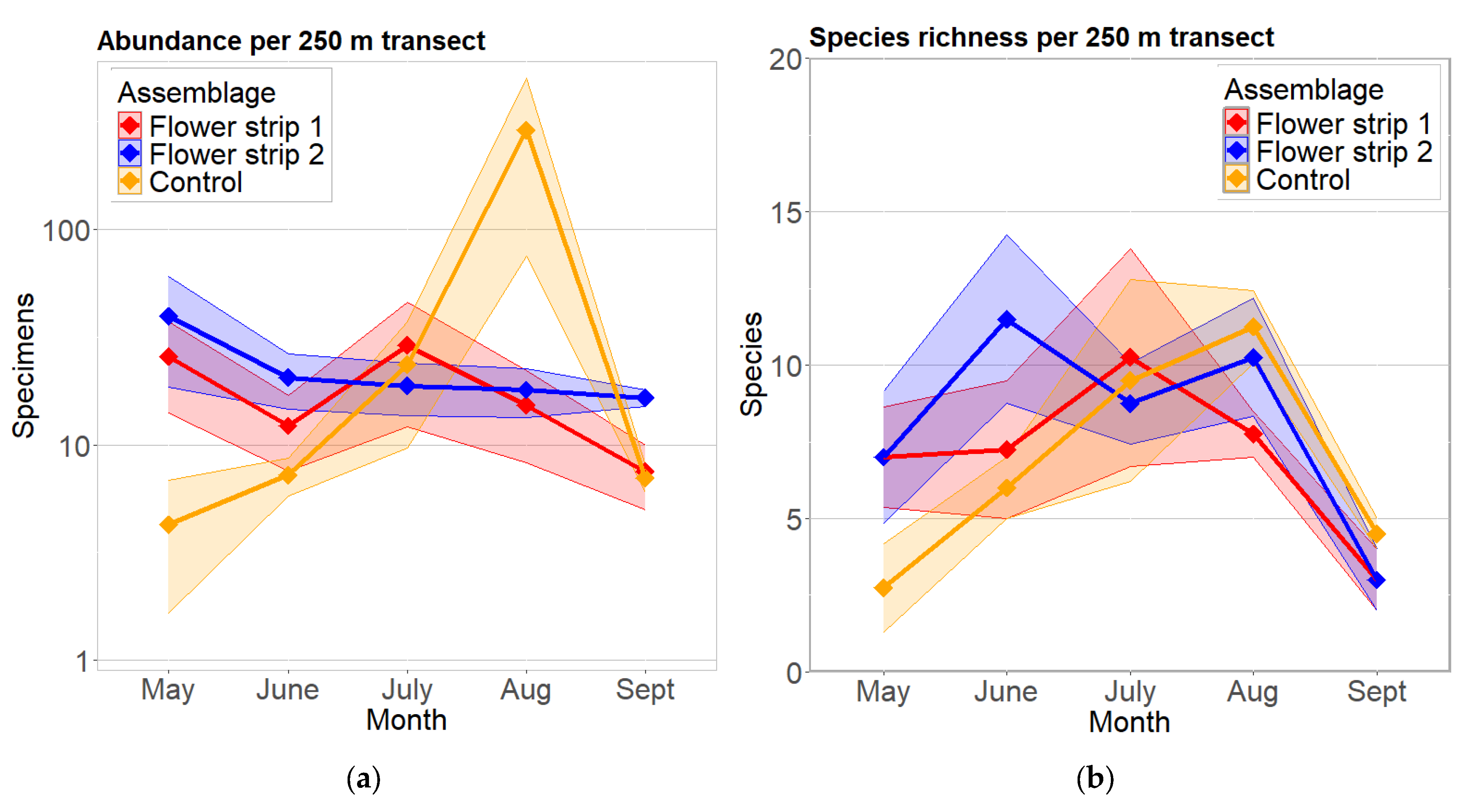
3.1. Species Richness and Abundance per 250 m Transect
3.2. Changes During Summer Season
3.3. Changes over Years
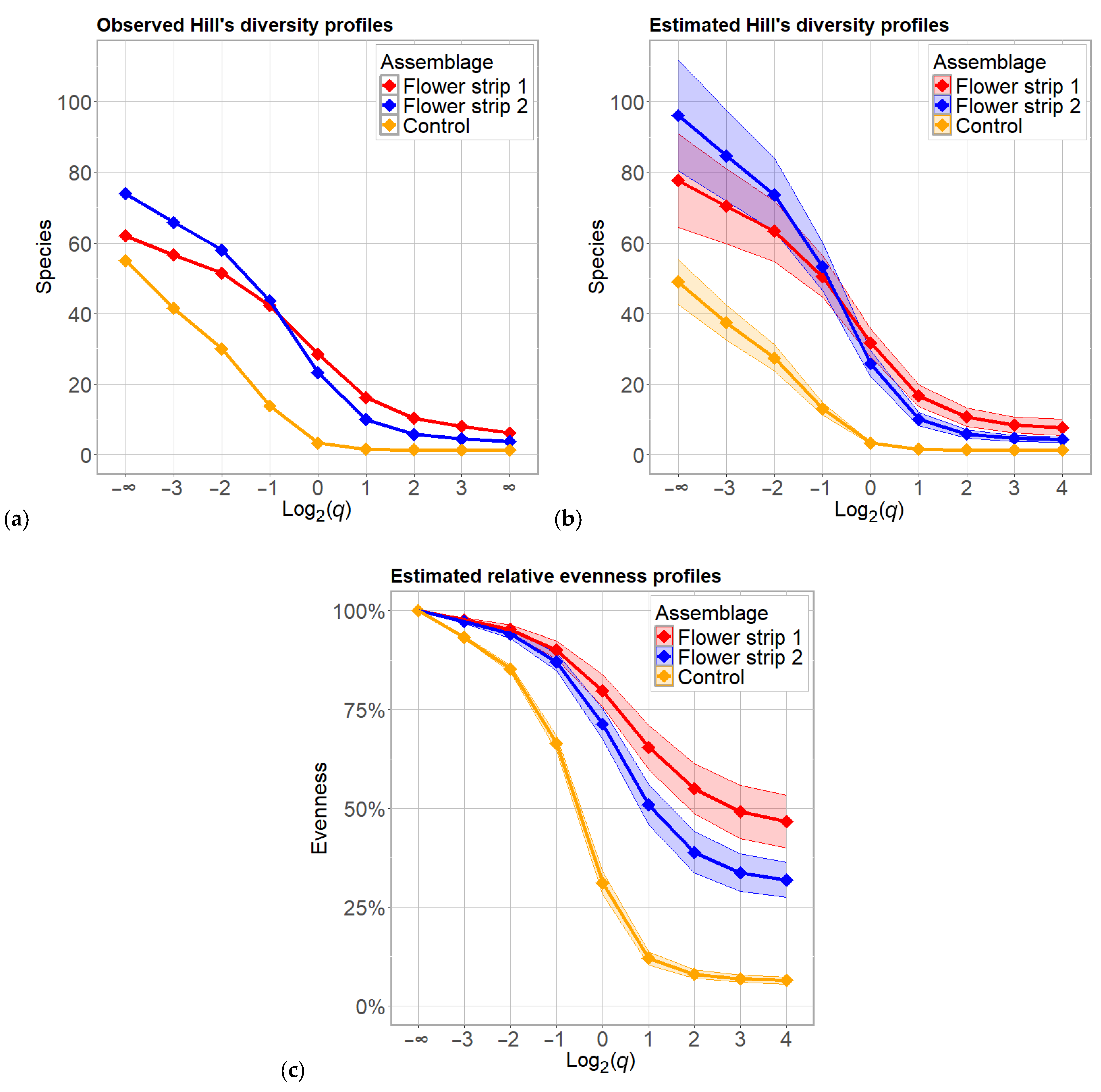
3.4. Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WFS | Wildflower strip |
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; LeBuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Pardo, A.; Borges, P.A.V. Worldwide importance of insect pollination in apple orchards: A review. Agric. Ecosyst. Environ. 2020, 293, 106839. [Google Scholar] [CrossRef]
- Stanley, D.A.; Msweli, S.M.; Johnson, S.D. Native honeybees as flower visitors and pollinators in wild plant communities in a biodiversity hotspot. Ecosphere 2020, 11, e02957. [Google Scholar] [CrossRef]
- Bishop, J.; Garratt, M.P.D.; Nakagawa, S. Animal pollination increases stability of crop yield across spatial scales. Ecol. Lett. 2022, 25, 2034–2047. [Google Scholar] [CrossRef]
- Devkota, K.; Ferreira, A.B.; Timberlake, T.P.; dos Santos, C.F. The impact of pollinator decline on global protein production: Implications for livestock and plant-based products. Glob. Ecol. Conserv. 2024, 50, e02815. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Zattara, E.E.; Aizen, M.A. Worldwide Occurrence Records Suggest a Global Decline in Bee Species Richness. One Earth 2021, 4, 114–123. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agriculture intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R.; Bartomeus, I.; Cariveau, D.P. Native pollinators in anthropogenic habitats. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 1–22. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; The Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Cole, L.J.; Kleijn, D.; Dicks, L.V.; Stout, J.C.; Potts, S.G.; Albrecht, M.; Balzan, M.V.; Bartomeus, I.; Bebeli, P.J.; Bevk, D.; et al. A critical analysis of the potential for EU Common Agricultural Policy measures to support wild pollinators on farmland. J. Appl. Ecol. 2020, 57, 681–694. [Google Scholar] [CrossRef]
- Heuel, K.C.; Ayasse, M.; Burger, H. Bee-diverse habitats positively affect seed set in wild plant species. Front. Ecol. Evol. 2024, 12, 1343885. [Google Scholar] [CrossRef]
- Pontarp, M.; Runemark, A.; Friberg, M.; Opedal, Ø.H.; Persson, A.S.; Wang, L.; Smith, H.G. Evolutionary plant–pollinator responses to anthropogenic land-use change: Impacts on ecosystem services. Biol. Rev. 2024, 99, 372–389. [Google Scholar] [CrossRef]
- Haaland, C.; Naisbit, R.E.; Bersier, L.-F. Sown wildflower strips for insect conservation: A review. Insect Conserv. Divers. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Ouvrard, P.; Transon, J.; Jacquemart, A.L. Flower-strip agri-environment schemes provide diverse and valuable summer flower resources for pollinating insects. Biodivers. Conserv. 2018, 27, 2193–2216. [Google Scholar] [CrossRef]
- Hellwig, N.; Schubert, L.F.; Kirmer, A.; Tischew, S.; Dieker, P. 2022. Effects of wildflower strips, landscape structure and agricultural practices on wild bee assemblages—A matter of data resolution and spatial scale? Agric. Ecosyst. Environ. 2022, 326, 107764. [Google Scholar] [CrossRef]
- Holland, J.M.; Bianchi, F.J.; Entling, M.H.; Moonen, A.C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest. Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef]
- Kleijn, D.; Bommarco, R.; Fijen, T.P.M.; Garibaldi, L.A.; Potts, S.G.; van der Putten, W.H. Ecological intensification: Bridging the gap between science and practice. Trends Ecol. Evol. 2019, 34, 154–166. [Google Scholar] [CrossRef]
- Kowalska, J.; Antkowiak, M.; Sienkiewicz, P. Flower strips and their ecological multifunctionality in agricultural fields. Agriculture 2022, 12, 1470. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.J.; Schröder, B.; Dauber, J.; Hellwig, N. Flower strip effectiveness for pollinating insects in agricultural landscapes depends on established contrast in habitat quality: A meta-analysis. Ecol. Solut. Evid. 2023, 4, e12261. [Google Scholar] [CrossRef]
- Jachowicz, N.; Sigsgaard, L. Highly diverse flower strips promote natural enemies more in annual field crops: A review and meta-analysis. Agric. Ecosyst. Environ. 2025, 381, 109412. [Google Scholar] [CrossRef]
- Uyttenbroeck, R.; Hatt, S.; Paul, A.; Boeraeve, F.; Piqueray, J.; Francis, F.; Danthine, S.; Frederich, M.; Dufrêne, M.; Bodson, B.; et al. Pros and cons of flowers strips for farmers. A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 1. [Google Scholar] [CrossRef]
- Uyttenbroeck, R.; Piqueray, J.; Hatt, S.; Mahy, G.; Monty, A. Increasing plant functional diversity is not the key for supporting pollinators in wildflower strips. Agric. Ecosyst. Environ. 2017, 249, 144–155. [Google Scholar] [CrossRef]
- Krimmer, E.; Martin, E.A.; Krauss, J.; Holzschuh, A.; Steffan-Dewenter, I. Size, age and surrounding semi-natural habitats modulate the effectiveness of flower-rich agri-environment schemes to promote pollinator visitation in crop fields. Agric. Ecosyst. Environ. 2019, 284, 106590. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Capera-Aragones, P.; Cartar, R.; Foxall, E.; Tyson, R.C. How can we enhance crops with flowers to increase pollination services and stop bee decline? Agric. Ecosyst. Environ. 2024, 367, 108964. [Google Scholar] [CrossRef]
- Lowe, E.B.; Groves, R.; Gratton, C. Field edge flower plantings have variable effects on wild bee abundance, richness, nesting success, and crop pollination, independent of the surrounding landscape. Agric. Ecosyst. Environ. 2024, 362, 108811. [Google Scholar] [CrossRef]
- Sutcliffe, L.M.E.; Batáry, P.; Kormann, U.; Báldi, A.; Dicks, L.V.; Herzon, I.; Kleijn, D.; Tryjanowski, P.; Apostolova, I.; Arlettaz, R.; et al. Harnessing the biodiversity value of Central and Eastern European farmland. Divers. Distrib. 2015, 21, 722–730. [Google Scholar] [CrossRef]
- Holland, J.M.; Douma, J.C.; Crowley, L.; James, L.; Kor, L.; Stevenson, D.R.; Smith, B.M. Semi-natural habitats support biological control, pollination and soil conservation in Europe. A review. Agron. Sustain. Dev. 2017, 37, 31. [Google Scholar] [CrossRef]
- Schmidt, A.; Kirmer, A.; Kiehl, K.; Tischew, S. Seed mixture strongly affects species-richness and quality of perennial flower strips on fertile soil. Basic Appl. Ecol. 2020, 42, 62–72. [Google Scholar] [CrossRef]
- Schütz, L.; Wenzel, B.; Rottstock, T.; Dachbrodt-Saaydeh, S.; Golla, B.; Kehlenbeck, H. How to promote multifunctionality of vegetated strips in arable farming: A qualitative approach for Germany. Ecosphere 2022, 13, e4229. [Google Scholar] [CrossRef]
- Albrecht, M.; Knecht, A.; Riesen, M.; Rutz, T.; Ganser, D. Time since establishment drives bee and hoverfly diversity, abundance of crop-pollinating bees and aphidophagous hoverflies in perennial wildflower strips. Basic Appl. Ecol. 2021, 57, 102–114. [Google Scholar] [CrossRef]
- Harmon-Threatt, A.N.; Hendrix, S.D. Prairie restorations and bees: The potential ability of seed mixes to foster native bee communities. Basic Appl. Ecol. 2015, 16, 64–72. [Google Scholar] [CrossRef]
- Kuppler, J.; Neumüller, U.; Mayr, A.V.; Hopfenmüller, S.; Weiss, K.; Prosi, R.; Burger, H. Favourite plants of wild bees. Agric. Ecosyst. Environ. 2023, 342, 108266. [Google Scholar] [CrossRef]
- Amy, C.; Noël, G.; Hatt, S.; Uyttenbroeck, R.; Van de Meutter, F.; Genoud, D.; Francis, F. Flower Strips in wheat intercropping system: Effect on pollinator abundance and diversity in Belgium. Insects 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.M.; Lonsdorf, E.V. Selecting cost-effective plant mixes to support pollinators. Biol. Conserv. 2018, 217, 195–202. [Google Scholar] [CrossRef]
- Scheper, J.; Bukovinszky, T.; Huigens, M.E.; Kleijn, D. Attractiveness of sown wildflower strips to flower-visiting insects depends on seed mixture and establishment success. Basic Appl. Ecol. 2021, 56, 401–415. [Google Scholar]
- Carvell, C.; Mitschunas, N.; McDonald, R.; Hulmes, S.; Hulmes, L.; O’Connor, R.S.; Garratt, M.P.D.; Potts, S.G.; Fountain, M.T.; Sadykova, D.; et al. Establishment and management of wildflower areas for insect pollinators in commercial orchards. Basic Appl. Ecol. 2022, 58, 2–14. [Google Scholar] [CrossRef]
- Nichols, R.N.; Holland, J.M.; Goulson, D. Can novel seed mixes provide a more diverse, abundant, earlier, and longer-lasting floral resource for bees than current mixes? Basic Appl. Ecol. 2022, 60, 34–47. [Google Scholar] [CrossRef]
- Müller, U.; Bruninga-Socolar, B.; Brokaw, J.; Cariveau, D.P.; Williams, N.M. Integrating perspectives on ecology, conservation value, and policy of bee pollinator seed mixes. Front. Ecol. Environ. 2024, 22, e2715. [Google Scholar] [CrossRef]
- Williams, N.M.; Ward, K.L.; Pope, N.; Isaacs, R.; Wilson, J.; May, E.A.; Ellis, J.; Daniels, J.; Pence, A.; Ullmann, K.; et al. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol. Appl. 2015, 25, 2119–2131. [Google Scholar] [CrossRef]
- Buhk, C.; Oppermann, R.; Schanowski, A.; Bleil, R.; Lüdemann, J.; Maus, C. Flower strip networks offer promising long term effects on pollinator species richness in intensively cultivated agricultural areas. BMC Ecol. 2018, 18, 55. [Google Scholar] [CrossRef]
- Warzecha, D.; Diekötter, T.; Wolters, V.; Jauker, F.; Didham, R.; Batáry, P. Attractiveness of wildflower mixtures for wild bees and hoverflies depends on some key plant species. Insect Conserv. Divers. 2018, 11, 32–41. [Google Scholar] [CrossRef]
- Nichols, R.N.; Goulson, D.; Holland, J.M. The best wildflowers for wild bees. J. Insect Conserv. 2019, 23, 819–830. [Google Scholar] [CrossRef]
- Burkle, L.A.; Delphia, C.M.; O’Neill, K.M. Redundancy in wildflower strip species helps support spatiotemporal variation in wild bee communities on diversified farms. Basic Appl. Ecol. 2020, 44, 1–13. [Google Scholar] [CrossRef]
- von Königslöw, V.; Mupepele, A.C.; Klein, A.M. Overlooked jewels: Existing habitat patches complement sown flower strips to conserve pollinators. Biol. Conserv. 2021, 261, 109263. [Google Scholar] [CrossRef]
- Schoch, K.; Tschumi, M.; Lutter, S.; Ramseier, H.; Zingg, S. Competition and facilitation effects of semi-natural habitats drive total insect and pollinator abundance in flower strips. Front. Ecol. Evol. 2022, 10, 854058. [Google Scholar] [CrossRef]
- Sutter, L.; Albrecht, M.; Jeanneret, P. Landscape greening and local creation of wildflower strips and hedgerows promote multiple ecosystem services. J. Appl. Ecol. 2018, 55, 612–620. [Google Scholar] [CrossRef]
- Boetzl, F.A.; Krimmer, E.; Krauss, J.; Steffan-Dewenter, I. Agri-environmental schemes promote ground-dwelling predators in adjacent oilseed rape fields: Diversity, species traits and distance-decay functions. J. Appl. Ecol. 2019, 56, 10–20. [Google Scholar] [CrossRef]
- Alarcón-Segura, V.; Grass, I.; Breustedt, G.; Rohlfs, M.; Tscharntke, T. Strip intercropping of wheat and oilseed rape enhances biodiversity and biological pest control in a conventionally managed farm scenario. J. Appl. Ecol. 2022, 59, 1513–1523. [Google Scholar] [CrossRef]
- Serée, L.; Barbottin, A.; Chiron, F.; Valantin-Morison, M.; Gardarin, A. Within-field floral resources have the potential to increase parasitism rates in winter oilseed rape pests more than resources at field margins. Agric. Ecosyst. Environ. 2023, 344, 108288. [Google Scholar] [CrossRef]
- Diekötter, T.; Kadoya, T.; Peter, F.; Wolters, V.; Jauker, F. Oilseed rape crops distort plant–pollinator interactions. J. Appl. Ecol. 2010, 47, 209–214. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dainese, M.; Gonzalez-Varo, J.P.; Mudri-Stojnic, S.; Riedinger, V.; Rundlof, M.; Scheper, J.; Wickens, J.B.; Wickens, V.J.; Bommarco, R.; et al. Mass-flowering crops dilute pollinator abundance in agricultural landscapes across Europe. Ecol. Lett. 2016, 19, 1228–1236. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Ekroos, J.; Dänhardt, J.; Andersson, G.K.S.; Olsson, O.; Smith, H.G. Sown flower strips in southern Sweden increase abundances of wild bees and hoverflies in the wider landscape. Biol. Conserv. 2015, 184, 51–58. [Google Scholar] [CrossRef]
- Osterman, J.; Theodorou, P.; Radzevičiūtė, R.; Schnitker, P.; Paxton, R.J. Apple pollination is ensured by wild bees when honey bees are drawn away from orchards by a mass co-flowering crop, oilseed rape. Agric. Ecosyst. Environ. 2021, 315, 2021107383. [Google Scholar] [CrossRef]
- Harris, C.; Balfour, N.J.; Ratnieks, F.L.W. Floral Resource Wastage: Most Nectar Produced by the Mass-flowering Crop Oilseed Rape (Brassica napus) Is Uncollected by Flower-visiting Insects. Ecol. Evol. 2024, 14, e11453. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Marini, L.; Vaissière, B.E. Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia 2012, 169, 1025–1032. [Google Scholar] [CrossRef]
- Morandin, L.A.; Winston, M.L. Wild bee abundance and seed production in conventional, organica, and genetically modified canola. Ecol. Appl. 2005, 15, 871–881. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dormann, C.F.; Tscharntke, T.; Steffan-Dewenter, I. Expansion of mass-flowering crops leads to transient pollinator dilution and reduced wild plant pollination. Proc. R. Soc. B 2011, 278, 3444–3451. [Google Scholar] [CrossRef]
- Keitt, T.H. Habitat conversion, extinction thresholds, and pollination services in agroecosystems. Ecol. Appl. 2009, 19, 1561–1573. [Google Scholar] [CrossRef]
- Häussler, J.; Sahlin, U.; Baey, C.; Smith, H.G.; Clough, Y. Pollinator population size and pollination ecosystem service responses to enhancing floral and nesting resources. Ecol. Evol. 2017, 7, 1898–1908. [Google Scholar] [CrossRef]
- Ebeling, A.; Klein, A.-M.; Schumacher, J.; Weisser, W.W.; Tscharntke, T. How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 2008, 117, 1808–1815. [Google Scholar] [CrossRef]
- von Königslöw, V.; Fornoff, F.; Klein, A.-M. Wild bee communities benefit from temporal complementarity of hedges and flower strips in apple orchards. J. Appl. Ecol. 2022, 59, 2814–2824. [Google Scholar] [CrossRef]
- von Königslöw, V.; Fornoff, F.; Klein, A.-M. Pollinator enhancement in agriculture: Comparing sown flower strips, hedges and sown hedge herb layers in apple orchards. Biodivers. Conserv. 2022, 31, 433–451. [Google Scholar] [CrossRef]
- Sentil, A.; Reverté, S.; Lhomme, P.; Bencharki, Y.; Rasmont, P.; Christmann, S.; Michez, D. Wild vegetation and ‘farming with alternative pollinators’ approach support pollinator diversity in farmland. J. Appl. Entomol. 2022, 146, 1155–1168. [Google Scholar] [CrossRef]
- Sann, M.; Niehuis, O.; Peters, R.S.; Mayer, C.; Kozlov, A.; Podsiadlowski, L.; Bank, S.; Meusemann, K.; Misof, B.; Bleidorn, C.; et al. Phylogenomic analysis of Apoidea sheds new light on the sister group of bees. BMC Evol. Biol. 2018, 18, 71. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Home Page. 2025. Available online: https://www.gbif.org (accessed on 3 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2025. [Google Scholar]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity/differentiation measures through Hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Jost, L. The relation between evenness and diversity. Diversity 2010, 2, 207–232. [Google Scholar] [CrossRef]
- Chao, A.; Ricotta, C. Quantifying evenness and linking it to diversity, beta diversity, and similarity. Ecology 2019, 100, e02852. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Kubota, Y.; Zelený, D.; Chiu, C.-H.; Li, C.-F.; Kusumoto, B.; Yasuhara, M.; Thorn, S.; Wei, C.-L.; Costello, M.J.; et al. Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 2020, 35, 292–314. [Google Scholar] [CrossRef]
- da Silva, I.A.; Batalha, M.A. Taxonomic distinctness and diversity of a hyperseasonal savanna in central Brazil. Divers. Distrib. 2006, 12, 725–730. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Blaauw, B.R.; Isaacs, R. Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J. Appl. Ecol. 2014, 51, 890–898. [Google Scholar] [CrossRef]
- Westphal, C.; Steffan-Dewenter, I.; Tscharntke, T. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 2003, 6, 961–965. [Google Scholar] [CrossRef]
- Biegerl, C.; Holzschuh, A.; Tanner, B.; Sponsler, D.; Krauss, J.; Zhang, J.; Steffan-Dewenter, I. Landscape management can foster pollinator richness in fragmented high-value habitats. Proc. R. Soc. B 2025, 292, 20242686. [Google Scholar] [CrossRef] [PubMed]
- Hadrava, J.; Talašová, A.; Straka, J.; Benda, D.; Kazda, J.; Klečka, J. A comparison of wild bee communities in sown flower strips and semi-natural habitats: A pollination network approach. Insect Conserv. Divers. 2022, 15, 312–324. [Google Scholar] [CrossRef]
- Balfour, N.J.; Ratnieks, F.L. The disproportionate value of ‘weeds’ to pollinators and biodiversity. J. Appl. Ecol. 2022, 59, 1209–1218. [Google Scholar] [CrossRef]
- Fountain, M.T. Impacts of wildflower interventions on beneficial insects in fruit crops: A review. Insects 2022, 13, 304. [Google Scholar] [CrossRef]
- Twerski, A.; Albrecht, H.; Fründ, J.; Moosner, M.; Fischer, C. Effects of rare arable plants on flower-visiting wild bees in agricultural fields. Agric. Ecosyst. Environ. 2021, 323, 107685. [Google Scholar] [CrossRef]
- Vujanović, D.; Losapio, G.; Mészáros, M.; Popov, S.; Markov Ristić, Z.; Mudri Stojnić, S.; Jović, J.; Vujić, A. Forest and grassland habitats support pollinator diversity more than wildflowers and sunflower monoculture. Ecol. Entomol. 2023, 48, 421–432. [Google Scholar] [CrossRef]



| Plants from the Seed Mixture Lebensraum I in WFS | Wild Entomophilous Plants, Naturally Invading the WFS | Wild Entomophilous Plants in Semi-Natural Grassland of the Control Site |
|---|---|---|
| Achillea millefolium Agrimonia eupatoria Anethum graveolens * Anthriscus sylvestris Borago officinalis * Camelina sativa * Carthamus tinctorius * Carum carvi Centaurea cyanus Cerastium holosteoides Cichorium intybus Clinopodium vulgare Cota tinctoria Crepis biennis Daucus carota Dipsacus fullonum * Echium vulgare Fagopyrum esculentum * Galium album Galium verum Helianthus annuus * Heracleum sphondylium Leucanthemum ircutianum Linum usitatissimum * Malva moschata Malva sylvestris Medicago sativa * Papaver rhoeas Pastinaca sativa Phacelia tanacetifolia * Plantago lanceolata Poterium sanguisorba Salvia pratensis Silene dioica Silene flos-cuculi Silene latifolia Silene vulgaris Tanacetum vulgare Trifolium incarnatum * Trifolium resupinatum * | Arctium lappa Barbarea vulgaris Capsella bursa-pastoris Chaerophyllum aromaticum Cirsium arvense Erodium cicutarium Erysimum cheiranthoides Galeopsis tetrahit Hypericum perforatum Knautia arvensis Lamium purpureum Medicago lupulina Papaver dubium Polygonum aviculare Sonchus arvensis Stellaria media Taraxacum officinale Thlaspi arvense Trifolium repens Tripleurospermum inodorum Veronica filiformis Vicia cracca Viola arvensis | Achillea millefolium Aegopodium podagraria Angelica sylvestris Anthriscus sylvestris Arctium minus Barbarea vulgaris Centaurea cyanus Centaurea jacea Cichorium intybus Cirsium arvense Cirsium olearceum Cirsium vulgare Daucus carota Euphrasia officinalis Filipendula ulmaria Galium verum Hypericum perforatum Iris pseudacorus Knautia arvensis Lathyrus pratensis Lycopus europaeus Lysimachia vulgaris Lythrum salicaria Medicago falcata Melilotus albus Melilotus officinalis Papaver rhoeas Pentanema salicinum Ranunculus acris Silene flos-cuculi Silene latifolia Solidago virgaurea Taraxacum officinale Thalictrum flavum Thalictrum lucidum Tripleurospermum inodorum Tussilago farfara Valeriana officinalis Veronica officinalis Vicia cracca Viola arvensis |
| Species | Flower Strip 1 | Flower Strip 2 | Control | Species | Flower Strip 1 | Flower Strip 2 | Control |
|---|---|---|---|---|---|---|---|
| Apidae | Andrenidae | ||||||
| Anthophora furcata | 0 | 1 | 0 | Andrena alfkenella | 2 | 0 | 1 |
| Bombus bohemicus | 1 | 0 | 3 | Andrena bicolor | 0 | 2 | 0 |
| Bombus hortorum | 4 | 4 | 0 | Andrena cineraria | 0 | 1 | 0 |
| Bombus humilis | 0 | 1 | 1 | Andrena dorsata | 5 | 3 | 0 |
| Bombus jonellus | 0 | 2 | 1 | Andrena flavipes | 2 | 2 | 0 |
| Bombus lapidarius | 3 | 5 | 6 | Andrena fulvago | 1 | 2 | 0 |
| Bombus lucorum | 4 | 3 | 3 | Andrena haemorrhoa | 1 | 2 | 0 |
| Bombus magnus | 2 | 0 | 0 | Andrena helvola | 0 | 1 | 0 |
| Bombus muscorum | 0 | 1 | 0 | Andrena jacobi | 0 | 0 | 1 |
| Bombus norvegicus | 0 | 0 | 1 | Andrena minutula | 4 | 1 | 2 |
| Bombus pascuorum | 16 | 5 | 5 | Andrena minutuloides | 56 | 33 | 6 |
| Bombus ruderarius | 2 | 1 | 5 | Andrena nigroaenea | 1 | 1 | 1 |
| Bombus rupestris | 1 | 0 | 0 | Andrena ovatula | 0 | 3 | 0 |
| Bombus soroeensis | 4 | 3 | 1 | Andrena pilipes | 1 | 1 | 0 |
| Bombus sylvarum | 37 | 9 | 17 | Andrena praecox | 0 | 0 | 1 |
| Bombus terrestris | 7 | 35 | 8 | Andrena wilkella | 7 | 3 | 1 |
| Bombus vestalis | 0 | 1 | 0 | Melittidae | |||
| Bombus veteranus | 12 | 2 | 9 | Dasypoda hirtipes | 1 | 0 | 0 |
| Eucera longicornis | 3 | 4 | 1 | Melitta leporina | 8 | 1 | 0 |
| Nomada castellana | 0 | 3 | 0 | Bembicidae | |||
| Nomada flavoguttata | 2 | 0 | 0 | Gorytes quinquecinctus | 0 | 0 | 1 |
| Nomada lathburiana | 0 | 1 | 0 | Crabronidae | |||
| Nomada marshamella | 0 | 1 | 0 | Crabro cribrarius | 2 | 0 | 12 |
| Nomada panzeri | 0 | 1 | 0 | Crabro peltarius | 0 | 0 | 1 |
| Nomada rufipes | 1 | 0 | 0 | Crossocerus podagricus | 0 | 2 | 0 |
| Nomada succincta | 0 | 1 | 0 | Ectemnius continuus | 0 | 0 | 11 |
| Megachilidae | Ectemnius fossorius | 0 | 0 | 4 | |||
| Coelioxys conoidea | 0 | 1 | 0 | Ectemnius lapidarius | 1 | 0 | 0 |
| Heriades truncorum | 2 | 3 | 0 | Entomognatus brevis | 1 | 0 | 0 |
| Megachile centuncularis | 0 | 2 | 0 | Lindenius albilabris | 5 | 3 | 1 |
| Megachile versicolor | 0 | 1 | 0 | Oxybelus trispinosus | 0 | 2 | 1 |
| Osmia bicolor | 1 | 0 | 1 | Pemphredonidae | |||
| Osmia rufa | 2 | 2 | 0 | Diodontus luperus | 0 | 1 | 0 |
| Osmia spinulosa | 4 | 2 | 0 | Pemphredon inornata | 0 | 1 | 0 |
| Halictidae | Philanthidae | ||||||
| Halictus maculatus | 3 | 4 | 1 | Cerceris quinquefasciata | 0 | 2 | 0 |
| Halictus quadricinctus | 1 | 1 | 2 | Cerceris ruficornis | 0 | 0 | 1 |
| Halictus sexcinctus | 1 | 0 | 0 | Cerceris rybyensis | 1 | 0 | 0 |
| Halictus subauratus | 6 | 10 | 2 | Philanthus triangulum | 0 | 1 | 0 |
| Halictus tumulorum | 29 | 13 | 40 | Psenidae | |||
| Lasioglossum albipes | 0 | 0 | 1 | Mimumesa unicolor | 1 | 0 | 0 |
| Lasioglossum calceatum | 4 | 27 | 14 | Psenulus pallipes | 2 | 0 | 0 |
| Lasioglossum leucopus | 15 | 5 | 2 | Sphecidae | |||
| Lasioglossum leucozonium | 1 | 2 | 8 | Ammophila sabulosa | 0 | 1 | 0 |
| Lasioglossum morio | 1 | 1 | 11 | Chrysididae | |||
| Lasioglossum nitidiusculum | 2 | 0 | 1 | Pseudochrysis neglecta | 0 | 1 | 0 |
| Lasioglossum pauxillum | 24 | 111 | 1039 | Pompilidae | |||
| Lasioglossum quadrinotatum | 1 | 0 | 0 | Ceropales maculata | 0 | 0 | 1 |
| Lasioglossum sexnotatum | 1 | 0 | 1 | Arachnospila anceps | 0 | 1 | 0 |
| Lasioglossum zonulum | 4 | 3 | 4 | Tiphiidae | |||
| Sphecodes crassus | 0 | 4 | 0 | Tiphia femorata | 0 | 1 | 0 |
| Sphecodes ephippius | 0 | 1 | 4 | Vespidae | |||
| Sphecodes geoffrellus | 0 | 2 | 0 | Ancistrocerus nigricornis | 1 | 0 | 0 |
| Sphecodes gibbus | 0 | 2 | 0 | Dolichovespula saxonica | 0 | 0 | 25 |
| Sphecodes pellucidus | 1 | 0 | 0 | Dolichovespula sylvestris | 2 | 0 | 5 |
| Sphecodes scabricollis | 0 | 0 | 2 | Gymnomerus laevipes | 1 | 0 | 0 |
| Colletidae | Odynerus melanocephalus | 0 | 2 | 0 | |||
| Colletes daviesanus | 2 | 15 | 0 | Odynerus reniformis | 1 | 2 | 0 |
| Hylaeus brevicornis | 0 | 0 | 1 | Polistes dominula | 17 | 35 | 13 |
| Hylaeus communis | 0 | 1 | 1 | Polistes nimpha | 0 | 1 | 8 |
| Hylaeus confusus | 2 | 0 | 1 | Vespula germanica | 6 | 1 | 2 |
| Hylaeus nigritus | 11 | 8 | 0 | Vespula rufa | 2 | 0 | 3 |
| Vespula vulgaris | 0 | 1 | 12 | ||||
| Total: | 349 | 419 | 1311 |
| Estimator | Flower Strip 1 | Flower Strip 2 | Control |
|---|---|---|---|
| Observed richness | 62 | 74 | 55 |
| Estimated Chao richness | 79.2 ± 9.6 (68.2–109.8) | 102.2 ± 13.3 (85.7–142.0) | 96.1 ± 23.8 (69.4–172.7) |
| Observed Shannon | 28.5 | 23.3 | 3.30 |
| Estimated Chao Shannon | 32.4 ± 2.4 (28. 5–37.1) | 26.7 ± 2.4 (23.3–31.4) | 3.43 ± 0.21 (3.30–3.83) |
| Observed Simpson | 16.2 | 9.92 | 1.57 |
| Estimated Chao Simpson | 17.0 ± 1.4 (16.2–19.7) | 10.1 ± 1.0 (9.9–12.0) | 1.58 ± 0.04 (1.57–1.65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budrys, E.; Budrienė, A.; Lazauskaitė, M.; Skuja, J.A.; Skujienė, G. Wildflower Strips Increase Aculeate Pollinator Diversity but Not Abundance in Agricultural Landscapes with Rapeseed in Crop Rotations. Diversity 2025, 17, 263. https://doi.org/10.3390/d17040263
Budrys E, Budrienė A, Lazauskaitė M, Skuja JA, Skujienė G. Wildflower Strips Increase Aculeate Pollinator Diversity but Not Abundance in Agricultural Landscapes with Rapeseed in Crop Rotations. Diversity. 2025; 17(4):263. https://doi.org/10.3390/d17040263
Chicago/Turabian StyleBudrys, Eduardas, Anna Budrienė, Miglė Lazauskaitė, Jonas A. Skuja, and Grita Skujienė. 2025. "Wildflower Strips Increase Aculeate Pollinator Diversity but Not Abundance in Agricultural Landscapes with Rapeseed in Crop Rotations" Diversity 17, no. 4: 263. https://doi.org/10.3390/d17040263
APA StyleBudrys, E., Budrienė, A., Lazauskaitė, M., Skuja, J. A., & Skujienė, G. (2025). Wildflower Strips Increase Aculeate Pollinator Diversity but Not Abundance in Agricultural Landscapes with Rapeseed in Crop Rotations. Diversity, 17(4), 263. https://doi.org/10.3390/d17040263






