Abstract
Analyzing the nutritional and defensive chemistry of Quercus rugosa provides insight into gall wasp interactions. Quercus rugosa is the most widely distributed white oak species in Mexico. It is the dominant canopy species in its geographic distribution range and has the largest number of associated gall wasp species (Cynipidae: Cynipini). Our main aims were to characterize the nutritional and defensive chemistry of Q. rugosa leaves and determine whether this chemistry differed between leaves with and without galls. We evaluated 60 trees from six populations of Q. rugosa in central Mexico. For each tree, we analyzed the nutritional chemistry (nitrogen, phosphorus, carbon, and carbon/nitrogen ratio) and defensive chemistry (secondary metabolites). Also, we characterized the community of cynipids in the leaf tissue of each tree. We documented 18 cynipid species, and the cynipid communities differed in composition among localities. We recorded the presence of a total of ten phenolics. The composition of nutritional and defensive chemicals differed significantly between leaves with and without galls in each locality. The nutritional and defensive chemical compounds of Q. rugosa were influenced by their associated cynipids. Our results suggest that gall-inducing cynipids influence the production of secondary metabolites in leaves with galls through the reassignment of nutritional compounds by the hosts.
1. Introduction
Forest canopies contain a high number of organisms on Earth [1,2] and are primarily dominated by insects. For the study of insect communities associated with the canopy, trees serve as model organisms, primarily due to their straightforward physical delimitation and the fact that they provide resources and conditions that support the growth of insects [3,4].
Phytophagous insects are directly associated with a limited number of plants that fulfill their nutritional requirements because food quality is more important than quantity. Nitrogen, carbon, and phosphorus are the most limiting nutrients for the development and reproduction of phytophagous insects [5,6,7]; so, the content of these elements is expected to influence phytophagous insects’ selection of their host plants [7,8,9]. Therefore, plant species invest resources in producing substances to defend themselves, especially those that are difficult for their phytophagous insects to digest or excrete, making plant tissues less palatable [10]. The base of chemical defense (secondary metabolites, “SMs”) in plants consists of the accumulation of diverse types of toxic proteins, terpenes, alkaloids, and phenols. These SMs tend to be toxic, even to the plants themselves; so, they are often stored in cellular vacuoles and released only after the cells are damaged due to herbivory [11]. The production and storage of SMs is costly to the plant; so, it confronts a trade-off between the cost of defense and reproductive effort [12].
Secondary metabolites are involved in numerous processes that impact plants’ survival, defense, and reproduction [6,10,13]. It has been suggested that SMs may act as intermediaries in interactions between herbivores, pollinators, and predators, as well as in defense against abiotic stress [14,15]. Some specialist insects have evolved mechanisms that allow them to withstand and even utilize SMs to their advantage [16,17]. For these reasons, changes in the expression and production of SMs by the host plant can influence the structure of the community of herbivorous, parasitoid, and predatory insects [18,19].
The oaks (Fagaceae: Quercus) are commonly associated with gall-inducing wasps (Hymenoptera: Cynipidae: Tribe Cynipini). Cynipids are highly specialized, with each wasp species attacking a single tissue, organ, and oak species [20]. Gall inducers control and manipulate the host plant’s development, morphology, anatomy, physiology, and chemistry [21] to their benefit. Some benefits include a habitat that offers food and protects larvae from natural enemies [22,23]. As plant tissue, galls mobilize plant resources, thereby increasing the nutritional value for their inducers [22,23,24]. Because inducing insects control gall formation, these structures are considered an extension of their phenotype [23,25]. Galls result from very specific metabolic interactions between the gall inducers and their host plants [26], and gall inducers are often considered the most highly evolved herbivores [27]. Plants respond to galling herbivores by producing and accumulating SMs [28]. This accumulation is considered the initiator of the whole process of gall induction and development [29]. The developmental process involves calcium ion fluxes, phosphorylation cascades, and, specifically, the jasmonate pathway, which plays a central role in promoting resistance to a wide spectrum of insects [30].
It has therefore been proposed that the Cynipidae–Quercus interaction is one of the most specialized relationships [20,31]. Gall-inducing cynipids can affect the growth of the host plant [32] and can even control the chemical composition of the tissues that form the gall [33,34].
Galls induced by cynipids give rise to a closely associated community of occupants, including cynipids, flies, moths, beetles, and parasitoids [35]. It has been suggested that by inducing galls, cynipids act as ecosystem engineers [36], generating new habitats for arthropods [37]. In particular, it has been documented that the composition of the insect community that is associated with the oak canopy is one of the parameters that best responds to factors such as the species of the host tree [38], locality [37], seasonality [39,40], vegetation type [41], genetic class of the host plant [39], and biotic interactions [42].
The specialization of Cynipid in Q. rugosa, considered the best-represented oak species in Mexico due to its wide geographic distribution and dominance in the canopy, exhibits the highest number of associated gall-inducing species, comprising 52 Cynipini species [43]. It is also the Mexican oak species with the highest SMs, comprising 13 phenolic compounds and three terpenoids [44].
Several studies have shown that the initiation, growth, and maintenance of galls on leaves, stems, fruits, or buds can modify multiple host plant traits, including nutrient allocation [45], shoot growth [46], plant architecture [47], and metabolite production [34]. However, few studies have quantified the correlation between cynipid diversity and SM variation in dominant canopy species, such as Q. rugosa.
This study tested the hypothesis that gall induction in oak trees leads to a change in the chemical profiles of nutritional and defensive substances in the host oak plant, with a relevant role in the development of the Quercus–Cynipini interaction [34,48]. We addressed the following questions: (i) Do gall-inducing cynipids influence the nutritional and defensive chemical composition of the leaf tissue of the Q. rugosa? (ii) Does the composition of gall-inducing insects (Cynipini) vary among localities? (iii) Which nutritional and defensive elements of Q. rugosa influence the gall-inducing insects?
2. Materials and Methods
2.1. Study Species
Quercus rugosa (Neé) is a tree species whose height can reach 20 m and whose trunk diameter can reach 1 m. The species is distributed in the most important mountainous zones of Mexico, including the Sierra Madre Oriental, Sierra Madre Occidental, Sierra Madre del Sur, Sierra Norte de Oaxaca, Trans-Mexican Volcanic Belt (TMVB), and Sierra de Chiapas (these areas stand out for their geological, topographical, and climatic characteristics, which have been crucial for the establishment and diversification of oak species), at altitudes ranging from 1800 to 2900 m. It is considered the most widely distributed oak species in Mexico, and its presence has been documented in Central America and the United States [49]. Quercus rugosa is present in Pinus, Quercus, Pinus-Quercus, and tropical montane cloud forests.
2.2. Study Sites and Population Sampling
We performed our sampling during March–April and July–August of 2019 in six localities: Parque Nacional El Chico, Mineral del Monte, and Omitlán de Juárez in the state of Hidalgo; Sierra de Guadalupe in Mexico State; and Calcahualco and Tlaquetzaltitla in the state of Veracruz (Table 1). We selected these localities in order to decrease the influence of geological, historical, and environmental factors on the structure of the community of gall-inducing cynipids and their parasitoids because these locations share several important characteristics. All the sites shared these characteristics: they are in the TMVB and have a common geological history (formation during a single geological event during the Quaternary–Pliocene [50]); they have a temperate subhumid climate, are mature oak forests and have soil of volcanic origin or derived from igneous and sedimentary rock. We sampled 10 oak trees per locality. Each individual was identified morphologically, and a specimen from each individual was deposited at the HUMO herbarium of the Autonomous University of the State of Morelos (Universidad Autónoma del Estado de Morelos), Mexico.

Table 1.
Study sites of six Q. rugosa populations located across the Trans-Mexican Volcanic Belt in Mexico. Locality name, state, altitude, and geographic coordinates are given.
In each population, the individuals were selected randomly by sampling each 100 m of the nearest individual on a 1000 m transect. To establish the transect line at all collection sites, we used a GPS to mark the starting point and extend the measuring tape in a straight line along the north–south direction. The trees had a height between 8 and 10 m (mean ± standard error) (9.20 ± 0.55 m), and the DBH of the trees was 43.7 ± 8.3 cm (mean ± standard deviation). We collected leaf tissue using a “paired branches” protocol, which was standardized for this study. This consisted of first locating a secondary branch in the middle part of the canopy of each oak tree (approximately 6 m high) with cynipid galls in the leaf tissue and collecting its leaves (without galls). Then, we identified and collected leaves of a different secondary branch that did not contain cynipid-induced galls and with no apparent damage by other herbivores or physical damage; this branch was parallel to the first branch and attached to the same primary branch. An 8.5 m ladder was used to access the crown of each tree, and branch collection was limited to the middle canopy. This allowed us to evaluate the response of the plants to the herbivore attack (which previous work has shown is localized, not systemic [51,52]) while controlling other factors related to branch location, such as orientation and light exposition.
2.3. Nutritional Chemistry
A total of 120 samples of leaf tissue (mature leaves with no apparent damage were collected in late July and early August) from the two treatments (with and without galls) of Q. rugosa belonging to each of the six localities (10 individuals per population). The samples were dried at room temperature in the shade. The leaves were ground to obtain 5 g of leaf sample per individual. Then, the dried and ground material was used to determine the percentage of nitrogen (%N), percentage of carbon (%C), and total phosphorus (ppm P). The percentage of nitrogen was determined by the Kjeldahl method [53]. The C/N ratio was obtained by dividing the percent carbon by the percent nitrogen.
2.4. Defensive Chemistry: Secondary Metabolites
To identify the most abundant compounds (majority compounds), a pool of leaves of each treatment (with and without galls) from each population of individuals of Q. rugosa was dried at room temperature and ground to obtain 100 g of fine powder. The crude extract of each treatment (n = 12; six with galls and six without) was obtained by macerating 1 g of pulverized leaf tissue in acetone for 24 h. Following maceration, the mixture was filtered using Whatman filter paper number 20 (Merck, Darmstadt, Germany), and the solvent was removed by distillation under reduced pressure in a rotavapor Please include (BUCHI R-114, Flawil, Switzerland). We performed comparative tests on the extract using thin-layer chromatography (TLC). Then, the solvent was eliminated through distillation at reduced pressure with a rotary evaporator. This procedure was performed in triplicate. The average yield was 8%. The acetonic extract was then analyzed using TLC. We used normal-phase and reverse-phase silica gel plates (Merck, Darmstadt, Germany), and we employed the NP-PEG reagent to detect flavonoids and the Komorovsky reaction to detect terpenes [54].
Then, we analyzed 0.2 mg of crude extract per treatment from the six study populations using high-performance liquid chromatography (HPLC, Waters, Milford, MA, USA); for further detail, consult Castillo-Mendoza and colleagues [44]. This methodology identified seven major phenolic compounds by directly comparing retention times and spectral behavior with known commercial and internal standards (isolated and elucidated). The same methodology was used to characterize the seven main SMs at the individual level.
2.5. Collection of Gall and Determination of Insects
Ten additional oak trees per location were sampled to characterize the cynipid community. In total, we collected galls from the leaves of 20 individuals of Q. rugosa associated with each of the six localities. Previous studies have shown that 20 trees are an adequate sample size to represent the community of gall-inducing insects in Q. rugosa by location [55]. The galls were transported to the laboratory in paper bags, separated by morphotype into transparent plastic containers, and monitored constantly for the emergence of cynipids. After emerging, they were placed in 70% alcohol and processed and mounted for determination. The insects were determined using specialized keys for gall-inducing insects [56]. Finally, the abundance of each cynipid species was recorded.
2.6. Statistical Analysis
The data were subjected to Shapiro–Wilk tests to verify normality and Levene tests to confirm homoscedasticity. Since the data fulfilled the two assumptions mentioned, we used parametric analyses. To evaluate the effects of locality (L), treatment (T; leaves with and without galls), and their interaction (L × T) on the nutritional and defensive leaf chemistry in Q. rugosa, we performed a two-way analysis of variance. Then, we performed post hoc (Tukey) tests to detect significant differences between leaves with and without galls within each sampling locality [57].
To determine whether there were differences in the composition of the nutritional chemicals in Q. rugosa leaves with and without galls, we used a nonmetric multidimensional scaling analysis (NMDS) based on N, P, C, and C/N. The NMDS generated a dissimilarity matrix between the treatments using the Bray–Curtis dissimilarity coefficient [58]. Subsequently, we analyzed similarity to determine whether there were significant differences in the chemical composition of leaves with and without galls. A bootstrap analysis (ANOSIM) was used to test the differences between the groups, using 9999 random reordering and determining whether the generated dissimilarity matrix significantly differed from randomness [59]. In addition, we used a percent similarity analysis (SIMPER) to determine which of the nutritional chemical compounds contributed most to the dissimilarity between leaves with and without galls. The analyses were performed separately for each of the six localities. The same statistical analyses were performed to evaluate the composition of defensive chemicals in leaves of Q. rugosa with and without galls in the six localities.
To determine whether there were differences in the composition of gall-inducing wasps among the six localities, we used NMDS based on the presence of 18 species of cynipids. The NMDS generated a dissimilarity matrix among the localities using the Bray–Curtis dissimilarity index [58]. This index is influenced by both differences in composition and relative abundance of species. Then, we performed an ANOSIM using 9999 random reordering to evaluate the significance of differences in the composition of the community of gall-inducing wasps among the six localities [59]. To determine which species of gall-inducing wasps contributed the most to the dissimilarity in the abundance of species among populations of Q. rugosa, we used the percent similarity analysis. Finally, the results of the SIMPER analysis were used to select the ten species that contributed the most to the dissimilarity among localities.
Finally, to identify the influence of gall-inducing insects on the chemical variables (nutritional and defensive) of Q. rugosa, we correlated the two NMDS axes of the composition of gall-inducing insects with the N, P, C, and C/N and the concentrations of gallic acid, chlorogenic acid, 4-hydroxybenzoic acid, rutin, kaempferol glucoside, quercetin glucoside, and ellagic acid of leaves with galls employing the general linear model (GLM) based on the normal distribution.
The software used for the Shapiro–Wilk, Levene, ANOVA, Tukey, and GLM tests was STATISTICA 8.0 [60], and for NMDS, ANOSIM, and SIMPER, it was Past 4.0 [61].
3. Results
3.1. Leaf Nutritional Chemistry of Q. rugosa
We detected a significant effect of locality and treatment (leaves with and without galls) on the N, P, C, and C/N ratio of Q. rugosa leaves (Table 2).

Table 2.
Average (±standard deviation) foliar nutritional chemistry of Q. rugosa in six locations of the Trans-Mexican Volcanic Belt. Nested ANOVA to evaluate the effect of site and treatment (with and without galls).
In 66.7% (n = 4) of the localities studied, the leaves with and without galls differed significantly in terms of N, P, C, and C/N ratio, which were all higher in leaves with galls. The two remaining localities showed some of these effects; in the locality of Mineral del Monte, the difference was only significant for P, which was higher in leaves with galls, while in the Tlaquetzaltitla locality, N was higher in leaves with galls, and the C/N ratio was higher in leaves without galls (the opposite effect from the rest of the populations; Table 2).
3.2. Composition of the Nutritional Chemistry Between Q. rugosa Leaves with and Without Galls
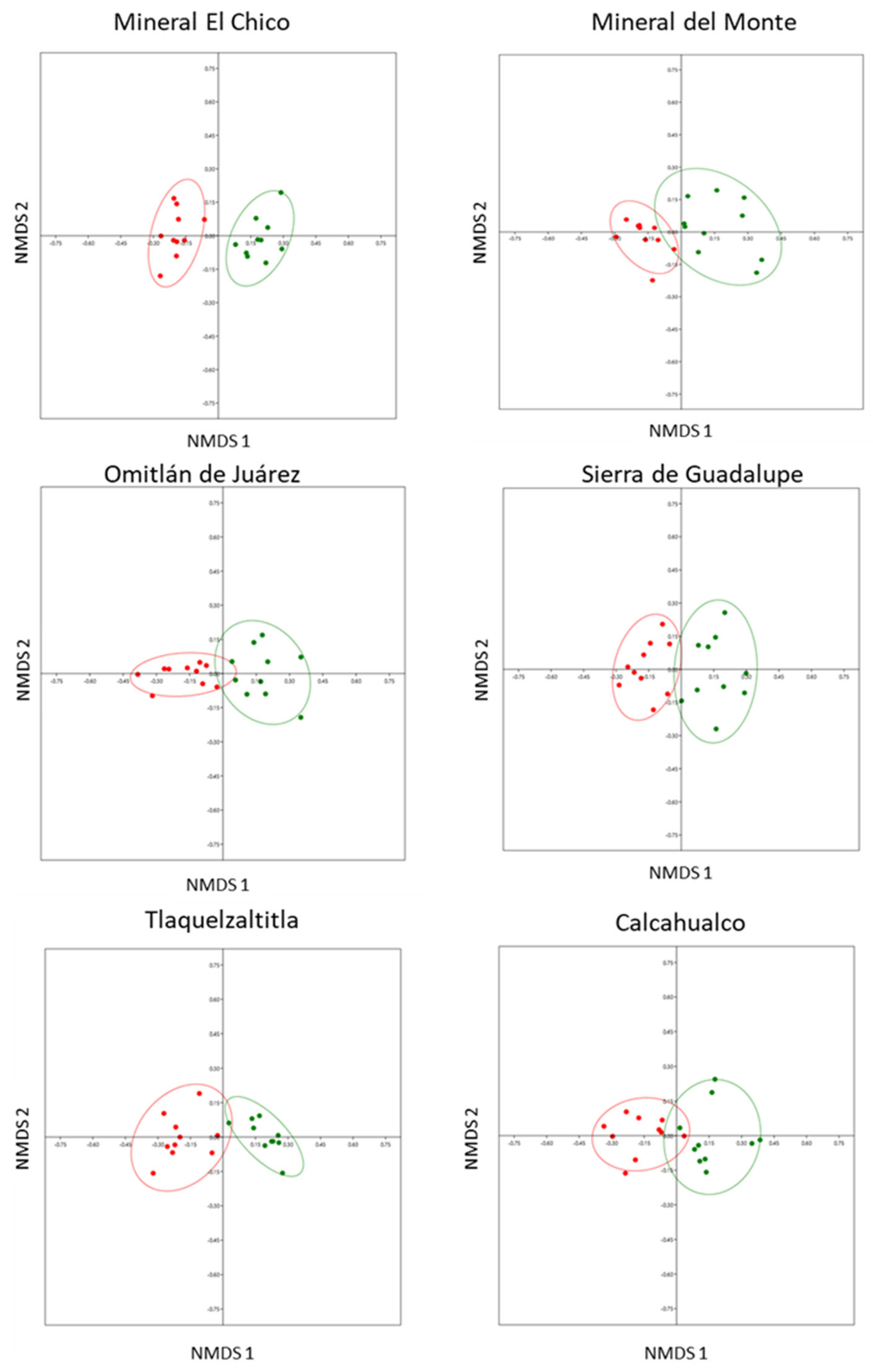
We found significant differences in the composition of the leaf nutritional chemistry between leaves with and without cynipid-induced galls in all six localities: Mineral El Chico (ANOSIM: r = 0.9522, n = 20, p < 0.0001); Mineral del Monte (ANOSIM: r = 0.6569, n = 20, p < 0.0001); Omitlán de Juárez (ANOSIM: r = 0.7402, n = 20, p < 0.0001); Sierra de Guadalupe (ANOSIM: r = 0.7033, n = 20, p < 0.0001); Tlaquetzaltitla (ANOSIM: r = 0.8971, n = 20, p < 0.0001); and Calcahualco (ANOSIM: r = 0.614, n = 20, p < 0.0002) (Figure 1).
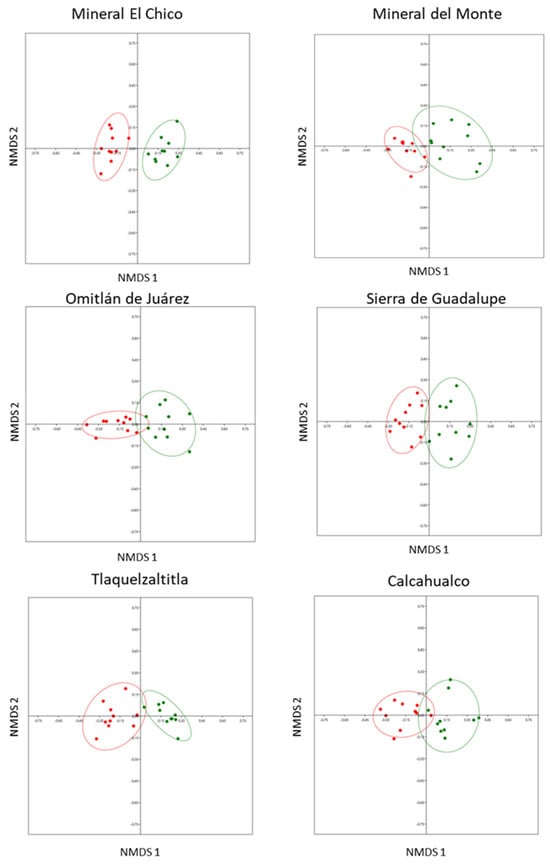
Figure 1.
Differences in the nutritional chemistry composition (N, P, C, C/N) of Q. rugosa between leaves with galls and leaves without galls in six localities, using the nonparametric multidimensional scaling (axis 1 and axis 2) in 10 trees per location. Distances between points reflect a dissimilarity matrix created using the Bray–Curtis dissimilarity coefficient. Points that are close together are more similar in leaf nutritional chemical composition compared to points that are far apart. Red dots = leaves with galls, green dots = leaves without galls.
The SIMPER analysis revealed that nitrogen (66.7%, n = 4 localities) and carbon (33.3%, n = 2 localities) were the compounds that contributed most to the differentiation between leaves with and without galls in the six study localities (Table 3).

Table 3.
Summary of SIMPER results: average value (% N, % C, ppm P) of discriminating element per treatment (with and without galls) in each locality, their contribution (%) to the dissimilarity between groups, and the cumulative total (%) of contributions.
3.3. Leaf Defensive Chemistry in Q. rugosa
In total, we identified ten major phenolic compounds (gallic acid, chlorogenic acid, 4-hydroxybenzoic acid, rutin, kaempferol glucoside, quercetin glucoside, ellagic acid, kaempferol sambubioside, tiliroside, and kaempferol-3-O-(3″,4″-diacetil-2″,6″-di-E-p-coumaroil)-glucopyranoside) in the leaf tissues of Q. rugosa (Figure S1; Table S1). The last three compounds have no known biological function, and it was not possible to determine their concentration since we did not have a reference standard. The seven remaining compounds (Table S1) have antiherbivore functions, such as reducing insect growth and biomass [62,63].
We detected a significant effect of locality and treatment (with and without galls) on the leaf defense chemistry of Q. rugosa (Table 4). Gallic acid (Calcahualco), chlorogenic acid (Mineral del Chico and Mineral del Monte), and 4-hydroxybenzoic acid (Calcahualco) were only produced in leaves with galls. Meanwhile, kaempferol glucoside and quercetin glucoside were present at significantly higher concentrations in leaves with galls. In contrast, the concentration of rutin was significantly higher in leaves without galls in the localities of Calcahualco, Mineral del Monte, and Omitlán de Juárez. At the same time, ellagic acid was significantly higher in leaves without galls in the localities of Calcahualco and Mineral del Chico (Table 4).

Table 4.
Average (±standard deviation) concentration (mg/kg) of foliar defensive chemistry of Q. rugosa in six locations of the Trans-Mexican Volcanic Belt. Nested ANOVA to evaluate the effect of site and treatment (with and without galls).
3.4. Composition of the Defensive Chemistry of Leaves with and Without Galls in Q. rugosa
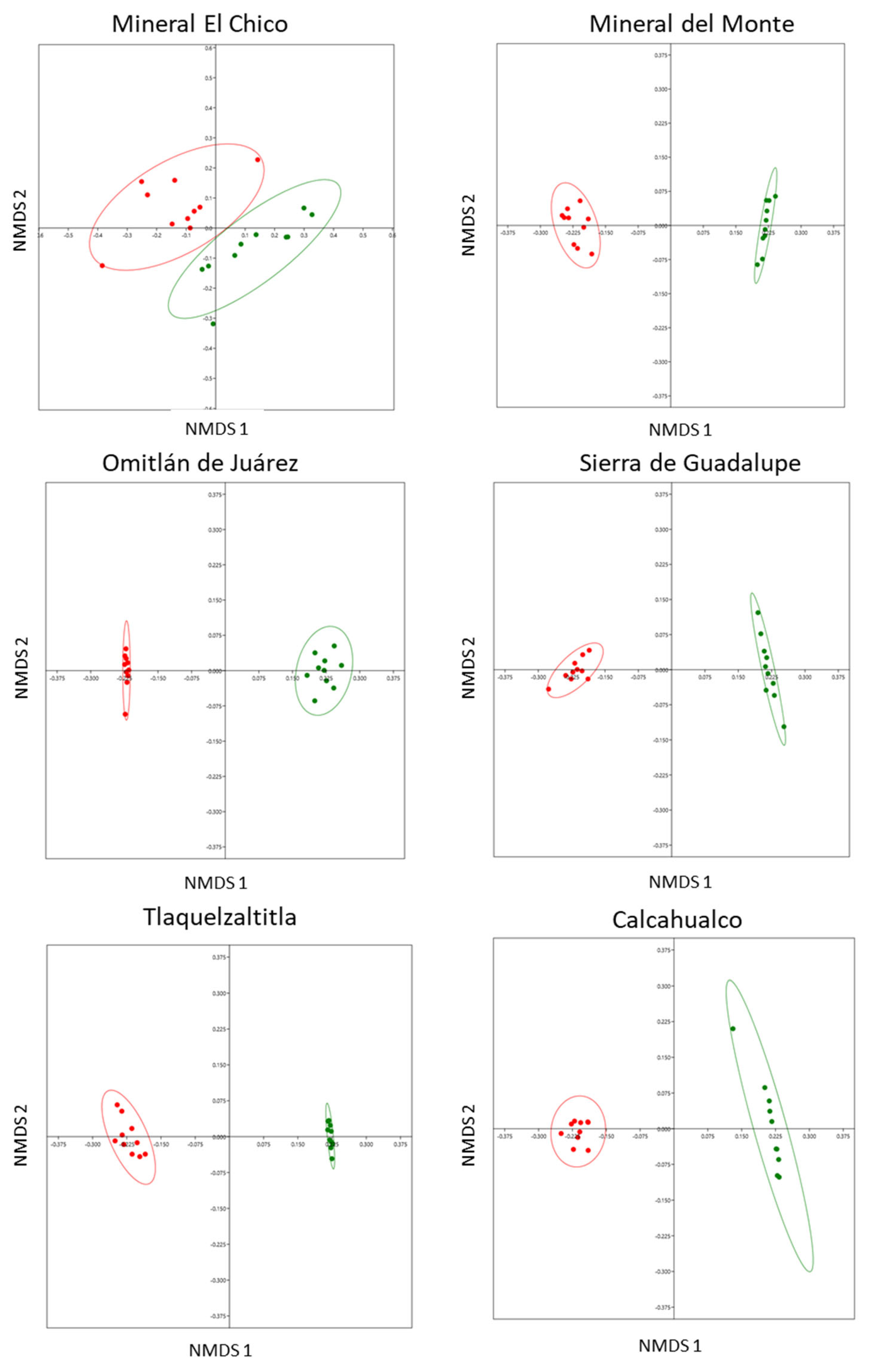
We documented significant differences in the composition of the defensive chemistry between leaves with and without cynipid-induced galls in each of the six localities. This showed that the chemical composition in terms of presence and concentration of SMs differed significantly between treatments (Figure 2) in Mineral El Chico (ANOSIM: r = 0.5884, n = 20, p < 0.0001); Mineral del Monte (ANOSIM: r = 0.99, n = 20, p < 0.0001); Omitlán de Juárez (ANOSIM: r = 0.9863, n = 20, p < 0.0001); Sierra de Guadalupe (ANOSIM: r = 1, n = 20, p < 0.0001); Tlaquetzaltitla (ANOSIM: r = 1, n = 20, p < 0.0001); and Calcahualco (ANOSIM: r = 0.981, n = 20, p < 0.0002).
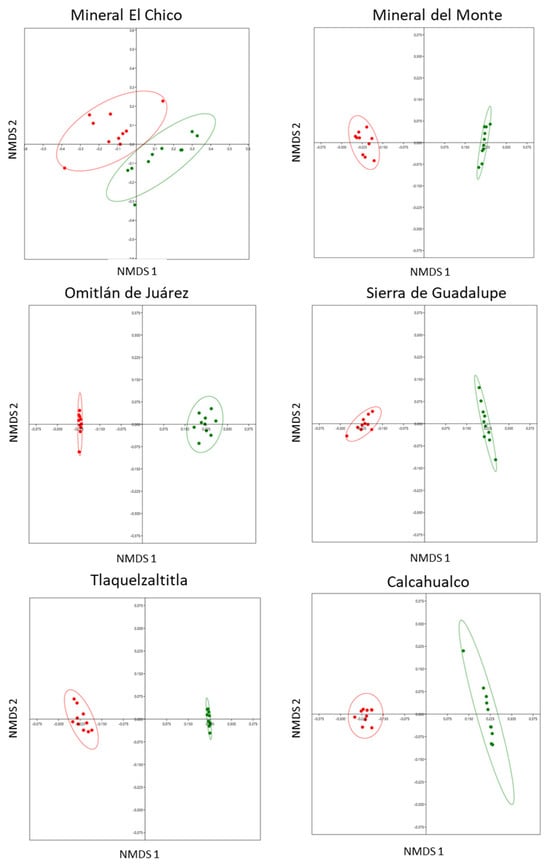
Figure 2.
Differences in the composition of defensive chemistry (seven SMs) of Q. rugosa between leaves with and without galls in six localities, using nonparametric multidimensional scaling analysis (axis 1 and axis 2) in 10 trees per location. Distances between points reflect a dissimilarity matrix created using the Bray–Curtis dissimilarity coefficient. Points that are close together are more similar in defensive chemistry compared to points that are far apart. Red dots = leaves with galls, green dots = leaves without galls.
The SIMPER analysis showed that the chemicals that most contributed to the differentiation between leaves with and without galls in the six study localities were rutin > quercetin glucoside = kaempferol glucoside > chlorogenic acid = gallic acid (Table 5).

Table 5.
Summary of SIMPER results: average concentration (mg/kg) of discriminating elements (SMs) per treatment (with and without galls) in each locality, their contribution (%) to the dissimilarity between groups, and the cumulative total (%) of contributions (75% cut-off).
3.5. Composition of Gall-Inducing Cynipids Associated with Q. rugosa
Fourteen species of gall-inducing wasps (Cynipidae: Cynipini) associated with Quercus rugosa were identified in six localities through the TMVB (Table S2 and Figure S2). According to the species richness, the genera of gall-inducing insects recorded the following pattern: Andricus (n = 4) = Disholcaspis (n = 4) > Atrusca (n = 2) = Neuropterus (n = 2) > Cynips (n = 1) = Striatoandricus (n = 1) (Table S2).
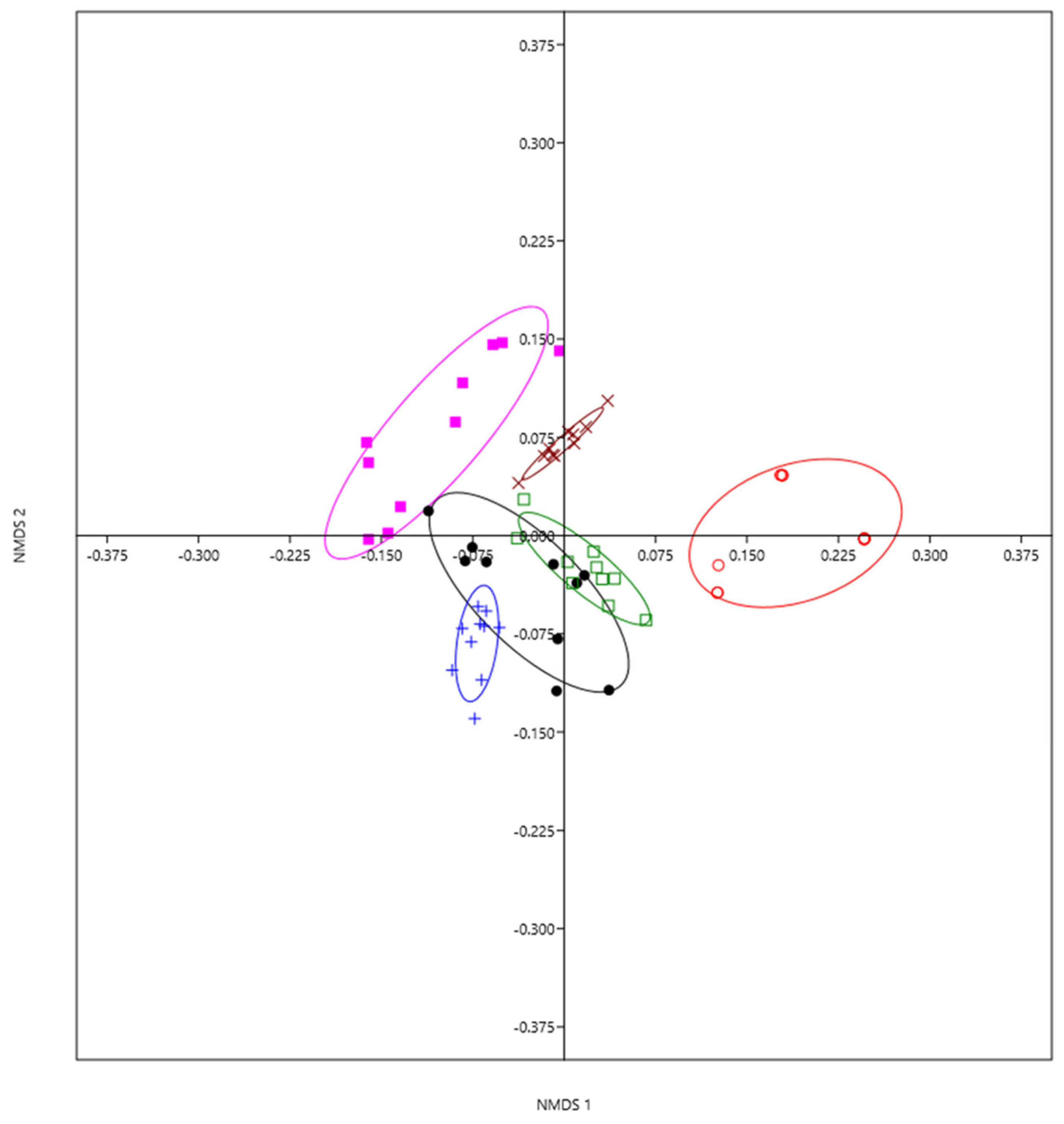
We detected significant differences in the composition of gall-inducing species among the six localities studied (ANOSIM: 0.8182, n = 60, p < 0.0001; Figure 3).
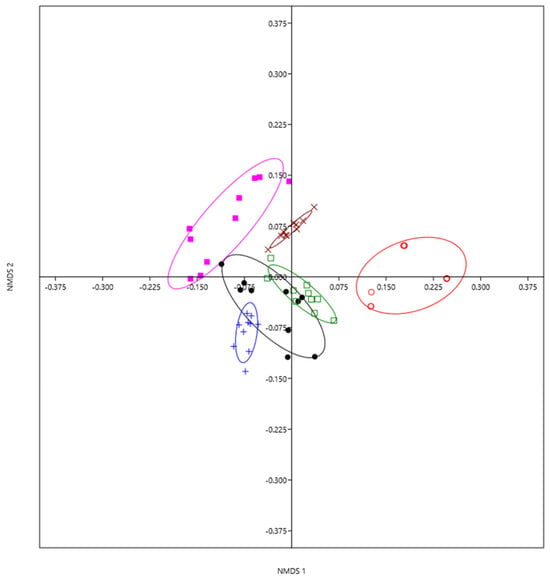
Figure 3.
Gall-inducing cynipid community composition differences in six localities of the Trans-Mexican Volcanic Belt using nonparametric multidimensional scaling (NMDS). Each point is a two-dimensional representation (axis 1 and axis 2) of the species composition of gall-inducing cynipids (10 points per locality). Distances between points reflect a dissimilarity matrix created using the Bray–Curtis dissimilarity coefficient. Points that are close together have arthropod communities that are more similar in composition compared to points that are far apart. Tlaquetzaltitla = pink, Sierra de Guadalupe = brown, Mineral del Monte = green, Mineral el Chico = blue, Omitlán de Juárez = black, Calcahualco = red.
SIMPER analysis showed that the eight gall-inducing insect species that most contributed to the dissimilarity in the abundance of species among populations of Q. rugosa are Andricus sp9, Atrusca pictor, Cynips sp1, Andricus sp2, Striatoandricus georgei, Andricus sphaericus, and Neuropterus sp4 (Table 6).

Table 6.
SIMPER results: average abundance of discriminating species in each locality, their contribution (%) to the dissimilarity between groups, and the cumulative total (%) of contributions (75% cut-off).
GLM analysis between nutritional (N, P, C and C/N) and defensive (4-hydroxybenzoic acid, gallic acid, chlorogenic acid, ellagic acid, quercetin glucoside, rutin, kaempferol glucoside) chemistry in Q. rugosa leaf tissue and NMDS axes showed that N, P, C/N proportion, gallic acid, 4-hydroxybenzoic acid, rutin, and ellagic acid were significantly correlated with NMDS 1. We documented that defensive chemistry in Q. rugosa is most correlated with NMDS 1, and the three SMs with the higher R2 values were gallic acid > 4-hydroxybenzoic acid > rutin. On the other side, chlorogenic acid, ellagic acid, quercetin glucoside, rutin, and kaempferol glucoside were significantly correlated with NMDS 2. The three SMs with the higher R2 values were kaempferol glucoside > chlorogenic acid = quercetin glucoside (Table 7).

Table 7.
General linear model (GLM) of nutritional and defensive chemistry and the first two NMDS axes of the composition of gall-inducing insects in Q. rugosa leaf tissue with galls.
4. Discussion
4.1. Leaf Nutritional Chemistry of Q. rugosa and Composition Between Leaves with and Without Galls
The total performance of herbivorous insects depends on the nutritional qualities of their host plants, principally the available nitrogen concentrations of the leaves or other target tissues [64]. Leaf nitrogen is a critical component for phytophagous insects [6], as there are records of a positive relationship between leaf nitrogen concentration and the rate of survival, growth, and reproduction of herbivorous insects [65]. It has been documented that a low nitrogen content is associated with a reduced preference and performance of insects, as the palatability of the plant for herbivores depends on the ratio of carbon/nitrogen in the leaves [66].
In addition, non-nutritive chemicals, such as plant secondary compounds, also determine host plant choice and insect feeding behavior [6].
This work revealed significant differences in the quality of individuals of Q. rugosa (in terms of C, N, P, and C/N ratio) among localities and between treatments (leaves with and without galls). These differences in nutritional quality among localities may be because populations of Q. rugosa are distributed along an altitudinal gradient [67] and, therefore, experience variations in abiotic factors such as temperature, precipitation, radiation, water availability, and soil conditions, among others [67,68,69]. These changes can promote intraspecific variation in host plant traits that could affect the nutritional quality of herbivorous insects [70]. The low foliar phosphorus content in leaves without galls suggests that they are less attractive food resources for herbivores. A negative correlation between altitude and foliar phosphorus content has been documented [71]. Therefore, the low foliar phosphorus content in leaves without galls suggests that they are less attractive as a food resource for herbivores. For example, altitudinal changes in the leaves of perennial herbaceous species, such as Alchemilla alpina [72], and tree species, including Picea alba [73] and Betula papyrifera [74], result in changes in nitrogen content. In oaks, particularly, a study in the United Kingdom demonstrated that temperature changes lead to alterations in the nitrogen content of Q. robur leaves [75]. These studies support the detected changes in the nutritional quality of Q. rugosa populations in this work. Genetic characteristics of the host plant populations may also affect nutritional quality [76]. This has been documented in different genetic categories of Mexico’s Q. castanea × Q. crassipes complex [77].
Variation in the nutritional characteristics of host plants is significant in defining their interactions with their associated herbivorous insects [5]. There is evidence that intraspecific variation in the nutritional quality of leaf tissue occurs both at the within-population and within-individual levels [78]. This has been documented among different leaves from the same individual in Nicotiana tabacum, which differed in N and C content, measured directly as the quantity of soluble proteins and carbohydrates in the plant tissue [5]. This study revealed that the concentrations of the analyzed nutrients, N, P, and C, are higher in leaves with galls, whereas the C/N ratio is higher in leaves without galls. Of the nutrients analyzed, the N and C contents contributed most strongly to the differentiation between leaves with galls and those without galls in the six study localities. In other words, leaves with galls presented a higher nutritional quality than leaf tissue without galls.
Such findings suggest two different scenarios. First, for cynipids, the leaf tissue of this host plant represents a heterogeneous nutritional landscape where natural selection could favor insects with the capacity to regulate a balanced nutrient intake. In this sense, it has been suggested that the behavioral strategies of oviposition site selection by females and feeding sites by larvae are given by the leaf quality in the search, favoring the performance of the herbivore and its fitness (for more detail, see [79,80]).
Another possibility is that the differences in nutritional quality between leaves with and without galls occur because gall-inducing Hymenoptera and Diptera manipulate the plant into diverting resources toward specific feeding locations [22,81]. In this sense, it has been shown that during the growth of the gall, there is a manipulation of the plant since the gall acts as an essential reservoir of the nutrients, minerals, and photoassimilates (carbon fixed during photosynthesis) [82,83] that reach the affected leaves through the mobilization of these resources from neighboring areas of the plant [20,82]. Our results suggest the possibility that cynipids are modifying the nutritional quality of the specific sites where the galls develop, which is congruent with the nutrition hypothesis that states that galls provide enhanced nutrition over other feeding modes [23] to satisfy their larvae’s nutritional requirements and therefore favor the development and fecundity of their descendants.
Finally, these findings suggest that variations in leaf nutrient content in Q. rugosa result from a combination of environmental factors associated with different localities along an altitudinal gradient and the alterations caused by gall induction. Thus, both locality and the presence of galls influence the nutritional chemistry of Q. rugosa, as evidenced by the significant interaction between variables.
4.2. Leaf Defensive Chemistry of Q. rugosa and Composition Between Leaves with and Without Galls
We documented significant differences in the composition of the leaf defensive chemistry (phenols) between leaves with and without galls in all six localities. Gallic acid, chlorogenic acid, and 4-hydroxybenzoic acid were produced in leaves with galls. Meanwhile, kaempferol glucoside and quercetin glucoside were at a significantly higher concentration in leaves with galls. In contrast, rutin and ellagic acid concentrations were significantly higher in leaves without galls.
It has been documented that plants exhibit qualitative and/or quantitative differences in their chemical defense characteristics, which may be influenced by genotype, environment, and even herbivory. In the case of some gall-inducing herbivores [11,81], the control of the chemical defenses of host plants is determined mainly by the genetic factors of the plant [81,84,85]. From this perspective, the analysis of metabolites with high heritability, such as phenolic compounds [86,87], could help to clarify the specific manner by which the establishment of herbivorous insects (especially specialist herbivores like cynipids) affects the patterns of SM expression.
Phenolics are classified as constitutive compounds. However, the variation in quantitative expression of some of these compounds can be caused by a source of external stress (e.g., herbivory). In this sense, diverse studies have found that the concentrations of some phenols (e.g., cinnamic acid, vanillic acid, syringic acid, and p-cumaric acid) increased when the plant was infested by herbivores [88,89,90]. Considering that cynipids did not infest the study populations, this may explain why not all phenols were documented or that it was impossible to quantify their concentrations.
Frequently, it has been documented that phenols can interrupt egg development and the fecundity of herbivores that ingest them, reducing the weight of larvae and prolonging their development period [91,92], which also makes them more vulnerable to the attack of parasitoids. The chemical profile of Q. rugosa characterized in this study includes several of the phenols most frequently reported to have diverse antiherbivore functions, including phenolic acids, chlorogenic acid, flavanone glucoside, and quercetin glucoside, among other flavonoids [62,63,91,93].
However, there may be interactions among phenolic compounds in which the expression of some decreases or inhibits the expression of other phenols. For example, gallic acid production negatively impacts shikimic acid synthesis and its products (tannins, flavonoids, caffeic acid, and coumaric acid derivatives) [94]. This could explain why the expression of other compounds was generally lower or absent when gallic acid was expressed in the study populations. Meanwhile, the lack of detectable phenols in some of the populations documented in this study could be explained by three possible scenarios: (a) the low concentration of the compound present in the leaf tissue, which made it undetectable (limit of detection) in some populations, (b) changes in the metabolic pathway due to local conditions (concentration and availability of nutrients), and (c) point mutations in biosynthetic genes.
4.3. Composition of the Gall-Inducing Insect Communities and Its Influence on Nutritional and Defensive Chemical Composition of the Leaf Tissue of Q. rugosa
It is estimated that the host plant Q. rugosa houses at least 52 species of cynipids [37,43,95,96], making it the oak species with the most significant number of gall-inducing species. Therefore, in this study, we documented 46% of the cynipid species recorded for Q. rugosa. The high richness of gall-inducing insects recorded in Q. rugosa can be explained by the geographic distribution of the species, which is the widest of any oak in Mexico, stretching from the northern border with the United States to the southern border with Guatemala; in addition, some have been recorded in Central America (Honduras) [49]. This has been reflected in the high morphological plasticity in the species. For example, Uribe and collaborators [97] analyzed morphological and genetic variation over a geographic gradient comprising 25 populations of Q. rugosa. The authors documented a significant effect of the variation among populations. This is evidence of the heterogeneity in conditions and resources represented by the canopy structure of Q. rugosa for its insects compared to oaks with a more restricted geographic distribution. In addition, it has been documented that when tree species with wide geographic distributions occur in sympatry with phylogenetically closely related species, the species of gall-inducing insects can change the species of host plants, which is a common process in the speciation of herbivorous insects [34].
In addition, we observed correlations between the nutritional and defense chemicals of Q. rugosa leaves with galls and the NMDS axes of gall-inducing cynipid species composition. These results suggested that both nutritional and defensive chemicals of gall tissues in Q. rugosa are influenced by the gall-inducing insects associated with the canopy of Q. rugosa. When a gall is induced, changes in cell metabolism and differentiation occur. The principal metabolic changes during gall development include changes in the biosynthesis of nutritional chemicals (the nutrition hypothesis, [98,99]) and secondary metabolites (the enemy hypothesis [99,100]). For example, evaluation of ungalled leaves and oak galls induced by cynipid wasps of the genera Andricus and Neuroterus showed that the concentration of defense phenolic compounds changes among ungalled and galled tissues [32]. These changes have been used to explain the adaptive significance of gall induction as a life history trait of insects [20]. It has been documented that leaves and oak galls contain phenols principally in the outer layers [101]. For example, phenolic concentrations are reduced in nutritive tissues compared with the protective outer layers of the gall or other tissues [23,102]. The accumulation of phenolic compounds in galls has been associated with defense mechanisms against parasitoids, generalist folivores, or fungal infection [100,103,104] because they can have a deterrent or toxic effect when ingested by herbivores. In this sense, a positive effect of leaf tannin levels in oak galls on the species richness of leaf-galling cynipid wasps was reported by Taper and Case [105]. This evidence suggests a protective role of phenolics against parasitic wasp attack. Consequently, the concentration of and variation in oak phenols contribute to explaining the variation in herbivore community structure [106]. Our results showed that gall-inducing insects associated with Q. rugosa modify plant chemistry, impacting the whole structure of the cynipid community.
In this study, we evaluated changes in the nutritional and defensive chemistry of Q. rugosa under the influence of gall-inducing wasps at a single point in time (average maximum peak density of galls and mature leaves in Q. rugosa). However, key metabolic alterations during gall development occur, involving fluctuations in the expression and concentration of SMs [107] and an increase in nutritional chemicals [108]. For example, in leaf galls induced by the wasp Leptocybe invasa on Eucalyptus, total phenolics and tannins strongly accumulated in early larval feeding stages [109]. For their part, Kariñho-Betancourt et al. [110] documented that the expression profile of Q. castanea galls triggered by the wasp Amphibolips michoacaensis reveals the changes in SMs throughout gall development. For these reasons, we suggest that further studies be carried out to evaluate the changes in the nutritional and defensive chemistry of Q. rugosa throughout the ontogeny of the galls.
5. Conclusions
The nutritional and defensive chemical compounds of Q. rugosa were influenced by gall-inducing wasps, and the composition of gall-inducing wasps was modified among populations of Q. rugosa. Considering that galls represent biodiversity hotspots and that cynipids have been proposed to act as ecosystem engineers, in the future it would be interesting to (1) evaluate the morphological variations in leaves of Q. rugosa to recognize the influence of those characteristics on the establishment of gall-inducing insects; (2) determine how levels of genetic diversity in populations of Q. rugosa influence the communities of gall-inducing insects in each of the localities evaluated in this study; and (3) evaluate the transfer of specialized metabolites through the multitrophic plant-cynipid-inquiline-parasitoid relationships.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17040288/s1, Figure S1: HPLC chromatograms of major phenolic compounds present in six populations of Q. rugosa distributed within the Trans-Mexican Volcanic Belt. Readings were at wavelengths of 280, 320, and 350 nm; Table S1: Phenolic compounds recorded in the leaf tissue of Q. rugosa, as well as the retention time (R.T.), chemical structure, and function of the compound in the plant–insect interaction; Table S2: Abundance of gall-inducing species in Q. rugosa in Trans-Mexican; Figure S2: Morphotypes of galls induced by cynipids in Q. rugosa leaves.
Author Contributions
Conceptualization, E.T.-S.; methodology, M.S.-M., E.C.-M. and A.Z.; validation, E.T.-S.; formal analysis, E.T.-S.; investigation, E.C.-M. and L.V.-C.; resources, E.T.-S. and P.M.-G.; data curation, M.S.-M., J.P.-V. and E.C.-M.; writing—original draft preparation, E.C.-M. and L.V.-C.; writing—review and editing, E.T.-S., L.V.-C. and P.M.-G.; supervision, E.T.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a scholarship from CONAHCyT-SEP Mexico (628761) to M.S.-M.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Gabriel Flores for his help with determining botanical specimens. We also thank the en Ciencias Naturales (UAEM). E.C.-M. thanks CONAHCyT for the postdoctoral fellowship granted (440788). L.V.-C. acknowledges CONAHCyT for the postdoctoral fellowship granted (42554).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lowman, M.D.; Wittman, P.K. Forest canopies: Methods, hypotheses and future directions. Ann. Rev. Ecol. Syst. 1996, 27, 55–81. [Google Scholar] [CrossRef]
- Lowman, M.D. Canopy research in the twenty-first century: A review of arboreal ecology. Trop. Ecol. 2009, 50, 125–136. [Google Scholar]
- Ribeiro, S.P.; Basset, Y. Gall-forming and free-feeding herbivory along vertical gradients in a lowland tropical rainforest: The importance of leaf sclerophylly. Ecography 2007, 30, 663–672. [Google Scholar] [CrossRef]
- Ribeiro, S.P.; Borges, P.A.V. Canopy habitat area effect on the arthropod species densities in the Azores: Pondering the contribution of tourist species and other life histories. In Terrestrial arthropods of Macaronesia—Biodiversity, Ecology and Evolution; Serrano, R.M., Borges, P.A.V., Boieiro, M.O.P., Eds.; Sociedade Portuguesa de Entomologia: Lisboa, Portugal, 2010; pp. 81–106. [Google Scholar]
- Wilson, J.K.; Ruiz, L.; Duarte, J.; Davidowitz, G. The nutritional landscape of host plants for a specialist insect herbivore. Ecol. Evol. 2019, 9, 13104–13113. [Google Scholar] [CrossRef]
- Strong, D.R.; Lawton, J.H.; Southwood, T.R.E. Insects on Plants. Community Patterns and Mechanisms; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Hansen, A.K.; Pers, D.; Russell, J.A. Symbiotic solutions to nitrogen limitation and amino acid imbalance in insect diets. In Advances in Insect Physiology; Oliver, K.M., Russell, J.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 161–205. [Google Scholar] [CrossRef]
- Heidorn, T.J.; Joern, A. Feeding preference and spatial distribution of grasshoppers (Acrididae) in response to nitrogen fertilization of Calamovilfa longifolia. Funct. Ecol. 1987, 1, 369–375. [Google Scholar] [CrossRef]
- Sudakaran, S.; Kost, C.; Kaltenpoth, M. Symbiont acquisition and replacement as a source of ecological innovation. Trends Microbiol. 2017, 25, 375–390. [Google Scholar] [CrossRef]
- Feeny, P. Plant apparency and chemical defense. In Biochemical Interaction Between Plants and Insects; Wallace, J.W., Mansell, R.L., Eds.; Springer: Boston, MA, USA, 1976; pp. 1–40. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, H.; Morquecho-Contreras, A. Chemical Plant Defense against Herbivores. In Herbivores; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Hartley, S.E.; Eschen, R.; Horwood, J.M.; Robinson, L.; Hill, E.M. Plant secondary metabolites and the interactions between plants and other organisms: The potential of a metabolomic approach. In Ecology of Plant Secondary Metabolites: From Genes to Landscapes; Ianson, G.I., Dick, M., Hartley, S.E., Eds.; Cambridge University Press: Cambridge, MA, USA, 2012; pp. 204–225. [Google Scholar]
- Iason, G.R.; Moore, B.D.; Lennon, J.J.; Stockan, J.A.; Osler, G.H.; Campbell, C.D.; Sim, D.A.; Beaton, J.R.; Russell, J.R. Plant secondary metabolite polymorphisms and the extended chemical phenotype. In The Ecology of Plant Secondary Metabolites: From Genes to Global Processes; Ianson, G.I., Dick, M., Hartley, S.E., Eds.; Cambridge University Press: Cambridge, MA, USA, 2012; pp. 247–268. [Google Scholar]
- Dyer, L.A.; Philbin, C.S.; Ochsenrider, K.M.; Richards, L.A.; Massad, T.J.; Smilanich, A.M.; Forister, M.L.; Parchman, T.L.; Galland, L.M.; Hurtado, P.J.; et al. Modern approaches to study plant–insect interactions in chemical ecology. Nat. Rev. Chem. 2018, 2, 50–64. [Google Scholar] [CrossRef]
- Aliabadi, A.; Renwick, J.A.A. Sequestration of glucosinolates by harlequin bug Murgantia histriónica. J. Chem. Ecol. 2002, 28, 49–62. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Van Loon, J.J.A.; Dicke, M. Insect effects on ecosystem services-introduction. Basic Appl. Ecol. 2005, 26, 1–7. [Google Scholar] [CrossRef]
- Wimp, G.M.; Wooley, S.; Bangert, R.K.; Young, W.P.; Martinsen, G.D.; Keim, P.; Rehill, B.; Lindroth, R.L.; Whitham, T.G. Plant genetics predicts intra-annual variation in phytochemistry and arthropod community structure. Mol. Ecol. 2007, 16, 5057–5069. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Barbosa, D.; Van Loon, J.J.A.; Dicke, M. The effects of herbivore-induced plant volatiles on interactions between plants and flower-visiting insects. Phytochemistry 2022, 72, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.N.; Schönrogge, K.; Atkinson, R.J.; Pujade-Villar, J. The population biology of gall wasp (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 2002, 47, 633–668. [Google Scholar] [CrossRef] [PubMed]
- Shorthouse, J.D.; Rohfritsch, O. Biology of Insect-Induced Galls; Oxford University Press: New York, NY, USA, 1992. [Google Scholar]
- Price, P.W.; Fernandes, G.W.; Waring, G.L. Adaptive nature of insect galls. Environ. Entomol. 1987, 16, 15–24. [Google Scholar] [CrossRef]
- Stone, G.N.; Schönrogge, K. The adaptative significance of insect gall morphology. Trends Ecol. Evol. 2003, 18, 512–522. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Coelho, M.S.; Santos, J.C. Neotropical Insect Galls: Status of Knowledge and Perspectives; Springer: Dordrecht, The Netherlands, 2014; pp. 1–14. [Google Scholar]
- Dawkins, R. The Extended Phenotype; Oxford University Press: Oxford, UK, 1982. [Google Scholar]
- Giron, D.; Huguet, E.; Stone, G.N.; Body, M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J. Insect Physiol. 2016, 84, 70–89. [Google Scholar] [CrossRef]
- Csóka, G.; Stone, G.N.; Melika, G. Non-native gall-inducing insects on forest trees: A global review. Biol. Invasions 2017, 19, 3161–3181. [Google Scholar] [CrossRef]
- Coelho, K.V.; Costa, R.U.; Fernandes, C.J.C.; dos Santos, I.R.M.; Coelho de Oliveira, D. How Galling Organisms Manipulate the Secondary Metabolites in the Host Plant Tissues? A Histochemical Overview in Neotropical Gall Systems. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Moreira, A.S.F.P.; Isaias, R.M.S. Functional gradients in insect gall tissues, studies on neotropical host plants. In Neotropical Insect Galls; Fernandes, G.W., Santos, J.C., Eds.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Kariñho-Betancourt, E. Coevolution: Plant-herbivore interactions and secondary metabolites of plants. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 47–76. [Google Scholar]
- Hartley, S.E. The chemical composition of plant galls: Are levels of nutrients and secondary compounds controlled by the gall-former? Oecologia 1998, 113, 492–501. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Price, P.W. Biogeographical gradients in galling species richness: Tests of hypotheses. Oecologia 1988, 76, 161–167. [Google Scholar] [CrossRef]
- Abrahamson, W.G.; Hunter, M.D.; Melika, G.; Price, P.W. Cynipid gall-wasp communities correlate with oak chemistry. J. Chem. Ecol. 2003, 29, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Villar, J. Las agallas de los encinos: Un ecosistema en miniatura que hace posibles estudios multidiciplinares. Entomol. Mex. 2013, 12, 2–22. [Google Scholar]
- Wetzel, S.R.M.; Li, I.; McKenzie, J.; Phillips, K.A.; Cruz, M.; Zhang, W.; Greene, A.; Lee, E.; Singh, N.; Tran, C.; et al. Ecosystem engineering by a gall-forming wasp indirectly suppresses diversity and density of herbivores on oak trees. Ecology 2016, 97, 427–438. [Google Scholar] [CrossRef]
- Serrano-Muñoz, M.; Pujade-Villar, J.; Lobato-Vila, I.; Valencia-Cuevas, L.; Mussali-Galante, P.; Castillo-Mendoza, E.; Tovar-Sánchez, E. Influence of elevation gradient on Cynipid galls and their associated insect communities: The case of Quercus rugosa (Fagaceae). Arthropod Plant Interact. 2022, 16, 401–421. [Google Scholar] [CrossRef]
- Tovar-Sanchez, E. Canopy arthropods community within and among oak species in central Mexico. Curr. Zool. 2009, 55, 132–144. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Oyama, K. Effect of hybridization of the Quercus crassifolia × Quercus crassipes complex on the community structure of endophagous insects. Oecologia 2006, 147, 702–713. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Valencia-Cuevas, L.; Castillo-Mendoza, E.; Mussali-Galante, P.; Pérez-Ruiz, R.V.; Mendoza, A. Association between individual genetic diversity of two oak host species and canopy arthropod community structure. Eur. J. For. Res. 2013, 132, 165–179. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Martí-Flores, E.; Valencia-Cuevas, L.; Mussali-Galante, P. Influence of forest type and host plant genetic relatedness on the canopy arthropod community structure of Quercus crassifolia. Rev. Chil. Hist. Nat. 2015, 88, 7. [Google Scholar] [CrossRef]
- Valencia-Cuevas, L.; Tovar-Sánchez, E. Oak canopy arthropod communities: Which factors shape its structure? Rev. Chil. Hist. Nat. 2015, 88, 15. [Google Scholar] [CrossRef]
- Martínez-Romero, A.; Cuesta-Porta, V.; Equihua-Martínez, A.; Estrada-Venegas, E.D.; Barrera-Ruiz, U.M.; Cibrián-Tovar, D.; Pujade-Villar, J. Aportación al conocimiento de las especies de Cynipini (Hymenoptera: Cynipidae) en los estados mexicanos. Rev. Mex. Biodiver. 2022, 93, 21. [Google Scholar] [CrossRef]
- Castillo-Mendoza, E.; Zamilpa, A.; González-Cortazar, M.; Ble-González, E.A.; Tovar-Sánchez, E. Chemical constituents and their production in Mexican oaks (Q. rugosa, Q. glabrescens and Q. obtusata). Plants 2022, 11, 2610. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, W.G.; McCrea, K.D. Nutrient and biomass allocation in Solidago altissima: Effects of two stem gallmakers, fertilization, and ramet isolation. Oecologia 1986, 68, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Vuorisalo, T.; Walls, M.; Kuitunen, H. Gall mite (Eriophyes laevis) infestation and leaf removal affect growth of leaf area in black alder (Alnus glutinosa) short shoots. Oecologia 1990, 84, 122–125. [Google Scholar] [CrossRef]
- Larson, K.; Whitham, T. Competition between gall aphids and natural plant sinks: Plant architecture affects resistance to galling. Oecologia 1997, 109, 575–582. [Google Scholar] [CrossRef]
- Price, P.W.; Abrahamson, W.G.; Hunter, M.D.; Melika, G. Using gall wasps on oaks to test broad ecological concepts. Conserv. Biol. 2004, 18, 1405–1416. [Google Scholar] [CrossRef]
- Valencia, A.S. Diversidad del género Quercus (Fagaceae) en México. Bol. Soc. Bot. Méx. 2004, 75, 33–53. [Google Scholar] [CrossRef]
- Challenger, A. Utilización y Conservación de los Ecosistemas Terrestres de México: Pasado, Presente, y Futuro; Conabio, IBUNAM y Agrupación Sierra Madre: Mexico City, Mexico, 1998. [Google Scholar]
- Clavijo McCormick, A.; Irmisch, S.; Boeckler, G.A.; Gershenzon, J.; Köllner, T.G.; Unsicker, S.B. Herbivore-induced volatile emission from old-growth black poplar trees under field conditions. Sci. Rep. 2019, 9, 7714. [Google Scholar] [CrossRef]
- Volf, M.; Volfová, T.; Seifert, C.L.; Ludwig, A.; Engelmann, R.A.; Jorge, L.R.; Richter, R.; Sched, A.; Weinhold, A.; Wirth, C.; et al. A mosaic of induced and non-induced branches promotes variation in leaf traits, predation and insect herbivore assemblages in canopy trees. Ecol. Lett. 2022, 25, 729–739. [Google Scholar] [CrossRef]
- Kjeldahl, J. A New Method for the Determination of Nitrogen in Organic Matter. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Wagner, H.; Blandt, S.; Zgainski, E.M. A thin layer chromatography. In Plant Drug Analysis; Springer: Berlín, Germany, 1996; 384 p. [Google Scholar]
- Castillo-Mendoza, E.; Valencia-Cuevas, L.; Mussali-Galante, P.; Ramos-Quintana, F.; Zamilpa, A.; Serrano-Muñoz, M.; Tovar-Sánchez, E. White Oaks Genetic and Chemical Diversity Affect the Community Structure of Canopy Insects Belonging to Two Trophic Levels. Diversity 2025, 17, 62. [Google Scholar] [CrossRef]
- Nieves-Aldrey, J.L. Hymenoptera: Cynipidae; Editorial CSIC-CSIC Press: Madrid, Spain, 2001; Volume 16. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R.; Suharsono. A statistical analysis of coral community responses to the 1982–1983 El Nino in the thousand islands, Indonesia. Coral Reefs 1990, 8, 171–179. [Google Scholar] [CrossRef]
- Statsoft Inc. Statistica for Windows; STATISTICA: Tulsa, OK, USA, 2007. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Rani, P.U.; Sambangi, P.; Sandhyarani, K. Impact of plant phenolics as semiochemicals on the performance of Trichogramma chilonis Ishii. J. Insect Behav. 2017, 30, 16–31. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Gaffoor, I.; Sattar, S.; Dixon, C.W.; Frock, N.; Moen, J.; De Moraes, C.M.; Mescher, M.C.; Thompson, G.A.; Chopra, S. Sorghum 3-deoxyanthocyanidin flavonoids confer resistance against corn leaf aphid. J. Chem. Ecol. 2019, 45, 502–514. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chapman, R.F. Evolution of host range. In Host Plant Selection in Phytophagous Insects; Chapman and Hall: New York, NY, USA, 1994; pp. 258–263. [Google Scholar]
- Mattson, W.J. Herbivory in relation to plant nitrogen content. Ann. Rev. Ecol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Schädler, M.; Jung, G.; Auge, H.; Brandl, R. Palatability, decomposition and insect herbivory: Patterns in a successional old-field plant community. Oikos 2003, 103, 121–132. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Pellissier, L.; Roger, A.; Bilat, J.; Rasmann, S. High elevation Plantago lanceolata plants are less resistant to herbivory than their low elevation conspecifics: Is it just temperature? Ecography 2014, 37, 950–959. [Google Scholar] [CrossRef]
- De Long, J.R.; Sundqvist, M.K.; Gundale, M.J.; Giesler, R.; Wardle, D.A. Effects of elevation and nitrogen and phosphorus fertilization on plant defense compounds in subarctic tundra heath vegetation. Funct. Ecol. 2016, 30, 314–325. [Google Scholar] [CrossRef]
- Pellissier, L.; Moreira, X.; Danner, H.; Serrano, M.; Salamin, N.; van Dam, N.M.; Rasmann, S. The simultaneous inducibility of phytochemicals related to plant direct and indirect defenses against herbivores is stronger at low elevation. J. Ecol. 2016, 104, 1116–1125. [Google Scholar] [CrossRef]
- Pillacela Zhunio, D.P. Evaluación de la Regeneración Natural su Relación con Variables Ambientales y de Cobertura Arbórea en Ecosistemas Naturales Alto Andinos de la Provincia del Azuay. Bachelor’s Thesis, Facultad de Ciencias Agropecuarias, Universidad de Cuenca, Cuenca, Ecuador, 2017. [Google Scholar]
- Morecroft, M.D.; Woodward, F.I. Experiments on the causes of altitudinal differences in the leaf nutrient contents, size and δ13C of Alchemilla alpina. New Phytol. 1996, 134, 471–479. [Google Scholar] [CrossRef]
- Niemelä, P.; Rousi, M.; Saarenmaa, H. Topographical delimitation of Neodiprion sertifer (Hym., Diprionidae) outbreaks on Scots pine in relation to needle quality. J. Appl. Entomol. 1987, 103, 84–91. [Google Scholar] [CrossRef]
- Erelli, M.C.; Ayres, M.P.; Eaton, G.K. Altitudinal patterns in host suitability for forest insects. Oecologia 1998, 117, 133–142. [Google Scholar] [CrossRef]
- Buse, A.; Good, J.E.G.; Dury, S.; Perrins, C.M. Effects of elevated temperature and carbon dioxide on the nutritional quality of leaves of oak (Quercus robur L.) as food for the winter moth (Operophtera brumata L.). Funct. Ecol. 1998, 12, 742–749. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kull, K. Leaf structure vs. nutrient relationships vary with soil conditions in temperate shrubs and trees. Acta Oecol. 2003, 24, 209–219. [Google Scholar] [CrossRef]
- Valencia-Cuevas, L.; Mussali-Galante, P.; Piñero, D.; Castillo-Mendoza, E.; Tovar-Sánchez, E. Hybridization of Quercus castanea (Fagaceae) across a red oak species gradient in Mexico. Plant Syst. Evol. 2015, 301, 1085–1097. [Google Scholar] [CrossRef]
- Jakobs, R.; Müller, C. Effects of intraspecific and intra-individual differences in plant quality on preference and performance of monophagous aphid species. Oecologia 2018, 186, 173–184. [Google Scholar] [CrossRef]
- Behmer, S.T. Insect Herbivore Nutrient Regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef]
- Joern, A.; Provin, T.; Behmer, S.T. Not just the usual suspects: Insect herbivore populations and communities are associated with multiple plant nutrients. Ecology 2012, 93, 1002–1015. [Google Scholar] [CrossRef]
- Abrahamson, W.G.; Anderson, S.S.; McCrea, K.D. Effects of manipulation of plant carbon nutrient balance on tall goldenrod resistance to a gallmaking herbivore. Oecologia 1988, 77, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Paquette, L.C.; Bagatto, G.; Shorthouse, J.D. Distribution of mineral nutrients within the leaves of common dandelion (Taraxacum officinale) galled by Phanacis taraxaci (Hymenoptera: Cynipidae). Can. J. Bot. 1993, 71, 1026–1031. [Google Scholar] [CrossRef]
- Bagatto, G.; Shorthouse, J.D. Seasonal acquisition of mineral nutrients by a chalcid gall on lowbush blueberry. Entomol. Exp. Appl. 1994, 73, 61–66. [Google Scholar] [CrossRef]
- McCrea, K.D.; Abrahamson, W.G. Variation in herbivore infestation: Historical vs. Genetic factors. Ecology 1987, 68, 822–827. [Google Scholar] [CrossRef]
- Gätjens-Boniche, O. The mechanism of plant gall induction by insects: Revealing clues, facts, and consequences in a cross-kingdom complex interaction. Rev. Biol. Trop. 2019, 67, 1359–1382. [Google Scholar] [CrossRef]
- Tsai, H.H.; Schmidt, W. Mobilization of Iron by Plant-Borne Coumarins. Trends Plant Sci. 2017, 22, 538–548. [Google Scholar] [CrossRef]
- Barker, H.L.; Holeski, L.M.; Lindroth, R.L. Genotypic variation in plant traits shapes herbivorous insect and ant communities on a foundation tree species. PLoS ONE 2018, 13, e0200954. [Google Scholar] [CrossRef]
- Usha-Rani, P.; Jyothsna, Y. Physiological changes in groundnut plants induced by pathogenic infection of Cercosporidium personatum Deighton. Allelopath. J. 2009, 23, 369–378. [Google Scholar]
- Usha-Rani, P.; Pratyusha, S. Defensive role of Gossypium hirsutum L. antioxidative enzymes and phenolic acids in response to Spodoptera litura F. feeding. J. Asia Pac. Entomol. 2013, 16, 131–136. [Google Scholar] [CrossRef]
- Pratyusha, S. Phenolic compounds in the plant development and defense: An overview. In Plant Stress Physiology-Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Sytykiewicz, H.; Durak, R.; Borowiak-Sobkowiak, B.; Chrzanowski, G. Role of phenolic compounds during antioxidative responses of winter triticale to aphid and beetle attack. Plant Physiol. Biochem. 2017, 118, 529–540. [Google Scholar] [CrossRef]
- Tayal, M.; Somavat, P.; Rodriguez, I.; Martinez, L.; Kariyat, R. Cascading effects of polyphenol-rich purple corn pericarp extract on pupal, adult, and offspring of tobacco hornworm (Manduca sexta L.). Commun. Integr. Biol. 2020, 13, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Zhang, S.; Wang, L.; Zhu, X.Z.; Luo, J.Y.; Wang, C.Y.; Lü, L.M.; Cui, J.J. Genetic regulation of defense responses in cotton to insect herbivores. AoB Plants 2017, 9, 10–1093. [Google Scholar] [CrossRef]
- Salminen, J.P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Pujade-Villar, J.; Equihua-Martínez, A.; Estrada-Venegas, E.G.; Chagoyán-García. Estado del Conocimiento de los Cynipini (Hymenoptera: Cynipidae) en México: Perspectivas de Estudio. Neotrop. Entomol. 2009, 38, 809–821. [Google Scholar] [CrossRef]
- Castillo-Mendoza, E. Efecto de la hibridación del complejo Q. glabrescens x Q. rugosa y Q. glabrescens x Q. obtsata sobre la comunidad de insectos inductores de agallas y sus parasitoides. PhD Dissertation, Universidad Nacional Autónoma de México, México City, Mexico, 2019. [Google Scholar]
- Uribe-Salas, D.; Saenz-Romero, C.; González-Rodríguez, A.; Tellez-Valdez, O.; Oyama, K. Foliar morphological variation in the white oak Quercus rugosa Née (Fagaceae) along a latitudinal gradient in Mexico: Potential implications for management and conservation. For. Ecol. Manag. 2008, 256, 2121–2126. [Google Scholar] [CrossRef]
- Bronner, R. Contribution a l’etude histochimique des tissue nourriciers des zoocecidies. Marcellia 1977, 40, 11–34. [Google Scholar]
- Price, P.W.; Waring, G.L.; Fernandes, G.W. Hypotheses on the adaptive nature of galls. Proc. Entomol. Soc. 1986, 88, 361–363. [Google Scholar]
- Cornell, H.V. The secondary chemistry and complex morphology of galls formed by the Cynipidae (Hymenoptera): Why and how? Am. Midl. Nat. 1983, 110, 225–234. [Google Scholar] [CrossRef]
- Allison, S.D.; Schultz, J.C. Biochemical responses of chestnut oak to a galling cynipid. J. Chem. Ecol. 2005, 31, 151–166. [Google Scholar] [CrossRef]
- Nyman, T.; Julkunen-Tiitto, R. Manipulation of the phenolic chemistry of willows by gall-inducing sawflies. Proc. Nat. Acad. Sci. USA 2000, 97, 13184–13187. [Google Scholar] [CrossRef]
- Schultz, B.B. Insect herbivores as potential causes of mortality and adaptation in gall forming insects. Oecologia 1992, 90, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Alvarado, E.; Cuevas-Reyes, P.; Quesada, M.; Oyama, K. Interactions between galling insects and leaf-feeding insects: The role of plant phenolic compounds and their possible interference with herbivores. J. Trop. Ecol. 2008, 24, 329–336. [Google Scholar] [CrossRef]
- Taper, M.L.; Case, T.J. Interactions between oak tannins and parasite community structure: Unexpected benefits of tannins to cynipid gall-wasps. Oecologia 1987, 71, 254–261. [Google Scholar] [CrossRef]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T. Feeny revisited: Condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of Quercus. Ecol. Entomol. 2004, 29, 174–187. [Google Scholar] [CrossRef]
- Tooker, J.F.; Rohr, J.R.; Abrahamson, W.G.; De Moraes, C.M. Gall insects can avoid and alter indirect plant defenses. New Phytol. 2008, 178, 657–671. [Google Scholar] [CrossRef]
- Brooner, R. The role of nutritive cells in the nutrition of cynipids and cecidomyiids. In Biology of Insectinduced Galls; Shorthouse, J.D., Rohfritsch, O., Eds.; Oxford University Press: New York, NY, USA, 1992; pp. 118–140. [Google Scholar]
- Li, X.Q.; Liu, Y.Z.; Guo, W.F.; Solanki, M.K.; Yang, Z.D.; Xiang, Y.; Ma, Z.C.; Wen, Y.G. The gall wasp Leptocybe invasa (Hymenoptera: Eulophidae) stimulates different chemical and phytohormone responses in two Eucalyptus varieties that vary in susceptibility to galling. Tree Physiol. 2017, 37, 1208–1217. [Google Scholar] [CrossRef]
- Kariñho-Betancourt, E.; Hernández-Soto, P.; Rendón-Anaya, M.; Calderón-Cortés, N.; Oyama, K. Differential expression of genes associated with phenolic compounds in galls of Quercus castanea induced by Amphibolips michoacaensis. J. Plant Inter. 2019, 14, 177–186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).