Emerging Ranaviral Infectious Diseases and Amphibian Decline
Abstract
:1. Introduction
3. Viral Immunity in Mammals
4. The Xenopus Laevis Immune System
| Characteristics | Larva | Adult | |
|---|---|---|---|
| Molecules | MHC class I MHC class II | No to reduced expression B cell only | Present B and T cells |
| Cells | CD8 T-cells NK cells | Present Present at late stages | Better Yes |
| Immune functions | Mixed lymphocyte reaction Cytoxicity (CTL & NK cells) IgM to IgY switch Viral immunity | Poor Not demonstrated Incomplete Weak | Present Acute Better Potent |
5. Immunity to FV3 in X. laevis and S. tropicalis
5.1. Adults
5.2. Larvae
| Adults | Larvae | ||
|---|---|---|---|
| Primary Infection | Secondary Infection | ||
| Symptoms disappearance | 2−3 wks | 3−5 days | Long lasting, >80% death |
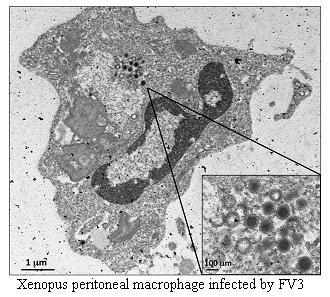
| Innate Immune responses | 1 dpi* Macrophages 3 dpi NK cells | PLs possible APCs | Up-regulation TNFα, IL-1β, Arg-1 |
| Clearance FV3 | 1 month | 1 week | Poor |
| CD8 T cell proliferation (BrdU) | 6 dpi | 3 dpi but no increase in amplitude | IFNγ |
| Cell infiltration in the kidneys | 6 dpi CD8 T cells | 3 dpi but less CD8 T cells | IFNγ |
| Antibody response | Undetectable | Detected 10−14 dpi memory >15 months | Not detected so far |
| AID up-regulation | 9 dpi | 3 dpi | 6−7 dpi. |
6. The Immunity to RVs in Other Amphibian Species
7. Role of Host Immune Defenses in Pathogenesis and Transmission of RV Infection
4. Conclusions and Speculation
Acknowledgements
References
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F. Zoonoses in wildlife integrating ecology into management. Adv. Parasitol. 2009, 68, 185–209. [Google Scholar] [PubMed]
- Daszak, P.; Berger, L.; Cunningham, A.A.; Hyatt, A.D.; Green, D.E.; Speare, R. Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 1999, 5, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Gewin, V. Riders of a modern-day Ark. PLoS. Biol. 2008, 6, e24. [Google Scholar] [CrossRef] [PubMed]
- Weyrauch, S.L.; Grubb, T.C., Jr. Effects of the interaction between genetic diversity and UV-B radiation on wood frog fitness. Conserv. Biol. 2006, 20, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.G.; Fonseca, C.R.; Haddad, C.F.; Batista, R.F.; Prado, P.I. Habitat split and the global decline of amphibians. Science 2007, 318, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, B.A.; Baker, N.J.; Blaustein, A.R. A meta-analysis of the effects of ultraviolet B radiation and its synergistic interactions with pH, contaminants, and disease on amphibian survival. Conserv. Biol. 2008, 22, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.R.; Diffendorfer, J.; Mendelson, J.R.; Sears, M.W. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008, 6, e72. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A.A.; Vonk, A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 5476–5480. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Garner, T.W.; Walker, S.F. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu. Rev. Microbiol. 2009, 63, 291–310. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Litvintseva, A.P.; Vilgalys, R.; Morgan, J.A.; Taylor, J.W.; Fisher, M.C.; Berger, L.; Weldon, C.; du Preez, L.; Longcore, J.E. Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathog. 2009, 5, e1000458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, A.M.; Briggs, C.J.; Daszak, P. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol. Evol. 2010, 25, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G. Ranaviruses (family Iridoviridae): emerging cold-blooded killers. Arch. Virol. 2002, 147, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Hyatt, A.; Miyazaki, T.; Williams, T. Family Iridoviridae: poor viral relations no longer. Curr. Top. Microbiol. Immunol. 2009, 328, 123–170. [Google Scholar] [PubMed]
- Green, D.E.; Converse, K.A.; Schrader, A.K. Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996−2001. Ann. N. Y. Acad. Sci. 2002, 969, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Rajeev, S.; Brookins, M.; Cook, J.; Whittington, L.; Baldwin, C.A. Concurrent infection with ranavirus, Batrachochytrium dendrobatidis, and Aeromonas in a captive anuran colony. J. Zoo Wildl. Med. 2008, 39, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Barbosa-Solomieu, V.; Chinchar, V.G. A decade of advances in iridovirus research. Adv. Virus Res. 2005, 65, 173–248. [Google Scholar] [PubMed]
- Hyatt, A.D.; Gould, A.R.; Zupanovic, Z.; Cunningham, A.A.; Hengstberger, S.; Whittington, R.J.; Kattenbelt, J.; Coupar, B.E. Comparative studies of piscine and amphibian iridoviruses. Arch. Virol. 2000, 145, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Granoff, A. Frog virus 3: a DNA virus with an unusual life-style. Prog. Med. Virol. 1984, 30, 187–198. [Google Scholar] [PubMed]
- Goorha, R.; Murti, K.G. The genome of frog virus 3, an animal DNA virus, is circularly permuted and terminally redundant. Proc. Natl. Acad. Sci. U.S.A. 1982, 79, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Hedrick, R.P.; Chinchar, V.G. Molecular characterization, sequence analysis, and taxonomic position of newly isolated fish iridoviruses. Virology 1997, 229, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.R.; Owens, L. Experimental challenge and clinical cases of Bohle iridovirus (BIV) in native Australian anurans. Dis. Aquat. Organ. 2002, 49, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Jancovich, J.K.; Mao, J.; Chinchar, V.G.; Wyatt, C.; Case, S.T.; Kumar, S.; Valente, G.; Subramanian, S.; Davidson, E.W.; Collins, J.P.; Jacobs, B.L. Genomic sequence of a ranavirus (family Iridoviridae) associated with salamander mortalities in North America. Virology 2003, 316, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, T.K.; Mao, J.; Schock, D.; Brigham, R.M.; Chinchar, V.G. Pathology, isolation, and preliminary molecular characterization of a novel iridovirus from tiger salamanders in Saskatchewan. J. Wildl. Dis. 1999, 35, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Travers, P; Walport, M. Janeway's Immunobiology, 7th ed.; Taylor and Francis Group: New York, NY, USA, 2008; p. 887. [Google Scholar]
- Lee, S.H.; Miyagi, T.; Biron, C.A. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007, 28, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Ellermann-Eriksen, S. Macrophages and cytokines in the early defence against herpes simplex virus. Virol. J. 2005, 2, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Doherty, P.C.; Christensen, J.P. Accessing complexity: the dynamics of virus-specific T cell responses. Annu. Rev. Immunol. 2000, 18, 561–592. [Google Scholar] [CrossRef] [PubMed]
- Du Pasquier, L.; Schwager, J.; Flajnik, M.F. The immune system of Xenopus. Annu. Rev. Immunol. 1989, 7, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Ohta, Y. Comparative and developmental study of the immune system in Xenopus. Dev. Dyn. 2009, 238, 1249–1270. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, U.; Harland, R.M.; Gilchrist, M.J.; Hendrix, D.; Jurka, J. The genome of the western clawed frog Xenopus tropicalis. Science 2010, in press. [Google Scholar]
- Du Pasquier, L.; Robert, J.; Courtet, M.; Mussmann, R. B-cell development in the amphibian Xenopus. Immunol. Rev. 2000, 175, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Kau, C.L.; Turpen, J.B. Dual contribution of embryonic ventral blood island and dorsal lateral plate mesoderm during ontogeny of hemopoietic cells in Xenopus laevis. J. Immunol. 1983, 131, 2262–2266. [Google Scholar] [PubMed]
- Du Pasquier, L.; Weiss, N. The thymus during the ontogeny of the toad Xenopus laevis: growth, membrane-bound immunoglobulins and mixed lymphocyte reaction. Eur. J. Immunol. 1973, 3, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Rollins-Smith, L.A.; Parsons, S.C.; Cohen, N. During frog ontogeny, PHA and Con A responsiveness of splenocytes precedes that of thymocytes. Immunology 1984, 52, 491–500. [Google Scholar] [PubMed]
- Rollins-Smith, L.A.; Parsons, S.C.; Cohen, N. Effects of thyroxine-driven precocious metamorphosis on maturation of adult-type allograft rejection responses in early thyroidectomized frogs. Differentiation 1988, 37, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, T.E.; Smith, P.B.; Turpen, J.B. Differential stem cell contributions to thymocyte succession during development of Xenopus laevis. J. Immunol. 1992, 148, 2975–2982. [Google Scholar] [PubMed]
- Flajnik, M.F.; Du Pasquier, L.; Cohen, N. Immune responses of thymus/lymphocyte embryonic chimeras: studies on tolerance and major histocompatibility complex restriction in Xenopus. Eur. J. Immunol. 1985, 15, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M.F.; Du Pasquier, L. MHC class I antigens as surface markers of adult erythrocytes during the metamorphosis of Xenopus. Dev. Biol. 1988, 128, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Rollins-Smith, L.A.; Flajnik, M.F.; Blair, P.J.; Davis, A.T.; Green, W.F. Involvement of thyroid hormones in the expression of MHC class I antigens during ontogeny in Xenopus. Dev. Immunol. 1997, 5, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Salter-Cid, L.; Nonaka, M.; Flajnik, M.F. Expression of MHC class Ia and class Ib during ontogeny: high expression in epithelia and coregulation of class Ia and lmp7 genes. J. Immunol. 1998, 160, 2853–2861. [Google Scholar] [PubMed]
- Hsu, E. Mutation, selection, and memory in B lymphocytes of exothermic vertebrates. Immunol. Rev. 1998, 162, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Gantress, J.; Maniero, G.D.; Cohen, N.; Robert, J. Development and characterization of a model system to study amphibian immune responses to iridoviruses. Virology 2003, 311, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Morales, H.; Buck, W.; Cohen, N.; Marr, S.; Gantress, J. Adaptive immunity and histopathology in frog virus 3-infected Xenopus. Virology 2005, 332, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Metzger, D.W.; Granoff, A.; Goorha, R. Localization of frog virus 3 proteins using monoclonal antibodies. Virology 1984, 137, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Maniero, G.D.; Morales, H.; Gantress, J.; Robert, J. Generation of a long-lasting, protective, and neutralizing antibody response to the ranavirus FV3 by the frog Xenopus. Dev. Comp. Immunol. 2006, 30, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Marr, S.; Morales, H.; Bottaro, A.; Cooper, M.; Flajnik, M.; Robert, J. Localization and differential expression of activation-induced cytidine deaminase in the amphibian Xenopus upon antigen stimulation and during early development. J. Immunol. 2007, 179, 6783–6789. [Google Scholar] [CrossRef] [PubMed]
- Morales, H.D.; Robert, J. Characterization of primary and memory CD8 T-cell responses against ranavirus (FV3) in Xenopus laevis. J. Virol. 2007, 81, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Morales, H.; Robert, J. In vivo and in vitro techniques for comparative study of antiviral T-cell responses in the amphibian Xenopus. Biol. Proced. Online 2008, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zupanovic, Z.; Lopez, G.; Hyatt, A.D.; Green, B.; Bartran, G.; Parkes, H.; Whittington, R.J.; Speare, R. Giant toads Bufo marinus in Australia and Venezuela have antibodies against 'ranaviruses'. Dis. Aquat. Organ. 1998, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Majji, S.; LaPatra, S.; Long, S.M.; Sample, R.; Bryan, L.; Sinning, A.; Chinchar, V.G. Rana catesbeiana virus Z (RCV-Z): a novel pathogenic ranavirus. Dis. Aquat. Organ. 2006, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Teacher, A.G.; Garner, T.W.; Nichols, R.A. Evidence for directional selection at a novel major histocompatibility class I marker in wild common frogs (Rana temporaria) exposed to a viral pathogen (Ranavirus). PLoS One 2009, 4, e4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, A.A.; Tems, C.A.; Russell, P.H. Immunohistochemical demonstration of Ranavirus Antigen in the tissues of infected frogs (Rana temporaria) with systemic haemorrhagic or cutaneous ulcerative disease. J. Comp. Pathol. 2008, 138, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Wang, J.; Murti, G.; Carey, C.; Rollins-Smith, L. Inactivation of frog virus 3 and channel catfish virus by esculentin-2P and ranatuerin-2P, two antimicrobial peptides isolated from frog skin. Virology 2001, 288, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Bryan, L.; Silphadaung, U.; Noga, E.; Wade, D.; Rollins-Smith, L. Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology 2004, 323, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Tweedell, K.; Granoff, A. Viruses and renal carcinoma of Rana pipiens. V. Effect of frog virus 3 on developing frog embryos and larvae. J. Natl. Cancer Inst. 1968, 40, 407–410. [Google Scholar]
- Greer, A.L.; Berrill, M.; Wilson, P.J. Five amphibian mortality events associated with ranavirus infection in south central Ontario, Canada. Dis. Aquat. Organ. 2005, 67, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.F.; Greer, A.L.; Torres-Cervantes, R.; Collins, J.P. First case of ranavirus-associated morbidity and mortality in natural populations of the South American frog Atelognathus patagonicus. Dis. Aquat. Organ. 2006, 72, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Balseiro, A.; Dalton, K.P.; Del Cerro, A.; Marquez, I.; Parra, F.; Prieto, J.M.; Casais, R. Outbreak of common midwife toad virus in alpine newts (Mesotriton alpestris cyreni) and common midwife toads (Alytes obstetricans) in Northern Spain: A comparative pathological study of an emerging ranavirus. Vet. J. 2009, in press. [Google Scholar]
- Gray, M.J.; Miller, D.L.; Schmutzer, A.C.; Baldwin, C.A. Frog virus 3 prevalence in tadpole populations inhabiting cattle-access and non-access wetlands in Tennessee, USA. Dis. Aquat. Organ. 2007, 77, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Une, Y.; Sakuma, A.; Matsueda, H.; Nakai, K.; Murakami, M. Ranavirus outbreak in North American bullfrogs (Rana catesbeiana), Japan, 2008. Emerg. Infect. Dis. 2009, 15, 1146–1147. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Rajeev, S.; Gray, M.J.; Baldwin, C.A. Frog virus 3 infection, cultured American bullfrogs. Emerg. Infect. Dis. 2007, 13, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, R.; de Mesquita, A.; Fleury, L.; de Brito, W.; Nunes, I.; Robert, J.; Morales, H.; Coelho, A.; Barthasson, D.; Galli, L.; Catroxo, M. Mass mortality associated with a FV3-like ranavirus infection in farmed tadploels Rana catesbiana from Brazil. Dis. Aquat. Organ. 2009, 86, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.L.; Richards, K.; Collins, J.P. Dose and host characteristics influence virulence of ranavirus infections. Oecologia 2005, 144, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Forson, D.D.; Storfer, A. Atrazine increases ranavirus susceptibility in the tiger salamander, Ambystoma tigrinum. Ecol. Appl. 2006, 16, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Jancovich, J.K.; Davids, E.W.; Seiler, A.; Jacobs, B.L.; Collins, J.P. Transmission of the Ambystoma tigrinum virus to alternative hosts. Dis. Aquat. Organ. 2001, 46, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.L.; Schock, D.M.; Collins, J.P. Transmission dynamics of the amphibian ranavirus Ambystoma tigrinum virus. Dis. Aquat. Organ. 2007, 77, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Docherty, D.E.; Meteyer, C.U.; Wang, J.; Mao, J.; Case, S.T.; Chinchar, V.G. Diagnostic and molecular evaluation of three iridovirus-associated salamander mortality events. J. Wildl. Dis. 2003, 39, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Richards, K.; Jancovich, J.K.; Davidson, E.W. Influence of temperature on Ranavirus infection in larval salamanders Ambystoma tigrinum. Dis. Aquat. Organ. 2005, 63, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Sheafor, B.; Davidson, E.W.; Parr, L.; Rollins-Smith, L. Antimicrobial peptide defenses in the salamander, Ambystoma tigrinum, against emerging amphibian pathogens. J. Wildl. Dis. 2008, 44, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Cotter, J.D.; Storfer, A.; Page, R.B.; Beachy, C.K.; Voss, S.R. Transcriptional response of Mexican axolotls to Ambystoma tigrinum virus (ATV) infection. BMC Genomics 2008, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Tournefier, A.; Laurens, V.; Chapusot, C.; Ducoroy, P.; Padros, M.R.; Salvadori, F.; Sammut, B. Structure of MHC class I and class II cDNAs and possible immunodeficiency linked to class II expression in the Mexican axolotl. Immunol. Rev. 1998, 166, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Koniski, A. Axolotl immunology: Lymphocytes, cytokines, and alloincompatibility reactions. Axolotl Newsletter 1994, 23, 24–33. [Google Scholar]
- Essbauer, S.; Bremont, M.; Ahne, W. Comparison of the eIF-2alpha homologous proteins of seven ranaviruses (Iridoviridae). Virus Genes 2001, 23, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.; Cohen, N.; Rollins-Smith, L. Amphibian declines: an immunological perspective. Dev. Comp. Immunol. 1999, 23, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.; Robert, J.; Salo, H.; Dautremepuits, C.; Brousseau, P. Immunotoxicology of amphibians. Rev. Applied Herpetology 2005, 2, 297–309. [Google Scholar] [CrossRef]
- Langerveld, A.J.; Celestine, R.; Zaya, R.; Mihalko, D.; Ide, C.F. Chronic exposure to high levels of atrazine alters expression of genes that regulate immune and growth-related functions in developing Xenopus laevis tadpoles. Environ. Res. 2009, 109, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Christin, M.S.; Menard, L.; Gendron, A.D.; Ruby, S.; Cyr, D.; Marcogliese, D.J.; Rollins-Smith, L.; Fournier, M. Effects of agricultural pesticides on the immune system of Xenopus laevis and Rana pipiens. Aquat. Toxicol. 2004, 67, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Gray, M.J.; Rajeev, S.; Schmutzer, A.C.; Burton, E.C.; Merrill, A.; Baldwin, C.A. Pathologic findings in larval and juvenile anurans inhabiting farm ponds in Tennessee, USA. J. Wildl. Dis. 2009, 45, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Driskell, E.A.; Miller, D.L.; Swist, S.L.; Gyimesi, Z.S. PCR detection of ranavirus in adult anurans from the Louisville Zoological Garden. J. Zoo Wildl. Med. 2009, 40, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.L.; Brunner, J.L.; Collins, J.P. Spatial and temporal patterns of Ambystoma tigrinum virus (ATV) prevalence in tiger salamanders Ambystoma tigrinum nebulosum. Dis. Aquat. Organ. 2009, 85, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.R.; Brem, F.; Brenes, R.; Reeve, J.D.; Alford, R.A.; Voyles, J.; Carey, C.; Livo, L.; Pessier, A.P.; Collins, J.P. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 3165–3170. [Google Scholar] [CrossRef] [PubMed]
- Voyles, J.; Young, S.; Berger, L.; Campbell, C.; Voyles, W.F.; Dinudom, A.; Cook, D.; Webb, R.; Alford, R.A.; Skerratt, L.F.; Speare, R. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 2009, 326, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, E.B.; Poorten, T.J.; Settles, M.; Murdoch, G.K.; Robert, J.; Maddox, N.; Eisen, M.B. Genome-wide transcriptional response of Silurana (Xenopus) tropicalis to infection with the deadly chytrid fungus. PLoS One 2009, 4, e6494. [Google Scholar] [CrossRef] [PubMed]
- Rollins-Smith, L.A. The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim. Biophys. Acta 2009, 1788, 1593–1599. [Google Scholar]
- Tennessen, J.A.; Woodhams, D.C.; Chaurand, P.; Reinert, L.K.; Billheimer, D.; Shyr, Y.; Caprioli, R.M.; Blouin, M.S.; Rollins-Smith, L.A. Variations in the expressed antimicrobial peptide repertoire of northern leopard frog (Rana pipiens) populations suggest intraspecies differences in resistance to pathogens. Dev. Comp. Immunol. 2009, 33, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Robert, J. Emerging Ranaviral Infectious Diseases and Amphibian Decline. Diversity 2010, 2, 314-330. https://doi.org/10.3390/d2030314
Robert J. Emerging Ranaviral Infectious Diseases and Amphibian Decline. Diversity. 2010; 2(3):314-330. https://doi.org/10.3390/d2030314
Chicago/Turabian StyleRobert, Jacques. 2010. "Emerging Ranaviral Infectious Diseases and Amphibian Decline" Diversity 2, no. 3: 314-330. https://doi.org/10.3390/d2030314
APA StyleRobert, J. (2010). Emerging Ranaviral Infectious Diseases and Amphibian Decline. Diversity, 2(3), 314-330. https://doi.org/10.3390/d2030314