Grazer Functional Roles, Induced Defenses, and Indirect Interactions: Implications for Eelgrass Restoration in San Francisco Bay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mesocosm Experiment
2.2. Field Experiment
3. Results and Discussion
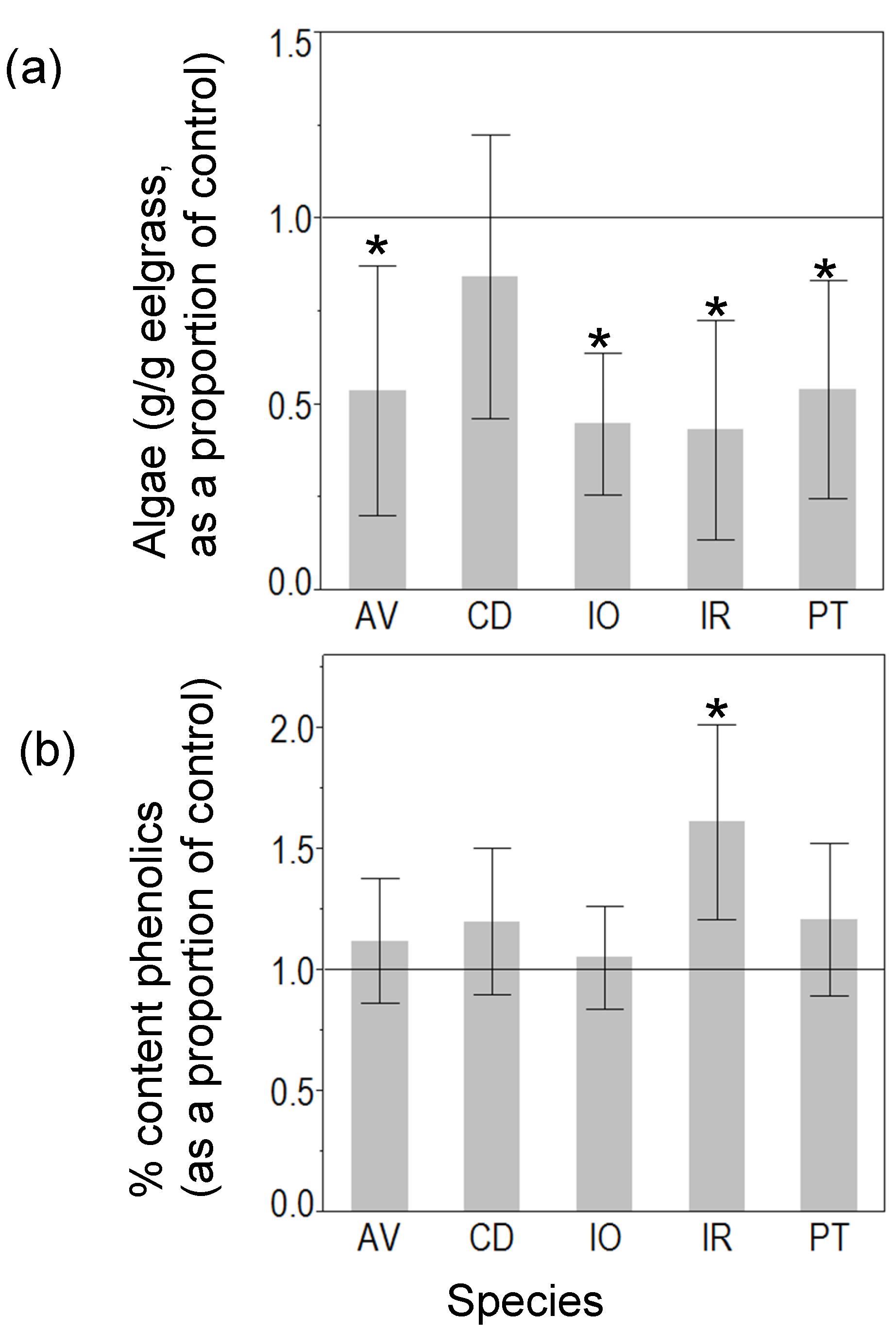
3.1. Mesocosm Experiment





3.2. Field Experiment
4. Conclusions
Acknowledgments
Author Contributions
Appendix
| Temporal Block | Date Initiated |
|---|---|
| 1 | 7/24/2010 |
| 2 | 8/2/2010 |
| 3 | 8/9/2010 |
| 4 | 8/14/2010 |
| 5 | 8/28/2010 |
| 6 | 9/2/2010 |
| 7 | 9/9/2010 |
| 8 | 9/19/2010 |
| 9 | 10/4/2010 |
| 10 | 11/15/2010 |
| 11 | 12/7/2010 |
| 12 | 12/29/2010 |
| 13 | 1/8/2011 |
| 14 | 1/20/2011 |
| 15 | 2/3/2011 |
| 16 | 2/8/2011 |
| 17 | 2/20/2011 |
| 18 | 3/1/2011 |
| 19 | 4/14/2011 |
| 20 | 5/9/2011 |
| 21 | 5/18/2011 |
| 22 | 6/2/2011 |
| 23 | 7/25/2011 |
| 24 | 8/16/2011 |
| 25 | 8/30/2011 |
| 26 | 9/14/2011 |
| 27 | 9/21/2011 |
Conflicts of Interest
References and Notes
- Cyr, H.; Pace, M.L. Allometric theory: Extrapolations from individuals to communities. Ecology 1993, 74, 1234–1245. [Google Scholar] [CrossRef]
- Andersen, A.N.; Majer, J.D. Ants show the way Down Under: Invertebrates as bioindicators in land management. Front. Ecol. Environ. 2004, 2, 291–298. [Google Scholar] [CrossRef]
- Ohgushi, T. Indirect interaction webs: Herbivore-Induced effects through trait change in plants. Annu. Rev. Ecol. Evol. Systemat. 2005, 36, 81–105. [Google Scholar] [CrossRef]
- Tomasko, D.K.; LaPointe, B.E. Productivity and biomass of Thalassia testudinum as related to water column nutrient availability and epiphyte levels—Field observations and experiemental studies. Mar. Ecol. Progr. 1991, 75, 9–17. [Google Scholar] [CrossRef]
- Sand-Jensen, K. Effect of epiphytes on eelgrass photosynthesis. Aquat. Bot. 1977, 3, 55–63. [Google Scholar] [CrossRef]
- Van Montfrans, J.; Orth, R.J.; Vay, S.A. Preliminary studies of grazing by Bitium varium on eelgrass periphyton. Aquat. Bot. 1982, 3, 75–89. [Google Scholar] [CrossRef]
- Robertson, A.I.; Mann, K.H. Population dynamics and life history adaptations of Littorina neglecta in an eelgrass meadow (Zostera marina L.) in Nova Scotia. J. Exp. Mar. Biol. Ecol. 1982, 63, 151–171. [Google Scholar] [CrossRef]
- Hootsmans, M.J.M.; Vermaat, J.E. The effect of periphyton grazing by three epifaunal species on the growth of Zostera marina L. under experimental conditions. Aquat. Bot. 1985, 22, 83–88. [Google Scholar] [CrossRef]
- Neckles, H.A.; Wetzel, R.L.; Orth, R.J. Relative effects of nutrient enrichment and grazing on epiphyte-macrophyte (Zostera marina L.) dynamics. Oecologia 1993, 93, 285–295. [Google Scholar] [CrossRef]
- Whalen, M.A.; Duffy, J.E.; Grace, J.B. Temporal shifts in top-down vs. bottom-up control of epiphytic algae in a seagrass ecosystem. Ecology 2013, 94, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.L.; Richardson, J.P.; Duffy, J.E. Field experimental evidence that grazers mediate transition between microalgal and seagrass dominance. Limnol. Oceanogr. 2014, 59, 1053–1064. [Google Scholar] [CrossRef]
- Moksnes, P.; Tryman, K.; Baden, S. Trophic cascades in a temperate seagrass community. Oikos. 2008, 117, 767–777. [Google Scholar] [CrossRef]
- Duffy, J.E.; MacDonald, K.S.; Rhode, J.M.; Parker, J.D. Grazer diversity, functional redundancy, and productivity in seagrass beds: An experimental test. Ecology 2001, 82, 2417–2434. [Google Scholar] [CrossRef]
- Holzer, K.K.; Rueda, J.L.; McGlathery, K.J. Differences in the feeding ecology of two seagrass-associated snails. Estuar. Coast. 2011, 34, 1140–1149. [Google Scholar] [CrossRef]
- Lewis, L.S.; Anderson, T.W. Top-down control of epifauna by fishes enhances seagrass production. Ecology 2012, 93, 2746–2757. [Google Scholar] [CrossRef] [PubMed]
- Ecklof, J.S.; Alsterberg, C.; Havenhand, J.N.; Sundback, K.; Wood, H.L.; Gamfeldt, L. Experimental climate change weakens the insurance effect of biodiversity. Ecol. Lett. 2012, 15, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Best, R.J.; Stachowicz, J.J. Trophic cascades in seagrass meadows depend on mesograzer variation in feeding rates, predation susceptibility, and abundance. Mar. Ecol. Progr. 2012, 456, 29–42. [Google Scholar] [CrossRef]
- Todd, J.S.; Zimmerman, R.C.; Crew, P.; Alberte, R.S. The antifouling activity of natural and synthetic phenol acid sulfate esters. Phytochemistry 1993, 34, 401–404. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defense mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Verges, A.; Becerro, M.A.; Alcoverro, T.; Romero, J. Variation in multiple traits of vegetative and reproductive seagrass tissues influences plant-herbivore interactions. Oecologia 2007, 151, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Tomas, F.; Abbott, J.M.; Steinberg, C.; Balk, M.; Williams, S.L.; Stachowicz, J.J. Plant genotype and nitrogen loading influence seagrass productivity, biochemistry, and plant-herbivore interactions. Ecology 2011, 92, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Long, J.D.; Hamilton, R.S.; Mitchell, J.L. Asymmetric competition via induced resistance: Specialist herbivores indirectly suppress generalist preference and populations. Ecology 2007, 88, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Verges, A.; Perez, M.; Alcoverro, T.; Romero, J. Compensation and resistance to herbivory in seagrasses: Induced responses to simulated consumption by fish. Oecologia 2008, 155, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Steele, L.; Valentine, J.F. Idiosyncratic responses of seagrass phenolic production following sea urchin grazing. Mar. Ecol. Progr. 2012, 466, 81–92. [Google Scholar] [CrossRef]
- Bos, A.R.; Bouma, T.J.; de Kort, G.L.J.; van Katwijk, M.M. Ecosystem engineering by annual intertidal seagrass beds: Sediment accretion and modification. Estuar. Coast. Shelf. Sci. 2007, 74, 344–348. [Google Scholar] [CrossRef]
- Heck, K.L.; Hays, G.; Orth, R.J. Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar. Ecol. Progr. 2003, 253, 123–136. [Google Scholar] [CrossRef]
- Fourqurean, J.; Duarte, C.M.; Kennedy, H.; Marba, N.; Holmer, M.; Mateo, M.A.; Apostalaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Short, F.T.; Wyllie-Echeverria, S. Natural and human-induced disturbance of seagrass. Environ. Conservat. 1996, 23, 17–27. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A global crisis for seagrass ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourquerean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merkel and Associates, Inc. Baywide Eelgrass (Z. marina L.) Distribution and Status within San Francisco Bay: Program Development and Testing of a Regional Eelgrass Monitoring Strategy; California Department of Transportation: Sacramento, CA, USA, 2010.
- World Atlas of Seagrasses; Green, E.P.; Short, F.T. (Eds.) University of California Press: Berkeley, CA, USA, 2003.
- State Coastal Conservancy. San Francisco Bay Subtidal Habitat Goals Report. State Coastal Conservancy: Oakland, CA, USA, 2010. [Google Scholar]
- Boyer, K.E.; Wyllie-Echeverria, S. Subtidal Habitat Goals Project Report, Appendix 8–1: Eelgrass Conservation and Restoration in San Francisco Bay, Opportunities and Constraints. State Coastal Conservancy: Oakland, CA, USA, 2010. [Google Scholar]
- Cohen, A.N.; Carlton, J.T. Accelerating invasion rate in a highly invaded estuary. Science 1998, 279, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.A.; Boyer, K.E.; Brooks, A.J. Spatial patterns of epifaunal abundance in San Francisco Bay eelgrass (Zostera marina) beds. Mar. Ecol. 2011, 32, 88–103. [Google Scholar] [CrossRef]
- Reynolds, L.K.; Carr, L.A.; Boyer, K.E. A non-native amphipod consumes eelgrass inflorescences in San Francisco Bay. Mar. Ecol. Progr. 2012, 451, 107–118. [Google Scholar] [CrossRef]
- Carr, L.A.; Boyer, K.E. Variation at multiple trophic levels mediates a novel seagrass-grazer interaction. Mar. Ecol. Progr. 2014, 508, 117–128. [Google Scholar] [CrossRef]
- Douglass, J.G.; Duffy, J.E.; Canuel, E.A. Food web structure in a Chesapeake Bay eelgrass bed as determined through gut content and 13C and 15N isotope analysis. Estuar. Coast. 2011, 34, 701–711. [Google Scholar] [CrossRef]
- Chapman, J.W. Gammaridea. In The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon, 4th ed.; Carlton, J.E., Ed.; University of California Press: Berkely, CA, USA, 2007; pp. 545–618. [Google Scholar]
- Pilgrim, E.M.; Darling, J.A. Genetic diversity in two introduced biofouling amphipods (Ampithoe valida and Jassa marmorata) along the Pacific North American coast: Investigation into molecular identification and cryptic diversity. Divers. Distrib. 2010, 16, 827–839. [Google Scholar] [CrossRef]
- Sotka, E.; College of Charleston, Charleston, SC, USA; Scheinberg, L.; Romberg Tiburon Center, San Francisco State University, Tiburon, CA, USA; Boyer, K.E.; Romberg Tiburon Center, San Francisco State University, Tiburon, CA, USA. Unpublished data. 2014.
- Watling, L.; Carlton, J.T. Caprellidae. In The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon, 4th ed.; Carlton, J.T., Ed.; University of California Press: Berkeley, CA, USA, 2007; pp. 618–629. [Google Scholar]
- Carlton, J.T. Introduced marine and estuaring mollusks of North America: An end-of-the-20th-century perspective. J. Shellfish Res. 1992, 11, 489–505. [Google Scholar]
- Carlton, J.T. The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon, 4th ed.; University of California Press: Berkeley, CA, USA, 2007. [Google Scholar]
- Boyer, K.E.; Romberg Tiburon Center, San Francisco State University, Tiburon, CA, USA. Unpublished data. 2014.
- Folin, O.; Ciocalteu, V. Of tyrosine and tryptophan determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar]
- Bolser, R.C.; Hay, M.E.; Lindquist, N.; Fenical, W.; Wilson, D. Chemical defenses of freshwater macrophytes against crayfish herbivory. J. Chem. Ecol. 1998, 24, 1639–1658. [Google Scholar] [CrossRef]
- Quackenbush, R.C.; Bunn, D.; Lingren, W. HPLC determination of phenolic acids in the water soluble extract of Zostera marina L (eelgrass). Aquat. Bot. 1986, 24, 83–89. [Google Scholar] [CrossRef]
- Ruxton, G.D. The unequal variance t-test is an underused alternative to Student's t-test and the Mann-Whitney U test. Behav. Ecol. 2006, 17, 688–690. [Google Scholar] [CrossRef]
- Williams, S.L.; Ruckelshaus, M.A. Effects of nitrogen availablity and herbivory on eelgrass (Zostera marina) and epiphytes. Ecology 1993, 74, 904–918. [Google Scholar] [CrossRef]
- Hughes, A.R.; Best, R.J.; Stachowicz, J.J. Genotypic diversity and grazer identity interactively influence seagrass and grazer biomass. Mar. Ecol. Progr. 2010, 403, 43–51. [Google Scholar] [CrossRef]
- Yun, H.Y.; Rohde, S.; Linnane, K.; Wahl, M.; Molis, M. Seaweed-mediated indirect interaction between two species of meso-herbivores. Mar. Ecol. Progr. 2010, 408, 47–53. [Google Scholar] [CrossRef]
- Buchsbaum, R.; Valiela, I.; Swain, T. The role of phenolic compounds and other plant constituents in feeding by Canada geese in a coastal marsh. Oecologia 1984, 63, 343–349. [Google Scholar] [CrossRef]
- Harrison, P.G.; Durance, C. Seasonal variation in phenolic content of eelgrass shoots. Aquat. Bot. 1989, 35, 409–413. [Google Scholar] [CrossRef]
- Vergeer, L.H.T.; Aarts, T.L.; de Groot, J.D. The “wasting disease” and the effect of abiotic factors (light intensity, temperature, salinity) and infection with Labyrinthula zosterae on the phenolic content of Zostera marina shoots. Aquat. Bot. 1995, 52, 35–44. [Google Scholar] [CrossRef]
- Buchsbaum, R.N.; Short, F.T.; Cheney, D.P. Phenolic-nitrogen interactions in eelgrass, Zostera marina L.: Possible implications for disease resistence. Aquat. Bot. 1990, 37, 291–297. [Google Scholar] [CrossRef]
- Ceh, J.; Molis, M.; Dzeha, T.M.; Wohl, M. Induction and reduction of anti-herbivore defenses in brown and red macroalgae off the Kenyan Coast. J. Phycol. 2005, 41, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.B.; Sotka, E.; Hay, M.E. Tissue-specific induction of herbivore resistance: Seaweed response to amphipod grazing. Oecologia 2002, 132, 68–76. [Google Scholar] [CrossRef]
- Icely, J.D.; Nott, J.A. Feeding and digestion in Corophium volutator. Mar. Biol. 1985, 89, 183–195. [Google Scholar] [CrossRef]
- Ort, B.S.; Cohen, C.S.; Boyer, K.E.; Wyllie-Echeverria, S. Population structure and genetic diversity among eelgrass (Zostera marina) beds and depths in San Francisco Bay. J. Hered. 2012, 103, 533–546. [Google Scholar] [CrossRef]
- Bischoff, A.; Tremulot, S. Differentiation and adaptation in Brassica nigra populations: Interactions with related herbivores. Oecologia 2011, 165, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Vergeer, L.H.T.; Develi, A. Phenolic acids in healthy and infected leaves of Zostera marina and their growth-limiting properties toward Labyrinthula zosterae. Aquat. Bot. 1997, 58, 65–72. [Google Scholar] [CrossRef]
- Ramamurthy, M.S.; Maiti, B.; Thomas, P.; Nair, P.M. High-performance liquid chromatography determination of phenolic acids in potato tubers (Solanum tuberosum) during wound healing. J. Agr. Food Chem. 1992, 40, 569–572. [Google Scholar] [CrossRef]
- Li, J.Y.; Oulee, T.M.; Raba, R.; Last, R.L. Arabidopsis flavanoid mutants are hypersensitive to UV-B radiation. Plant Cell 1993, 5, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Landry, L.G.; Chapple, C.C.S.; Last, R.L. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant. Physiol. 1995, 109, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Close, D.C.; McArthur, C. Rethinking the role of many plant phenolics: protection from photodamage not herbivores? Oikos 2002, 99, 166–172. [Google Scholar] [CrossRef]
- Denno, R.F. Plant-mediated interactions in herbivorous insects: mechanisms, symmetry, and challenging the paradigms of competition past. In Ecological Communities: Plant Mediation in Indirect Interaction Webs; Ohgushi, T., Craig, T.P., Price, P.W., Eds.; Cambridge University Press: New York, NY, USA, 2007; pp. 19–50. [Google Scholar]
- Denno, R.F.; Peterson, M.A.; Gratton, C.; Cheng, J.; Langellotto, G.A.; Huberty, A.F.; Finke, D.L. Feeding-induced changes in plant quality mediate interspecific competition between sap-feeding herbivores. Ecology 2000, 81, 1814–1827. [Google Scholar] [CrossRef]
- Long, J.D.; Mitchell, J.L.; Sotka, E.E. Local consumers induce resistance differentially between Spartina alterniflora populations in the field. Ecology 2011, 92, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Molis, M.; Enge, A.; Karsten, U. Grazing impact of, and indirect interactions between mesograzers associated with kelp (Laminaria digitata). J. Phycol. 2010, 46, 76–84. [Google Scholar] [CrossRef]
- Viejo, R.M.; Arrontes, J. Interactions between mesograzers inhabiting Fucus vesiculosus in Northern Spain. J. Exp. Mar. Biol. Ecol. 1992, 162, 97–111. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, J.T.; Boyer, K.E. Grazer Functional Roles, Induced Defenses, and Indirect Interactions: Implications for Eelgrass Restoration in San Francisco Bay. Diversity 2014, 6, 751-770. https://doi.org/10.3390/d6040751
Lewis JT, Boyer KE. Grazer Functional Roles, Induced Defenses, and Indirect Interactions: Implications for Eelgrass Restoration in San Francisco Bay. Diversity. 2014; 6(4):751-770. https://doi.org/10.3390/d6040751
Chicago/Turabian StyleLewis, Jeffrey T., and Katharyn E. Boyer. 2014. "Grazer Functional Roles, Induced Defenses, and Indirect Interactions: Implications for Eelgrass Restoration in San Francisco Bay" Diversity 6, no. 4: 751-770. https://doi.org/10.3390/d6040751
APA StyleLewis, J. T., & Boyer, K. E. (2014). Grazer Functional Roles, Induced Defenses, and Indirect Interactions: Implications for Eelgrass Restoration in San Francisco Bay. Diversity, 6(4), 751-770. https://doi.org/10.3390/d6040751




