A GSH Fluorescent Probe with a Large Stokes Shift and Its Application in Living Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical Measurements and Instrumentation
2.3. Synthesis and Characterization of Probe GP
2.4. Preparation of Samples and Test Solution
2.5. Calculating Method of LOD
2.6. Confocal Laser Scanning Microscope (CLSM) Imaging
3. Results and Discussion
3.1. Preparation and Photo Physical Property
3.2. Selectivity Experiment
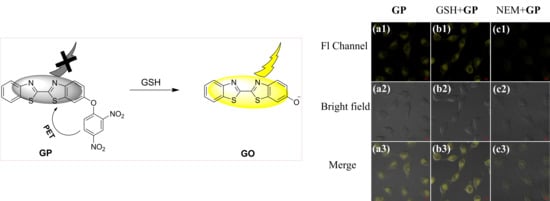
3.3. Cell Imaging Experiment and Its Application in the Analysis of Cell Healthy Status
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rui, H.; Gang, H.; Fernández, J.M.; Byoung-Jin, K.; Forbes, N.S.; Rotello, V.M. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 2006, 128, 1078–1079. [Google Scholar]
- Niu, H.; Ni, B.; Chen, K.; Yang, X.; Cao, W.; Ye, Y.; Zhao, Y. A long-wavelength-emitting fluorescent probe for simultaneous discrimination of H2S/Cys/GSH and its bio-imaging applications. Talanta 2019, 196, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tian, H.; Yang, L.; He, L.; Geng, Y.; Liu, X.; Song, X. Fluorescent probe for simultaneous discrimination of Cys/Hcy and GSH in pure aqueous media with a fast response under a single-wavelength excitation. Sens. Actuators B Chem. 2018, 273, 1170–1178. [Google Scholar] [CrossRef]
- Yang, R.; Tang, Y.; Zhu, W. Ratiometric Fluorescent Probe for the Detection of Glutathione in Living Cells. Chem. J. Chin. Univ.-Chin. 2016, 37, 643–647. [Google Scholar] [CrossRef]
- Niu, L.-Y.; Guan, Y.-S.; Chen, Y.-Z.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. BODIPY-Based Ratiometric Fluorescent Sensor for Highly Selective Detection of Glutathione over Cysteine and Homocysteine. J. Am. Chem. Soc. 2012, 134, 18928–18931. [Google Scholar] [CrossRef]
- Lee, S.; Li, J.; Xin, Z.; Yin, J.; Yoon, J. Recent progress on the development of glutathione (GSH) selective fluorescent and colorimetric probes. Coord. Chem. Rev. 2018, 366, 29–68. [Google Scholar] [CrossRef]
- Huang, R.; Wang, B.-B.; Si-Tu, X.-M.; Gao, T.; Wang, F.-F.; He, H.; Fan, X.-Y.; Jiang, F.-L.; Liu, Y. A lysosome-targeted fluorescent sensor for the detection of glutathione in cells with an extremely fast response. Chem. Commun. 2016, 52, 11579–11582. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Y.; Zhang, H.; Zhang, Z.; Li, M.-J.; Yi, C.; Yang, M. A dual-mode nanosensor based on carbon quantum dots and gold nanoparticles for discriminative detection of glutathione in human plasma. Biosens. Bioelectron. 2014, 56, 39–45. [Google Scholar] [CrossRef]
- Zampagni, M.; Wright, D.; Cascella, R.; D’Adamio, G.; Casamenti, F.; Evangelisti, E.; Cardona, F.; Goti, A.; Nacmias, B.; Sorbi, S.; et al. Novel S-acyl glutathione derivatives prevent amyloid oxidative stress and cholinergic dysfunction in Alzheimer disease models. Free Radic. Biol. Med. 2012, 52, 1362–1371. [Google Scholar] [CrossRef]
- Gong, D.; Ru, J.; Cao, T.; Qian, J.; Liu, W.; Iqbal, A.; Liu, W.; Qin, W.; Guo, H. Two-stage ratiometric fluorescent responsive probe for rapid glutathione detection based on BODIPY thiol-halogen nucleophilic mono- or disubstitution. Sens. Actuators B Chem. 2018, 258, 72–79. [Google Scholar] [CrossRef]
- Seghrouchni, I.; Drai, J.; Bannier, E.; Rivière, J.; Revol, A. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus; insulin treatment efficiency. Clin. Chim. Acta 2002, 321, 89–96. [Google Scholar] [CrossRef]
- Yuan, B.; Zeng, X.; Xu, C.; Lin, L.; Ma, Y.; Zhang, D.; Yong, F. Electrochemical modification of graphene oxide bearing different types of oxygen functional species for the electro-catalytic oxidation of reduced glutathione. Sens. Actuators B Chem. 2013, 184, 15–20. [Google Scholar] [CrossRef]
- Huang, Z.M.; Cai, Q.Y.; Ding, D.C.; Ge, J.; Hu, Y.L.; Yang, J.; Zhang, L.; Li, Z.H. A facile label-free colorimetric method for highly sensitive glutathione detection by using manganese dioxide nanosheets. Sens. Actuators B Chem. 2017, 242, 355–361. [Google Scholar] [CrossRef]
- Arindam, S.; Jana, N.R. Detection of cellular glutathione and oxidized glutathione using magnetic-plasmonic nanocomposite-based “turn-off” surface enhanced Raman scattering. Anal. Chem. 2013, 85, 9221–9228. [Google Scholar]
- Zhao, Z.; Fan, H.; Zhou, G.; Bai, H.; Liang, H.; Wang, R.; Zhang, X.; Tan, W. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheet-aptamer nanoprobe. J. Am. Chem. Soc. 2014, 136, 11220–11223. [Google Scholar] [CrossRef]
- Zhu, X.; Kalyanaraman, N.; Subramanian, R. Enhanced screening of glutathione-trapped reactive metabolites by in-source collision-induced dissociation and extraction of product ion using UHPLC-high resolution mass spectrometry. Anal. Chem. 2011, 83, 9516. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Z.; Qi, L.; Lai, J.; Kitte, S.A.; Xu, G. Ultrasensitive Glutathione Detection Based on Lucigenin Cathodic Electrochemiluminescence in the Presence of MnO2 Nanosheets. Anal. Chem. 2016, 88, 7654. [Google Scholar] [CrossRef]
- Yu, F.; Li, P.; Wang, B.; Han, K. Reversible near-infrared fluorescent probe introducing tellurium to mimetic glutathione peroxidase for monitoring the redox cycles between peroxynitrite and glutathione in vivo. J. Am. Chem. Soc. 2013, 135, 7674–7680. [Google Scholar] [CrossRef]
- Han, L.; Liu, S.G.; Zhang, X.F.; Tao, B.X.; Li, N.B.; Luo, H.Q. A sensitive polymer dots-manganese dioxide fluorescent nanosensor for “turn-on” detection of glutathione in human serum. Sens. Actuators B Chem. 2018, 258, 25–31. [Google Scholar] [CrossRef]
- Xia, X.; Qian, Y.; Shen, B. Synthesis of a BODIPY disulfonate near-infrared fluorescence-enhanced probe with high selectivity to endogenous glutathione and two-photon fluorescent turn-on through thiol-induced SNAr substitution. J. Mater. Chem. B 2018, 6, 3023–3029. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Z.-Y.; Du, Z.-F.; Cui, J.; Miao, J.-Y.; Zhao, B.-X. A colorimetric, ratiometric and water-soluble fluorescent probe for simultaneously sensing glutathione and cysteine/homocysteine. Anal. Chim. Acta 2015, 900, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huo, F.; Yue, Y.; Zhang, Y.; Yin, C. A coumarin-based “off-on” sensor for fluorescence selectivily discriminating GSH from Cys/Hcy and its bioimaging in living cells. Sens. Actuators B Chem. 2017, 253, 42–49. [Google Scholar] [CrossRef]
- Hou, X.; Li, Z.; Li, B.; Liu, C.; Xu, Z. An “off-on” fluorescein-based colormetric and fluorescent probe for the detection of glutathione and cysteine over homocysteine and its application for cell imaging. Sens. Actuators B Chem. 2018, 260, 295–302. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Xu, Q.H.; Loh, K.P.; Chen, Z.K. One- and two-photon turn-on fluorescent probe for cysteine and homocysteine with large emission shift. Org. Lett. 2009, 11, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Tang, Y.; Lin, W. Development of a Two-photon Ratiometric Fluorescent Probe for Glutathione and Its Applications in Living Cells. Chem. Res. Chin. Univ. 2018, 34, 523–527. [Google Scholar] [CrossRef]
- Xiong, K.; Huo, F.; Chao, J.; Zhang, Y.; Yin, C. Colorimetric and NIR Fluorescence Probe with Multiple Binding Sites for Distinguishing Detection of Cys/Hcy and GSH in Vivo. Anal. Chem. 2019, 91, 1472–1478. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Li, X.; Zhang, W.; Liu, X.; Zhu, Y.; Wang, H.-Q.; Fang, J. Improving performance and reducing hysteresis in perovskite solar cells by using F8BT as electron transporting layer. Sol. Energy Mater. Sol. Cells 2016, 157, 79–84. [Google Scholar] [CrossRef]
- Sun, W.; Li, W.; Li, J.; Zhang, J.; Du, L.; Li, M. A benzothiazole-based fluorescent probe for thiol bioimaging. Tetrahedron Lett. 2012, 53, 2332–2335. [Google Scholar] [CrossRef]
- Goswami, S.; Manna, A.; Paul, S.; Das, A.K.; Nandi, P.K.; Maity, A.K.; Saha, P. A turn on ESIPT probe for rapid and ratiometric fluorogenic detection of homocysteine and cysteine in water with live cell-imaging. Tetrahedron Lett. 2014, 55, 490–494. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Y.; Huang, J.; Zhang, X.; Fang, T.; Zhang, Y.; Zheng, X.; Yang, X. A benzothiazole-based fluorescent probe for selective detection of H2S in living cells and mouse hippocampal tissues. Dye. Pigment. 2017, 138, 112–118. [Google Scholar] [CrossRef]
- Ren, X.; Fang, W.; Jing, L.; Wei, T.; Wei, Z.; Yong, W.; Chen, X. An ESIPT-based fluorescent probe for highly selective detection of glutathione in aqueous solution and living cells. Dye. Pigment. 2016, 129, 156–162. [Google Scholar] [CrossRef]
- Mao, Y.; Du, H.; Wang, X.; Tian, M.; Wang, Y.; Liu, L.; Wei, J.; Xue, F.; Liu, G.; Zhang, X.; et al. A ratiometric fluorescent probe for rapidly detecting bio-thiols in vitro and in living cells. Dye. Pigment. 2019, 171, 107688. [Google Scholar] [CrossRef]
- Wang, X.-B.; Hao, X.; Zhang, D.; Jiang, Y. Reaction-based fluorescent turn-on probe for selective detection of thiophenols in aqueous solution and living cells. Dye. Pigment. 2017, 142, 167–174. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, Y.; Liu, Y.; Wang, W. Rational design of a highly selective and sensitive fluorescent PET probe for discrimination of thiophenols and aliphatic thiols. Chem. Commun. 2010, 46, 1944. [Google Scholar] [CrossRef]
- Lan, M.; Wu, J.; Liu, W.; Zhang, H.; Zhang, W.; Zhuang, X.; Wang, P. Highly sensitive fluorescent probe for thiols based on combination of PET and ESIPT mechanisms. Sens. Actuators B Chem. 2011, 156, 332–337. [Google Scholar] [CrossRef]
- Liu, K.; Shang, H.; Kong, X.; Lin, W. A novel near-infrared fluorescent probe with a large Stokes shift for biothiol detection and application in in vitro and in vivo fluorescence imaging. J. Mater. Chem. B 2017, 5, 3836–3841. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Xu, Y.; Li, Z.; Wang, Y.; Du, H.; Liu, L.; Ding, R.; Liu, G. A GSH Fluorescent Probe with a Large Stokes Shift and Its Application in Living Cells. Sensors 2019, 19, 5348. https://doi.org/10.3390/s19245348
Mao Y, Xu Y, Li Z, Wang Y, Du H, Liu L, Ding R, Liu G. A GSH Fluorescent Probe with a Large Stokes Shift and Its Application in Living Cells. Sensors. 2019; 19(24):5348. https://doi.org/10.3390/s19245348
Chicago/Turabian StyleMao, Yueyuan, Yediao Xu, Zhi Li, Yang Wang, Huanhuan Du, Lei Liu, Ran Ding, and Guodong Liu. 2019. "A GSH Fluorescent Probe with a Large Stokes Shift and Its Application in Living Cells" Sensors 19, no. 24: 5348. https://doi.org/10.3390/s19245348
APA StyleMao, Y., Xu, Y., Li, Z., Wang, Y., Du, H., Liu, L., Ding, R., & Liu, G. (2019). A GSH Fluorescent Probe with a Large Stokes Shift and Its Application in Living Cells. Sensors, 19(24), 5348. https://doi.org/10.3390/s19245348