Continuous Live-Cell Culture Imaging and Single-Cell Tracking by Computational Lensfree LED Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.1.1. Neuroblastoma Culture
2.1.2. Prorocentrum Minimum Culture
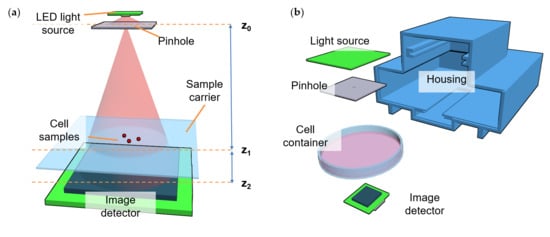
2.2. Microscopy Setup
2.3. Image Reconstruction and Examination
2.3.1. Image Back Propagation
2.3.2. Still Frame Cell Counting
2.3.3. Video Particle Tracking
3. Results
3.1. Microscopic Imaging Evaluation
3.2. Neuroblastoma Cells
3.3. Prorocentrum Minimum
3.4. Cell Tracking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kieninger, J.; Weltin, A.; Flamm, H.; Urban, G.A. Microsensor systems for cell metabolism—From 2D culture to organ-on-chip. Lab Chip 2018, 18, 1274–1291. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Henley, B.M.; Kim, C.H.; Lester, H.A.; Yang, C. Incubator embedded cell culture imaging system (EmSight) based on Fourier ptychographic microscopy. Biomed. Opt. Express 2016, 7, 3097. [Google Scholar] [CrossRef] [PubMed]
- Mudanyali, O.; Tseng, D.; Oh, C.; Isikman, S.O.; Sencan, I.; Bishara, W.; Oztoprak, C.; Seo, S.; Khademhosseini, B.; Ozcan, A. Compact, light-weight and cost-effective microscope based on lensless incoherent holography for telemedicine applications. Lab Chip 2010, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Shimobaba, T.; Yamanashi, H.; Kakue, T.; Oikawa, M.; Okada, N.; Endo, Y.; Hirayama, R.; Masuda, N.; Ito, T. In-line digital holographic microscopy using a consumer scanner. Sci. Rep. 2013, 3, 2664. [Google Scholar] [CrossRef] [PubMed]
- Scholz, G.; Xu, Q.; Schulze, T.; Boht, H.; Mattern, K.; Hartmann, J.; Dietzel, A.; Scherneck, S.; Rustenbeck, I.; Prades, J.; et al. LED-Based Tomographic Imaging for Live-Cell Monitoring of Pancreatic Islets in Microfluidic Channels. Proceedings 2017, 1, 552. [Google Scholar] [CrossRef]
- Scholz, G.; Mariana, S.; Syamsu, I.; Dharmawan, A.B.; Schulze, T.; Mattern, K.; Hörmann, P.; Hartmann, J.; Dietzel, A.; Rustenbeck, I.; et al. Continuous Live-Cell Culture Monitoring by Compact Lensless LED Microscopes. Proceedings 2018, 2, 877. [Google Scholar] [CrossRef]
- Roy, M.; Seo, D.; Oh, C.-H.; Nam, M.-H.; Kim, Y.J.; Seo, S. Low-cost telemedicine device performing cell and particle size measurement based on lens-free shadow imaging technology. Biosens. Bioelectron. 2015, 67, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chu, D. Coherence properties of different light sources and their effect on the image sharpness and speckle of holographic displays. Sci. Rep. 2017, 7, 5893. [Google Scholar] [CrossRef] [PubMed]
- Bishara, W.; Sikora, U.; Mudanyali, O.; Su, T.-W.; Yaglidere, O.; Luckhart, S.; Ozcan, A. Holographic pixel super-resolution in portable lensless on-chip microscopy using a fiber-optic array. Lab Chip 2011, 11, 1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Wu, J. Resolution enhancement method for lensless in-line holographic microscope with spatially-extended light source. Opt. Express 2017, 25, 24735. [Google Scholar] [CrossRef] [PubMed]
- Biedler, J.L.; Roffler-Tarlov, S.; Schachner, M.; Freedman, L.S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978, 38, 3751–3757. [Google Scholar] [PubMed]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential Treatment of SH-SY5Y Cells with Retinoic Acid and Brain-Derived Neurotrophic Factor Gives Rise to Fully Differentiated, Neurotrophic Factor-Dependent, Human Neuron-Like Cells. J. Neurochem. 2002, 75, 991–1003. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Lambert, G.; Andersen, R.A.; Coffroth, M.A.; Galbraith, D.W. Symbiodinium (Pyrrhophyta) genome sizes (DNA content) are smallest among dinoflagellates. J. Phycol. 2005, 41, 880–886. [Google Scholar] [CrossRef]
- Gaines, G.; Elbrächter, M. Heterotrophic nutrition. In The Biology of Dinoflagellates; Taylor, F., Ed.; Blackwell: Oxford, UK, 1987; pp. 224–269. [Google Scholar]
- Heil, C.A.; Glibert, P.M.; Fan, C. Prorocentrum minimum (Pavillard) Schiller: A review of a harmful algal bloom species of growing worldwide importance. Harmful Algae 2005, 4, 449–470. [Google Scholar] [CrossRef]
- Rodríguez, I.; Alfonso, A.; Alonso, E.; Rubiolo, J.A.; Roel, M.; Vlamis, A.; Katikou, P.; Jackson, S.A.; Menon, M.L.; Dobson, A.; et al. The association of bacterial C9-based TTX-like compounds with Prorocentrum minimum opens new uncertainties about shellfish seafood safety. Sci. Rep. 2017, 7, 40880. [Google Scholar] [CrossRef] [PubMed]
- Mandel, L.; Wolf, E. Optical Coherence and Quantum Optics; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Latychevskaia, T.; Fink, H.-W. Practical algorithms for simulation and reconstruction of digital in-line holograms. Appl. Opt. 2014, 54, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Wu, Y.; Tamamitsu, M.; Ozcan, A. Edge sparsity criterion for robust holographic autofocusing. Opt. Lett. 2017, 42, 3824. [Google Scholar] [CrossRef] [PubMed]
- Tamamitsu, M.; Zhang, Y.; Wang, H.; Wu, Y.; Ozcan, A. Comparison of Gini index and Tamura coefficient for holographic autofocusing based on the edge sparsity of the complex optical wavefront. arXiv, 2017; arXiv:1708.08055. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sbalzarini, I.F.; Koumoutsakos, P. Feature point tracking and trajectory analysis for video imaging in cell biology. J. Struct. Biol. 2005, 151, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Chenouard, N.; Smal, I.; de Chaumont, F.; Maška, M.; Sbalzarini, I.F.; Gong, Y.; Cardinale, J.; Carthel, C.; Coraluppi, S.; Winter, M.; et al. Objective comparison of particle tracking methods. Nat. Methods 2014, 11, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, T.; Rasband, W. ImageJ User Guide. Available online: https://imagej.nih.gov/ij/docs/guide/146-30.html#toc-Subsection-30.7 (accessed on 31 January 2019).
- Sobieranski, A.C.; Inci, F.; Tekin, H.C.; Yuksekkaya, M.; Comunello, E.; Cobra, D.; von Wangenheim, A.; Demirci, U. Portable lensless wide-field microscopy imaging platform based on digital inline holography and multi-frame pixel super-resolution. Light Sci. Appl. 2015, 4, e346. [Google Scholar] [CrossRef] [PubMed]
- Bishara, W.; Su, T.-W.; Coskun, A.F.; Ozcan, A. Lensfree on-chip microscopy over a wide field-of-view using pixel super-resolution. Opt. Express 2010, 18, 11181–11191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Ozcan, A. Lensless digital holographic microscopy and its applications in biomedicine and environmental monitoring. Methods 2018, 136, 4–16. [Google Scholar] [CrossRef] [PubMed]








| Low Concentration | High Concentration | |
|---|---|---|
| Concentration [1/mm2] | 15.2 ± 1.5 | 33.1 ± 2.1 |
| Relation round/non-round [%] | 52/48 | 26/74 |
| Area coverage [%] | 1.0 | 2.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholz, G.; Mariana, S.; Dharmawan, A.B.; Syamsu, I.; Hörmann, P.; Reuse, C.; Hartmann, J.; Hiller, K.; Prades, J.D.; Wasisto, H.S.; et al. Continuous Live-Cell Culture Imaging and Single-Cell Tracking by Computational Lensfree LED Microscopy. Sensors 2019, 19, 1234. https://doi.org/10.3390/s19051234
Scholz G, Mariana S, Dharmawan AB, Syamsu I, Hörmann P, Reuse C, Hartmann J, Hiller K, Prades JD, Wasisto HS, et al. Continuous Live-Cell Culture Imaging and Single-Cell Tracking by Computational Lensfree LED Microscopy. Sensors. 2019; 19(5):1234. https://doi.org/10.3390/s19051234
Chicago/Turabian StyleScholz, Gregor, Shinta Mariana, Agus Budi Dharmawan, Iqbal Syamsu, Philipp Hörmann, Carsten Reuse, Jana Hartmann, Karsten Hiller, Joan Daniel Prades, Hutomo Suryo Wasisto, and et al. 2019. "Continuous Live-Cell Culture Imaging and Single-Cell Tracking by Computational Lensfree LED Microscopy" Sensors 19, no. 5: 1234. https://doi.org/10.3390/s19051234
APA StyleScholz, G., Mariana, S., Dharmawan, A. B., Syamsu, I., Hörmann, P., Reuse, C., Hartmann, J., Hiller, K., Prades, J. D., Wasisto, H. S., & Waag, A. (2019). Continuous Live-Cell Culture Imaging and Single-Cell Tracking by Computational Lensfree LED Microscopy. Sensors, 19(5), 1234. https://doi.org/10.3390/s19051234