Nanoporous Gold as a VOC Sensor, Based on Nanoscale Electrical Phenomena and Convolutional Neural Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Electrochemcial Dealloying
2.3. Material Characterization
2.4. Functionalization
2.5. Electrical Measurement
2.6. Controlling VOC Concentration
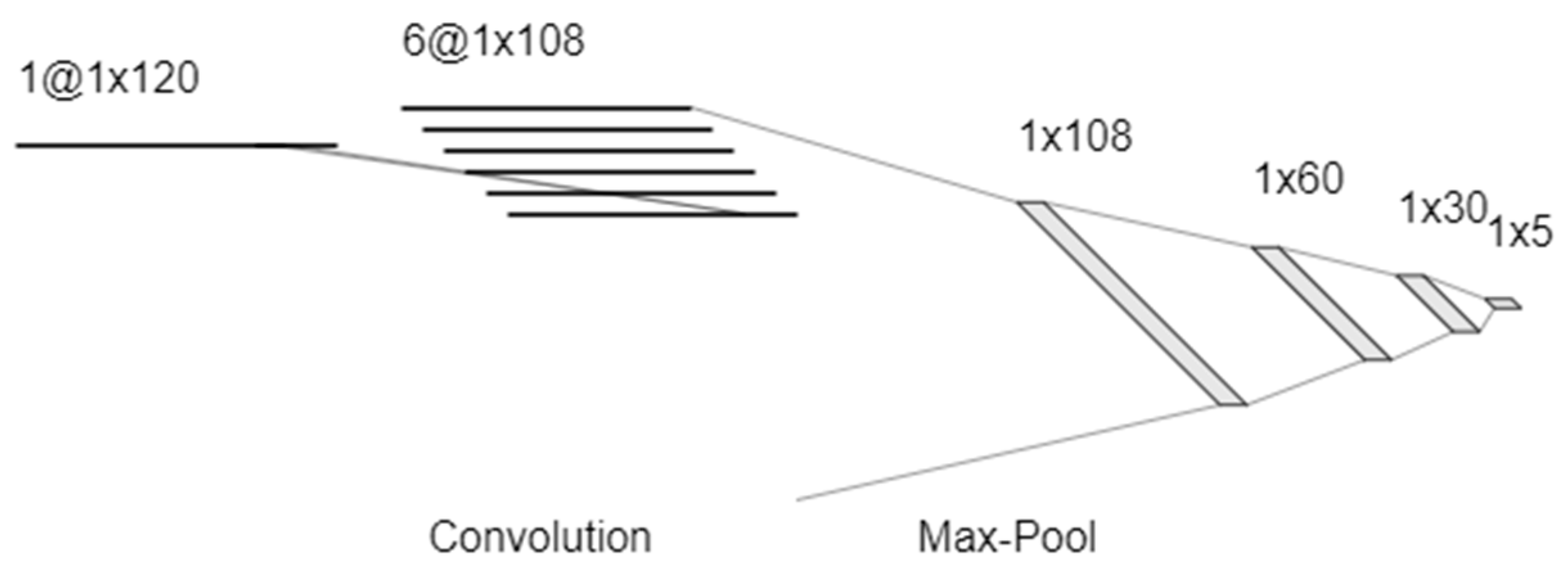
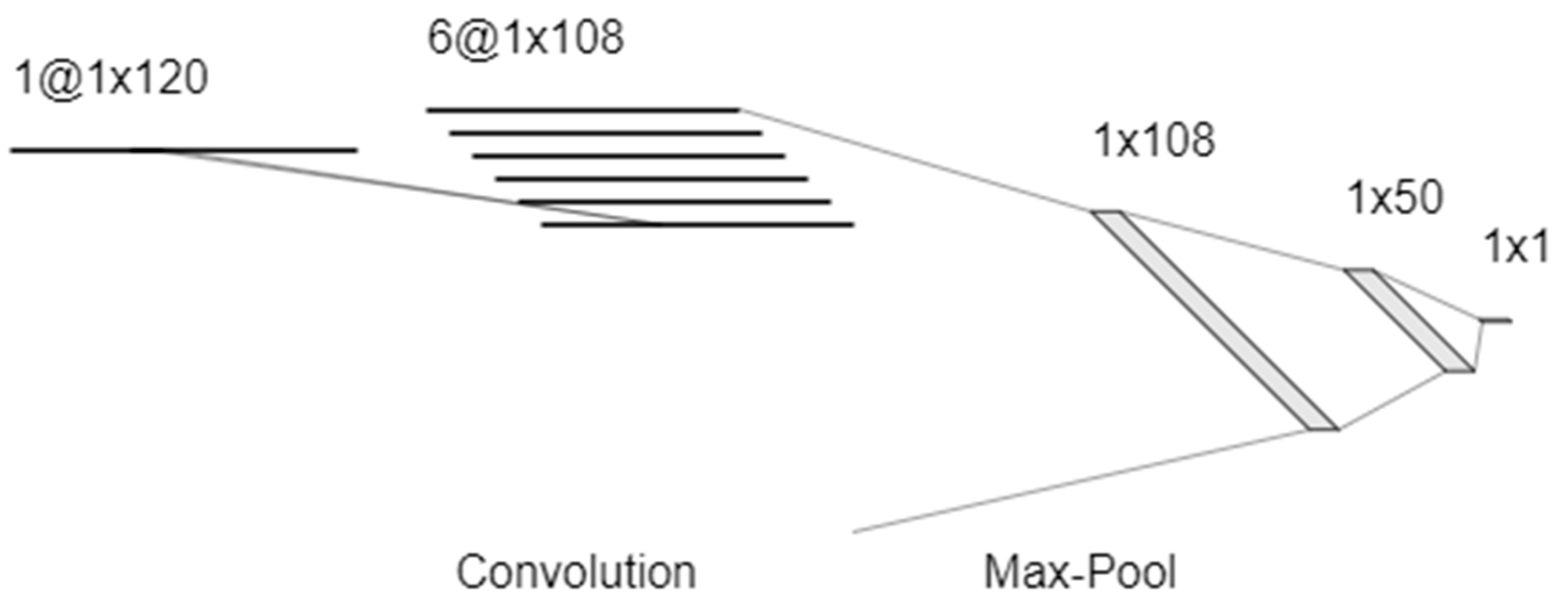
2.7. Data Processing and Analysis
3. Results and Discussion
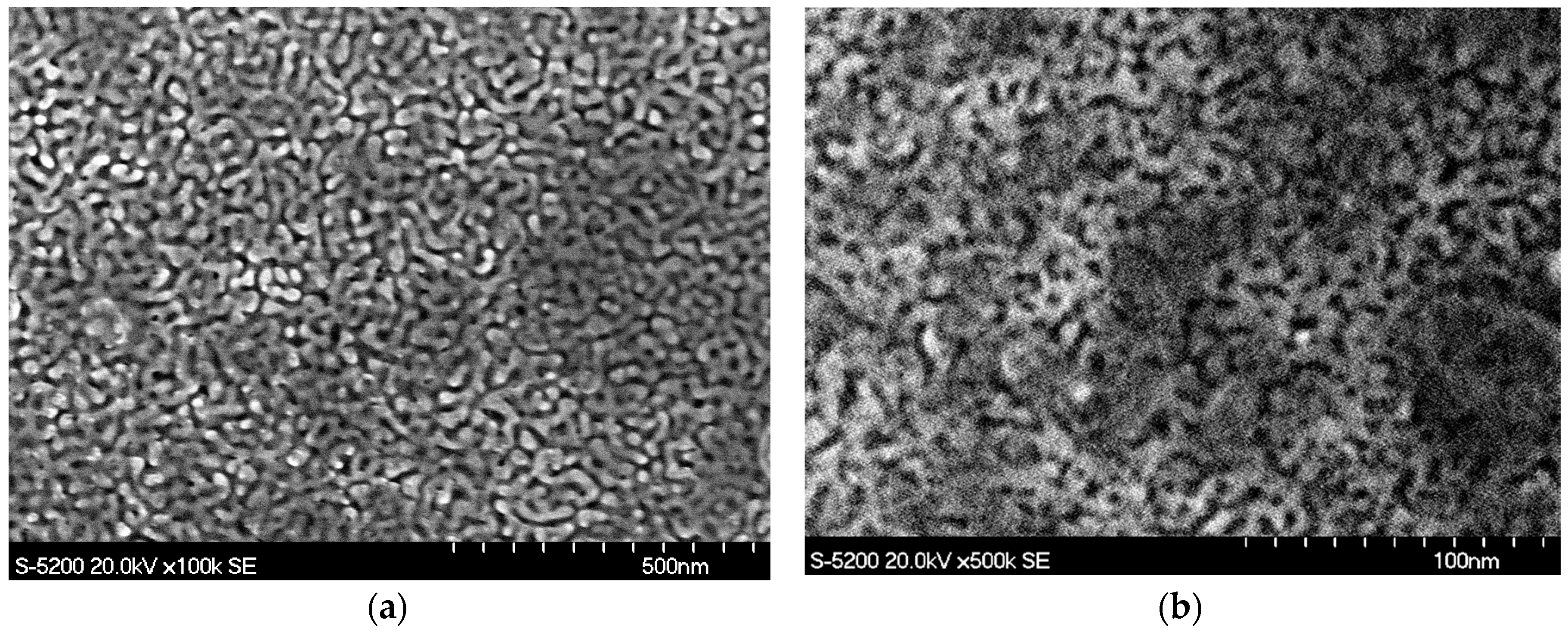
3.1. Material Characterization
3.2. Sensing Mechanism
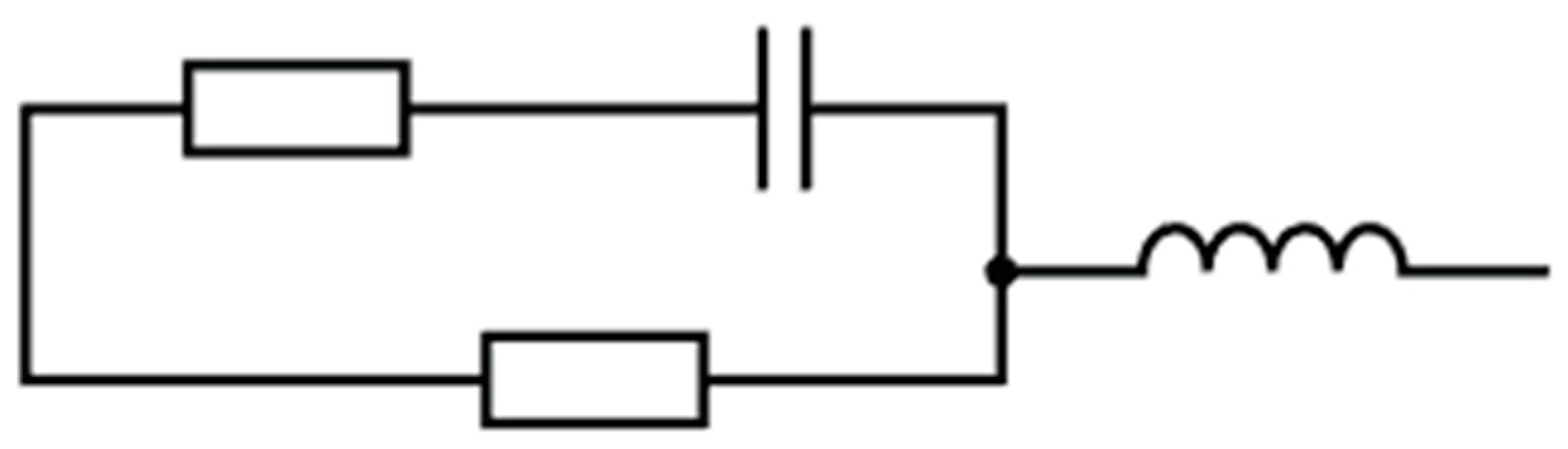
Proposed Circuit Model
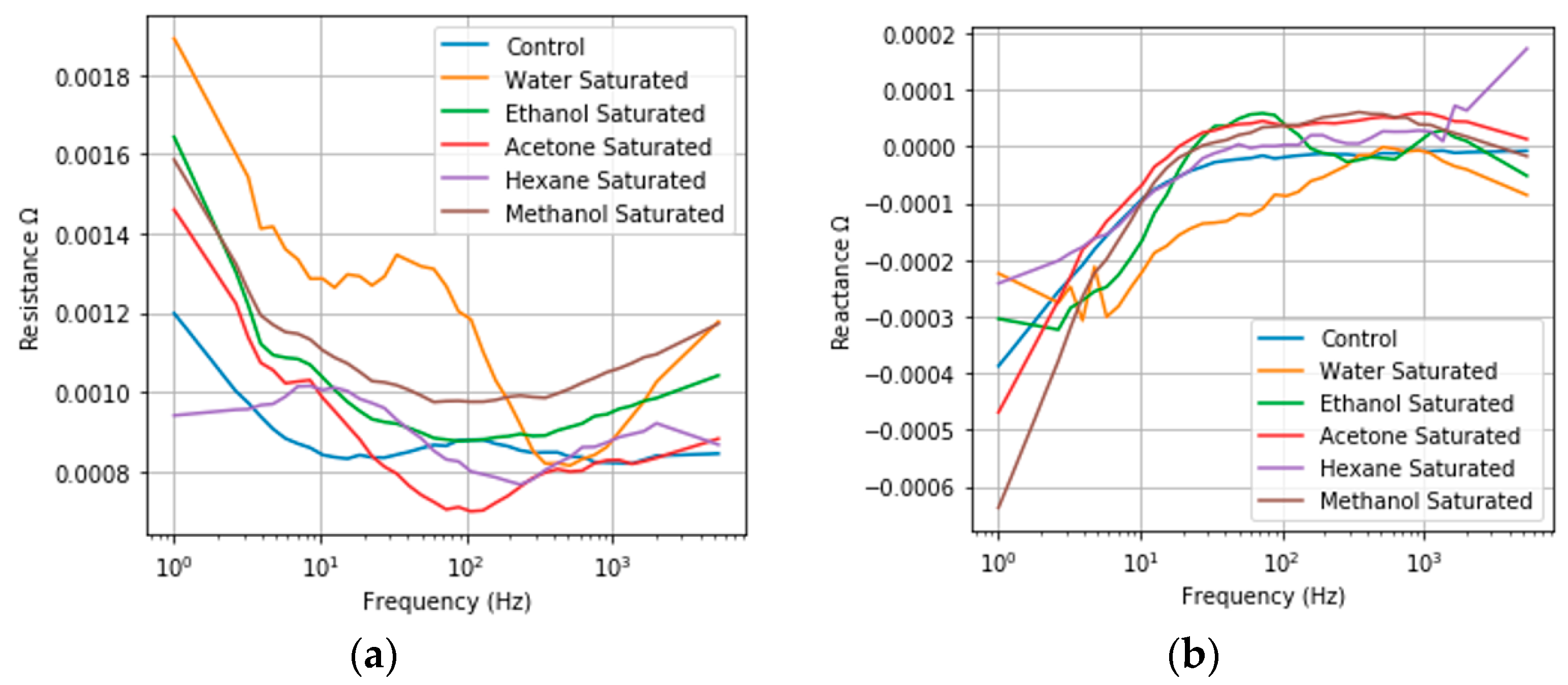
3.3. Saturated Reseponse
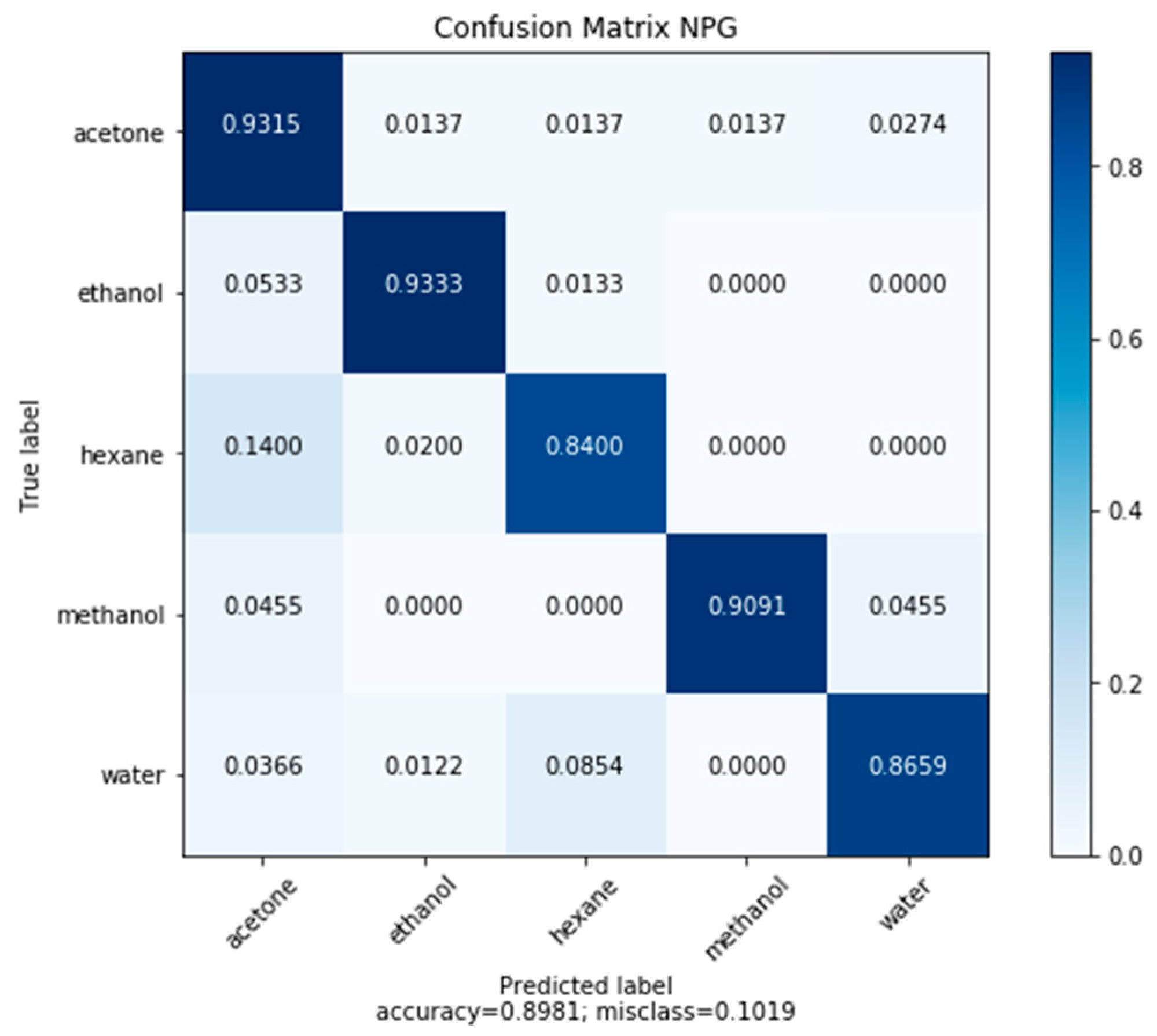
3.4. Classification of Model Compounds
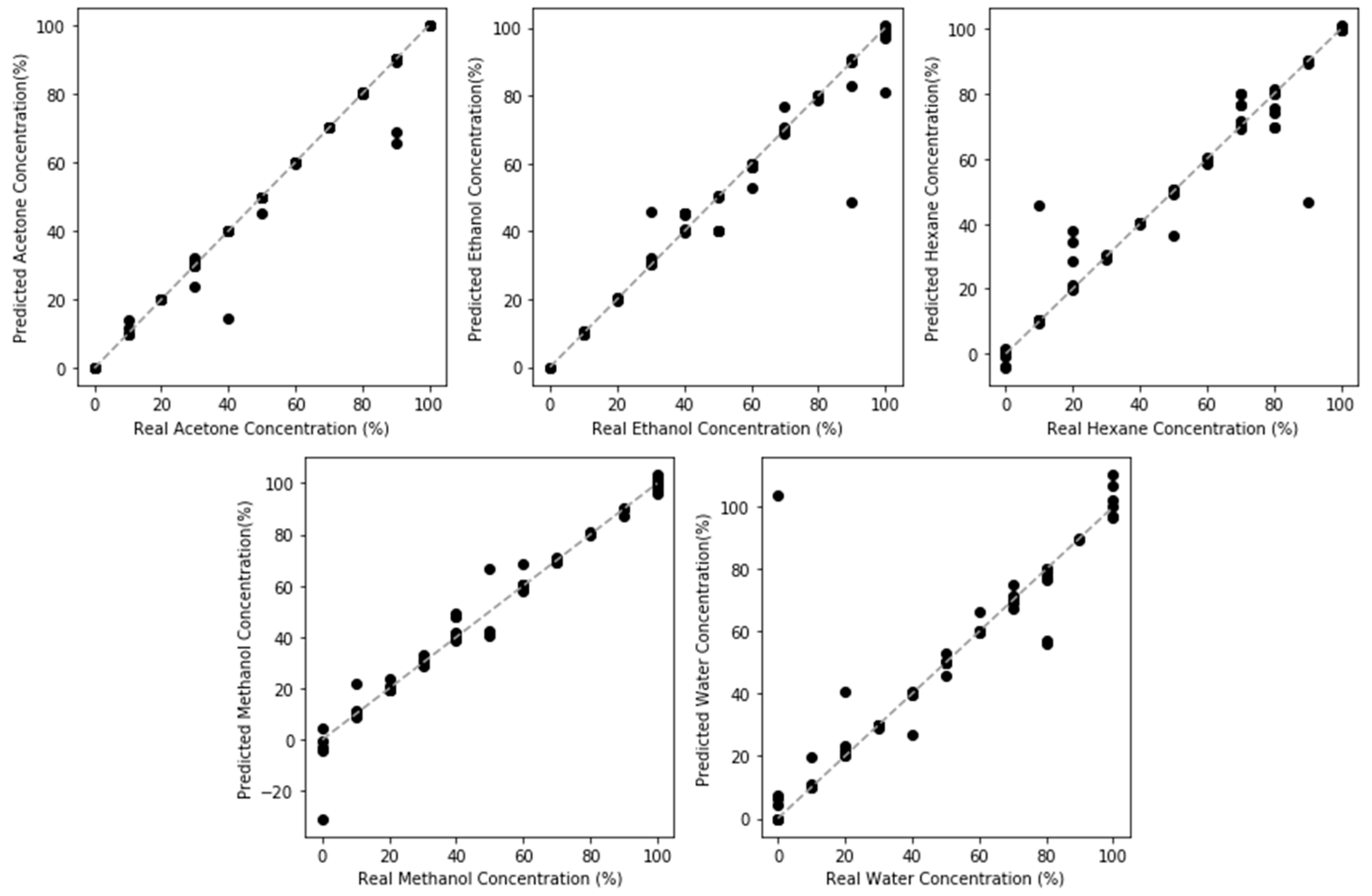
3.5. Regression of Volatile Concentration
3.6. Sensitivity Amplification by Pt Refinement
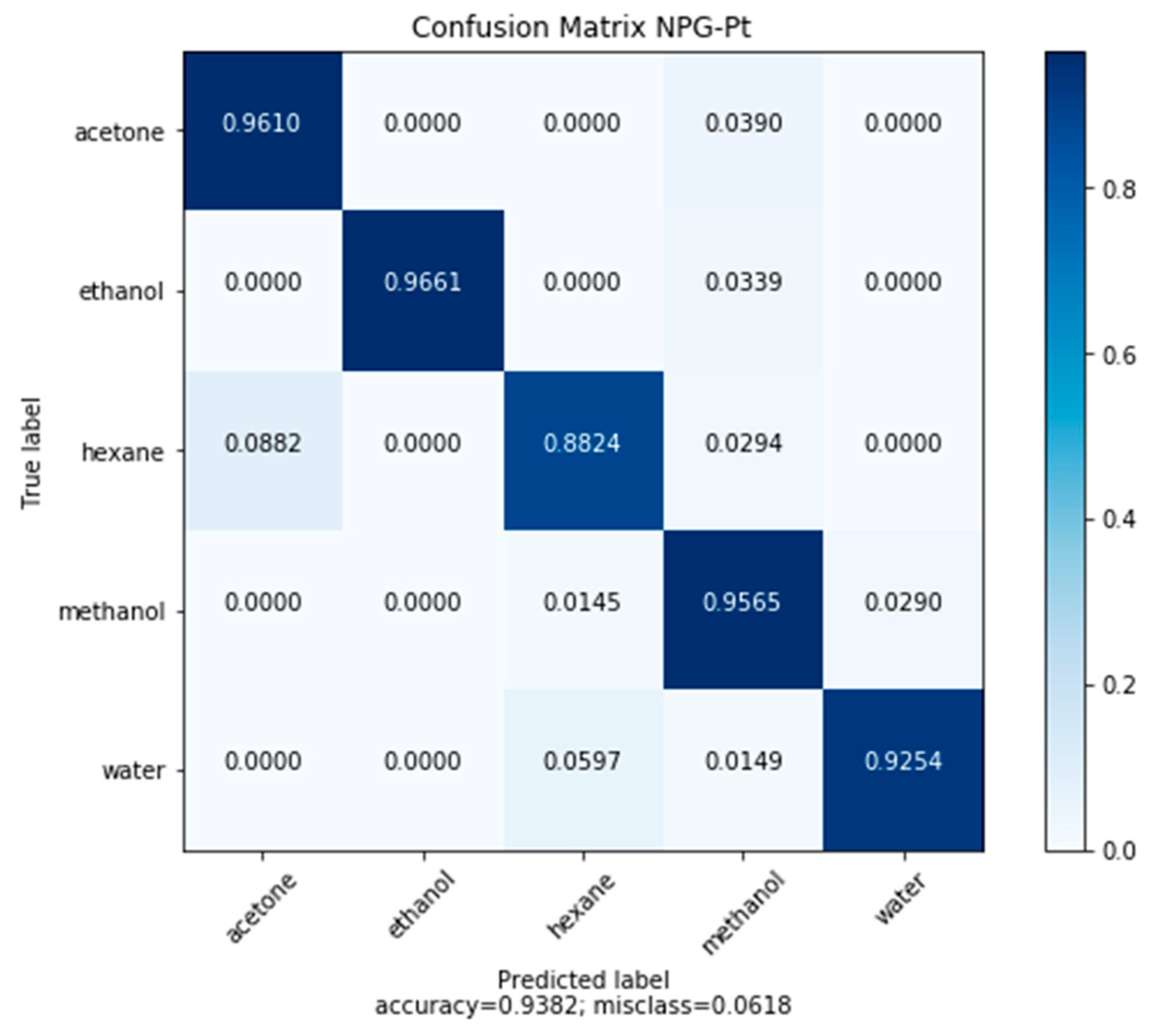
3.6.1. Classification by NPG-Pt
3.6.2. Regression Utilizing NPG-Pt Frequency Responses
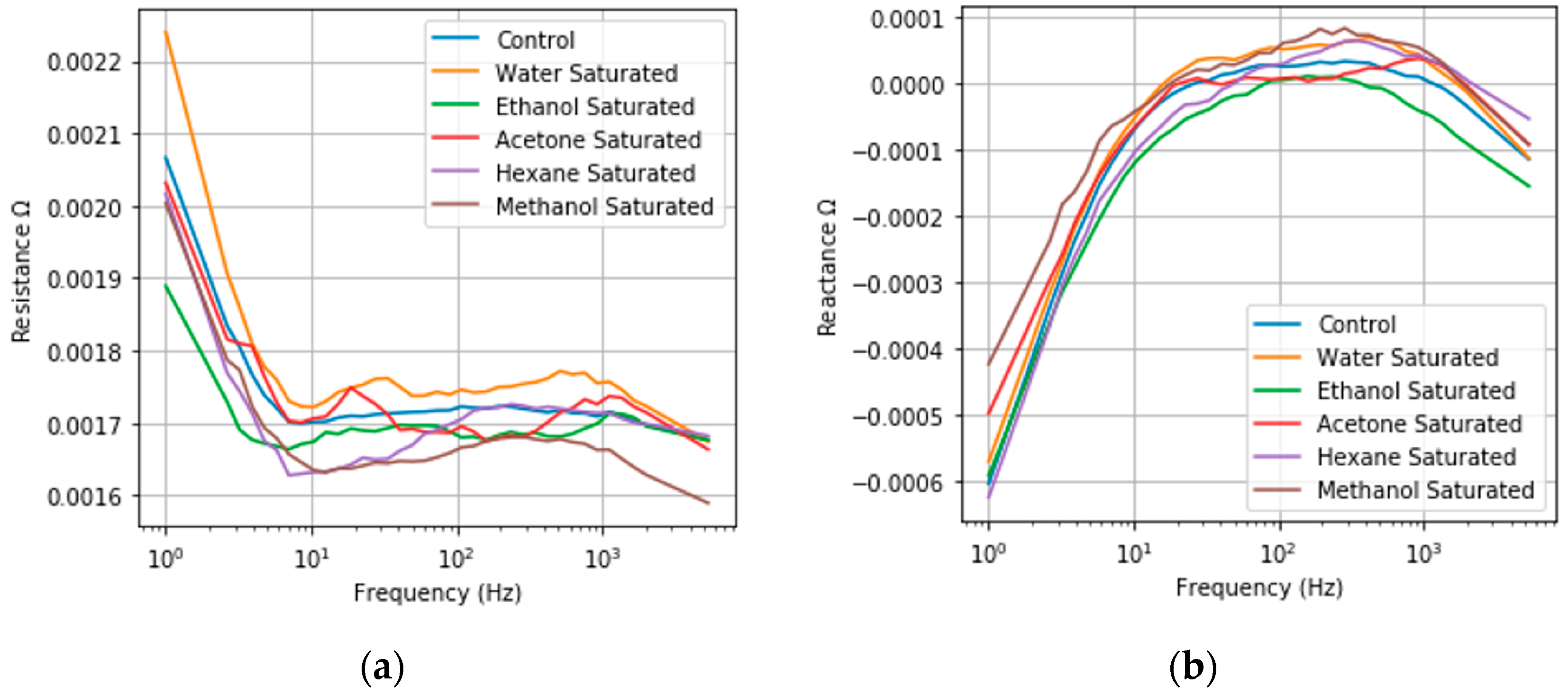
3.7. Effects of Thiol Functionalization
3.7.1. Classification of Model Compounds Utilizing tNPG-Pt Frequency Responses
3.7.2. Regression Utilizing tNPG-Pt Frequency Responses
4. Conclusions
- Convolutional neural networks were used to overcome the issues of nonlinearity and complex response behavior for the class of NPG sensors
- NPG demonstrated ability to classify and quantify five model volatile organic compounds (acetone, ethanol, hexane, methanol, water) through measurements of the frequency response of nanoporous gold, with an accuracy of 89.81%.
- CNN models produced nonbiased accurate quantification of the model compounds, except for hexane. The poorest performance occurred at low concentrations across all model compounds.
- Classification accuracy was improved by refining the pore size using NPG-Pt (93.82%). Regression performance for all model compounds improved at low concentrations.
- tNPG-Pt demonstrated an improved response for nonpolar compounds tested, with classification accuracy of hexane improving from 88.24% to 91.23%.
- Furthermore, regression of hexane concentration improved substantially.
- Improvements in sensing hexane came with a trade-off in water sensitivity and selectivity.
Author Contributions
Funding
Conflicts of Interest
References
- Jalal, A.H.; Alam, F.; Roychoudhury, S.; Umasankar, Y.; Pala, N.; Bhansali, S. Prospects and Challenges of Volatile Organic Compound Sensors in Human Healthcare. ACS Sens. 2018, 3, 1246–1263. [Google Scholar] [CrossRef]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical Application of Volatile Organic Compound Analysis for Detecting Infectious Diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, L.C.; Seabrook, B.S. Review: Indoor Concentrations of Volatile Organic Compounds: Implications for Comfort, Health and Regulation. Indoor Environ. 1995, 4, 7–26. [Google Scholar] [CrossRef]
- Hong, S.-H.; Shin, D.-C.; Lee, Y.-J.; Kim, S.-H.; Lim, Y.-W. Health risk assessment of volatile organic compounds in urban areas. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1454–1465. [Google Scholar] [CrossRef]
- Andrés, M.A.; Vijjapu, M.T.; Surya, S.G.; Shekhah, O.; Salama, K.N.; Serre, C.; Eddaoudi, M.; Roubeau, O.; Gascón, I. Methanol and Humidity Capacitive Sensors Based on Thin Films of MOF Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4155–4162. [Google Scholar] [CrossRef]
- van den Broek, J.; Abegg, S.; Pratsinis, S.E.; Güntner, A.T. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat. Commun. 2019, 10, 4220. [Google Scholar] [CrossRef]
- Muezzinoglu, M.K.; Vergara, A.; Huerta, R. A unified framework for Volatile Organic Compound classification and regression. In Proceedings of the 2010 International Joint Conference on Neural Networks (IJCNN), Budapest, Hungary, 18–23 July 2010; pp. 1–7. [Google Scholar]
- Huang, J.R.; Gu, C.P.; Meng, F.L.; Li, M.Q.; Liu, J.H. Detection of volatile organic compounds by using a single temperature-modulated SnO 2 gas sensor and artificial neural network. Smart Mater. Struct. 2007, 16, 701. [Google Scholar] [CrossRef]
- Jurs, P.C.; Bakken, G.A.; McClelland, H.E. Computational Methods for the Analysis of Chemical Sensor Array Data from Volatile Analytes. Chem. Rev. 2000, 100, 2649–2678. [Google Scholar] [CrossRef]
- Roy, N.; Mitra, S.; Das, N.M.; Mandal, N.; Bandyopadhyay, D.; Nemade, H.B.; Mandal, T.K. Paper Based Enzymatic Chemiresistor for POC Detection of Ethanol in Human Breath. IEEE Sens. J. 2020, 20, 2278–2286. [Google Scholar] [CrossRef]
- Alizadeh, N.; Jamalabadi, H.; Tavoli, F. Breath Acetone Sensors as Non-Invasive Health Monitoring Systems: A Review. IEEE Sens. J. 2020, 20, 5–31. [Google Scholar] [CrossRef]
- Quan, W.; Hu, X.; Min, X.; Qiu, J.; Tian, R.; Ji, P.; Qin, W.; Wang, H.; Pan, T.; Cheng, S.; et al. A Highly Sensitive and Selective ppb-Level Acetone Sensor Based on a Pt-Doped 3D Porous SnO2 Hierarchical Structure. Sensors 2020, 20, 1150. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, A.; Biener, J.; Bäumer, M. Nanoporous Gold: From an Ancient Technology to a High-Tech Material; RSC Publishing: Cambridge, UK, 2012; ISBN 1-84973-374-0. [Google Scholar]
- Detsi, E.; Chen, Z.G.; Vellinga, W.P.; Onck, P.R.; Hosson, J.T.M.D. Actuating and Sensing Properties of Nanoporous Gold. J. Nanosci. Nanotech. 2012, 12, 4951–4955. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H. Nanoporous gold: Preparation and applications to catalysis and sensors. Curr. Appl. Phys. 2018, 18, 810–818. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef]
- Ahangar, L.E.; Mehrgardi, M.A. Amplified detection of hepatitis B virus using an electrochemical DNA biosensor on a nanoporous gold platform. Bioelectrochemistry 2017, 117, 83–88. [Google Scholar] [CrossRef]
- Hakamada, M.; Kato, N.; Miyazawa, N.; Mabuchi, M. Water-adsorption effect on electrical resistivity of nanoporous gold. Scr. Mater. 2016, 123, 30–33. [Google Scholar] [CrossRef]
- Wong, T.S.B.; Newman, R.C. A novel application of nanoporous gold to humidity sensing: A framework for a general volatile compound sensor. Nanoscale Adv. 2020, 2, 777–784. [Google Scholar] [CrossRef]
- Vega, A.A.; Newman, R.C. Nanoporous Metals Fabricated through Electrochemical Dealloying of Ag-Au-Pt with Systematic Variation of Au:Pt Ratio. J. Electrochem. Soc. 2014, 161, C1–C10. [Google Scholar] [CrossRef]
- Hakamada, M.; Kato, N.; Mabuchi, M. Electrical resistivity of nanoporous gold modified with thiol self-assembled monolayers. Appl. Surf. Sci. 2016, 387, 1088–1092. [Google Scholar] [CrossRef]
- Vega, A.A.; Newman, R.C. Beneficial Effects of Adsorbate-Induced Surface Segregation of Pt in Nanoporous Metals Fabricated by Dealloying of Ag-Au-Pt Alloys. J. Electrochem. Soc. 2014, 161, C11–C19. [Google Scholar] [CrossRef]
- Sauer, E.; Gross, J. Prediction of Adsorption Isotherms and Selectivities: Comparison between Classical Density Functional Theory Based on the Perturbed-Chain Statistical Associating Fluid Theory Equation of State and Ideal Adsorbed Solution Theory. Langmuir 2019, 35, 11690–11701. [Google Scholar] [CrossRef] [PubMed]
- Kohonen, M.M.; Maeda, N.; Christenson, H.K. Kinetics of Capillary Condensation in a Nanoscale Pore. Phys. Rev. Lett. 1999, 82, 4667–4670. [Google Scholar] [CrossRef]
- Horikawa, T.; Do, D.D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface Sci. 2011, 169, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Chiang, C.E.; Li, C.-P.; Roshchin, I.V.; Ruminski, A.M.; Sailor, M.J.; Schuller, I.K. Gas adsorption and capillary condensation in nanoporous alumina films. Nanotechnology 2008, 19, 315709. [Google Scholar] [CrossRef] [PubMed]
- Cihan, A.; Tokunaga, T.K.; Birkholzer, J.T. Adsorption and Capillary Condensation-Induced Imbibition in Nanoporous Media. Langmuir 2019, 35, 9611–9621. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Staehle, R.W. Adsorption of Water on Gold. Corrosion 1996, 52, 843–852. [Google Scholar] [CrossRef]
- Wells, R.L.; Fort, T. Adsorption of water on clean gold by measurement of work function changes. Surf. Sci. 1972, 32, 554–560. [Google Scholar] [CrossRef]
- Wahl, P.; Traußnig, T.; Landgraf, S.; Jin, H.-J.; Weissmüller, J.; Würschum, R. Adsorption-driven tuning of the electrical resistance of nanoporous gold. J. Appl. Phys. 2010, 108, 073706. [Google Scholar] [CrossRef]
- Fawcett, W.R.; Filho, R.C.R.; Doubova, L.M. Double-layer studies in ethanolic solutions. Part 1.—Structure of the mercury/ethanol interface in the absence of specific adsorption. J. Chem. Soc. Faraday Trans. 1991, 87, 2967–2970. [Google Scholar] [CrossRef]
- Grahame, C. The Electrical Double Layer in Methanol. Zeitschrift für Elektrochemie Berichte der Bunsengesellschaft für Physikalische Chemie 1955, 4, 740–743. [Google Scholar]
- El-Zoka, A.A.; Langelier, B.; Botton, G.A.; Newman, R.C. Enhanced analysis of nanoporous gold by atom probe tomography. Mater. Charact. 2017, 128, 269–277. [Google Scholar] [CrossRef]
- Meng, S.; Wang, E.G.; Gao, S. Water adsorption on metal surfaces: A general picture from density functional theory studies. Phys. Rev. B 2004, 69, 195404. [Google Scholar] [CrossRef]
- El-Zoka, A.A.; Langelier, B.; Korinek, A.; Botton, G.A.; Newman, R.C. Advances in nanoscale characterization of refined nanoporous gold. Electrochim. Acta 2018, 283, 611–618. [Google Scholar] [CrossRef]




| Compound | R2 | Standard Deviation (P/Psat%) |
|---|---|---|
| Acetone | 0.764 | 15.6 |
| Ethanol | 0.971 | 5.2 |
| Hexane | 0.724 | 16.4 |
| Methanol | 0.884 | 10.4 |
| Water | 0.892 | 10.6 |
| Compound | R2 | Standard Deviation (P/Psat%) |
|---|---|---|
| Acetone | 0.961 | 5.4 |
| Ethanol | 0.974 | 5.1 |
| Hexane | 0.932 | 8.0 |
| Methanol | 0.902 | 9.2 |
| Water | 0.963 | 6.4 |
| Compound | R2 | Standard Deviation (P/Psat%) |
|---|---|---|
| Acetone | 0.975 | 4.6 |
| Ethanol | 0.960 | 6.1 |
| Hexane | 0.948 | 7.5 |
| Methanol | 0.972 | 5.0 |
| Water | 0.963 | 6.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, T.S.B.; Newman, R. Nanoporous Gold as a VOC Sensor, Based on Nanoscale Electrical Phenomena and Convolutional Neural Networks. Sensors 2020, 20, 2851. https://doi.org/10.3390/s20102851
Wong TSB, Newman R. Nanoporous Gold as a VOC Sensor, Based on Nanoscale Electrical Phenomena and Convolutional Neural Networks. Sensors. 2020; 20(10):2851. https://doi.org/10.3390/s20102851
Chicago/Turabian StyleWong, Timothy S.B., and Roger Newman. 2020. "Nanoporous Gold as a VOC Sensor, Based on Nanoscale Electrical Phenomena and Convolutional Neural Networks" Sensors 20, no. 10: 2851. https://doi.org/10.3390/s20102851
APA StyleWong, T. S. B., & Newman, R. (2020). Nanoporous Gold as a VOC Sensor, Based on Nanoscale Electrical Phenomena and Convolutional Neural Networks. Sensors, 20(10), 2851. https://doi.org/10.3390/s20102851




