Recent Progress in Flexible Wearable Sensors for Vital Sign Monitoring
Abstract
:1. Introduction
2. Bioelectric Signal Monitoring
2.1. Non-Implantable Flexible Electrophysiological Signal Sensor
2.1.1. Contact Sensors
2.1.2. Non-Contact Sensors
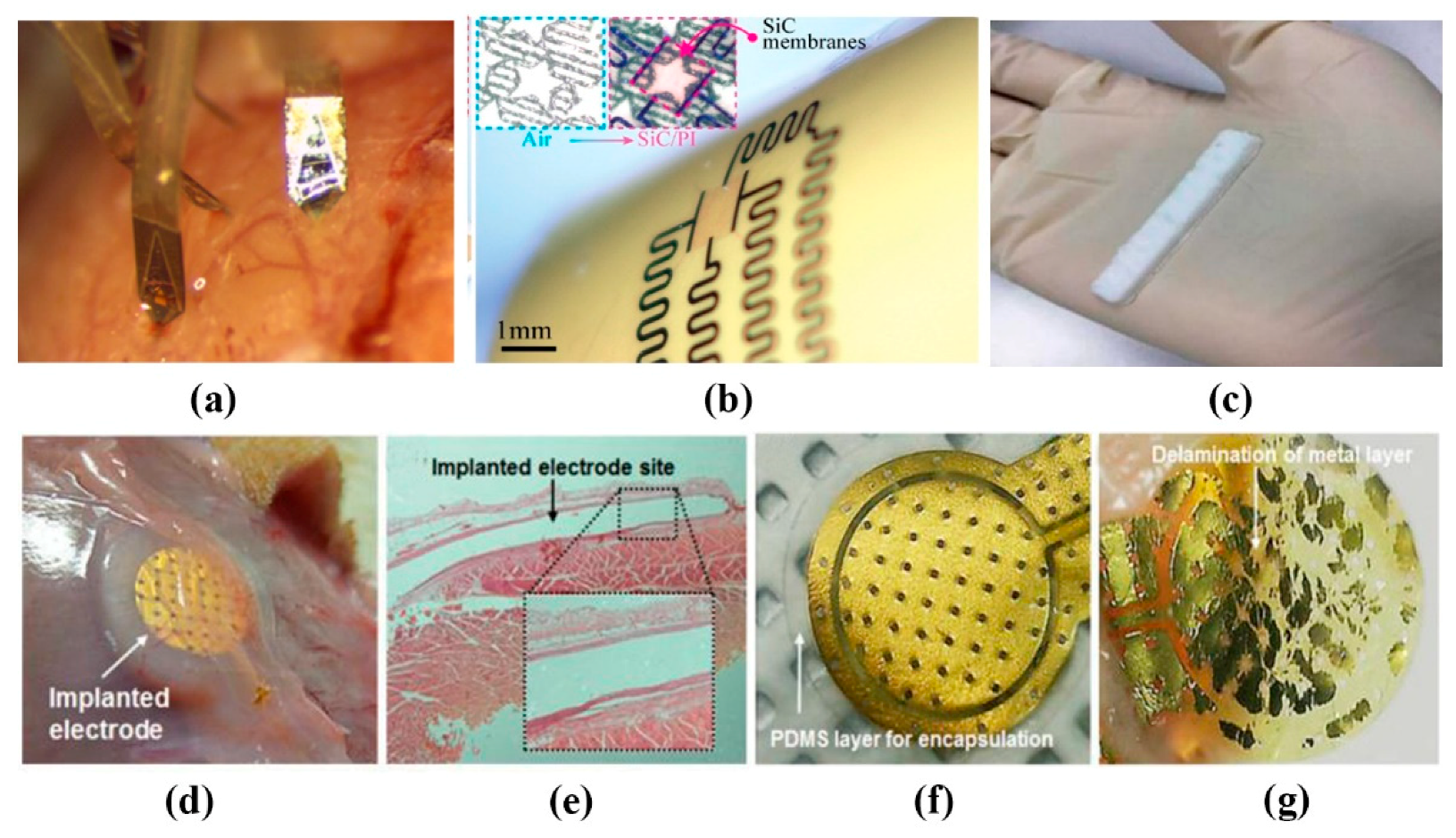
2.2. Implantable Flexible Electrophysiological Signal Sensor
2.3. Wearable Electrophysiological Monitoring System
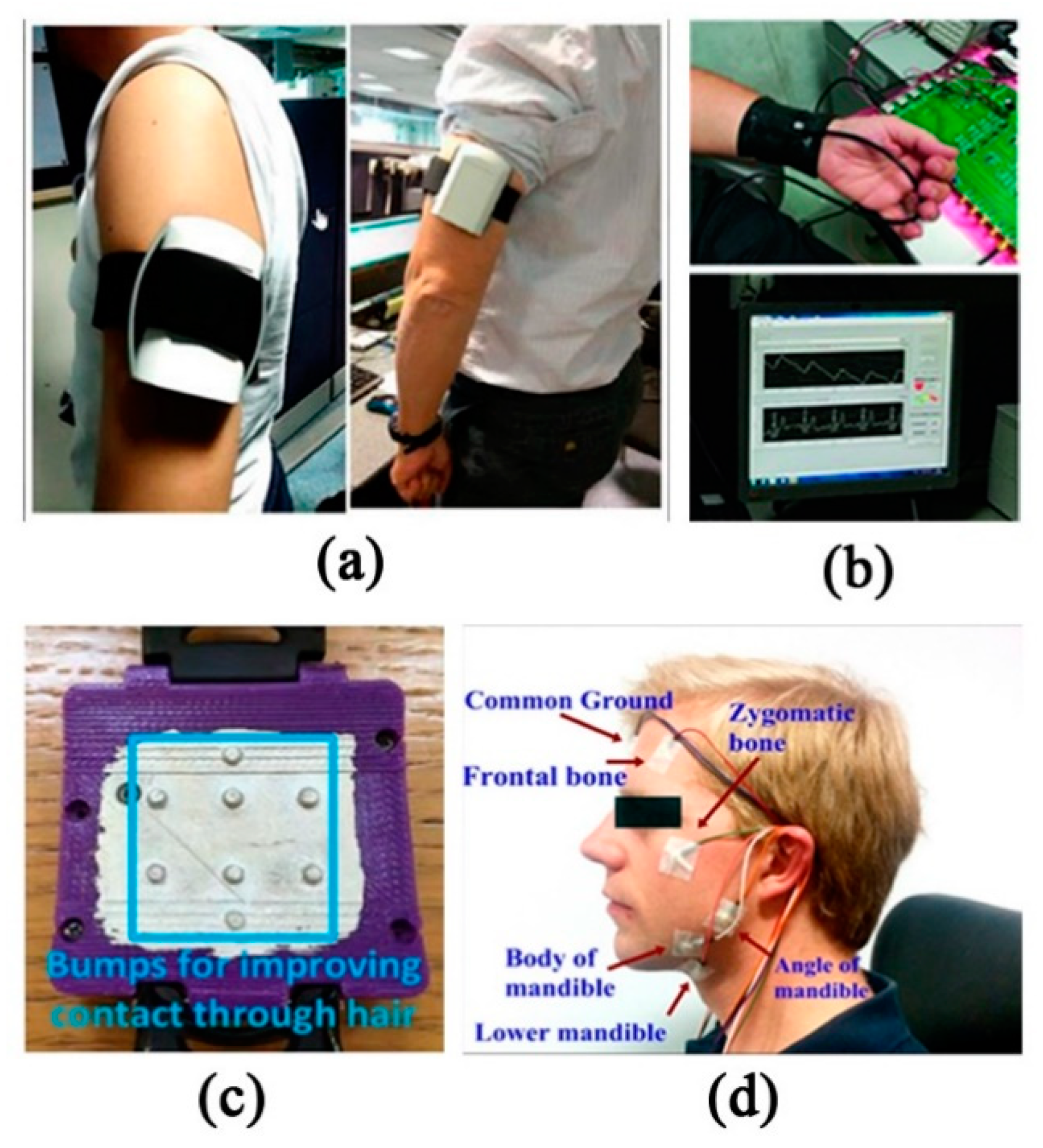
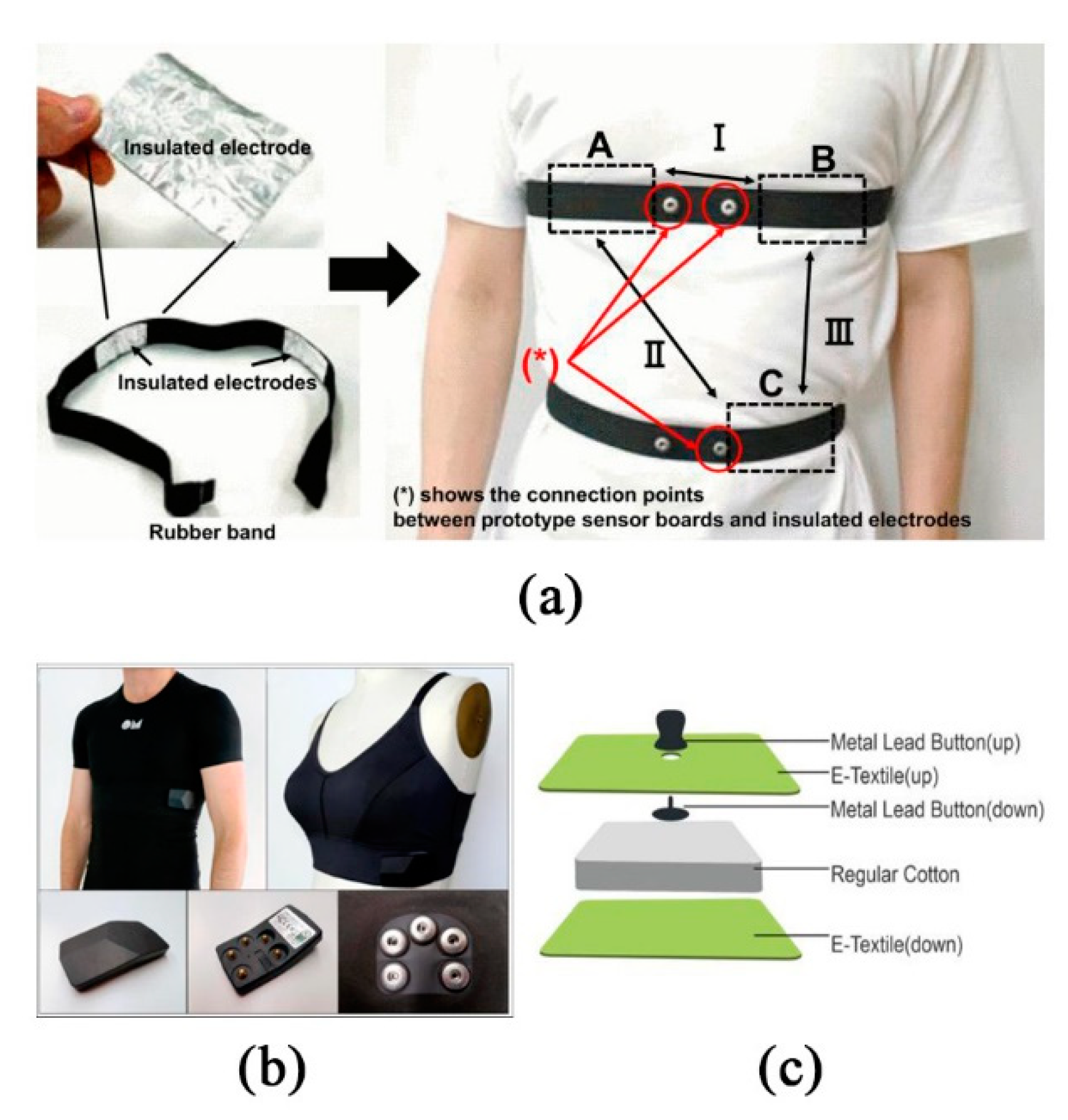
2.3.1. Contact Wearable Monitoring System
2.3.2. Non-Contact Wearable Monitoring System
2.3.3. Chemical–Electrophysiological Hybrid Biosensing System
2.4. Challenge and Improvement
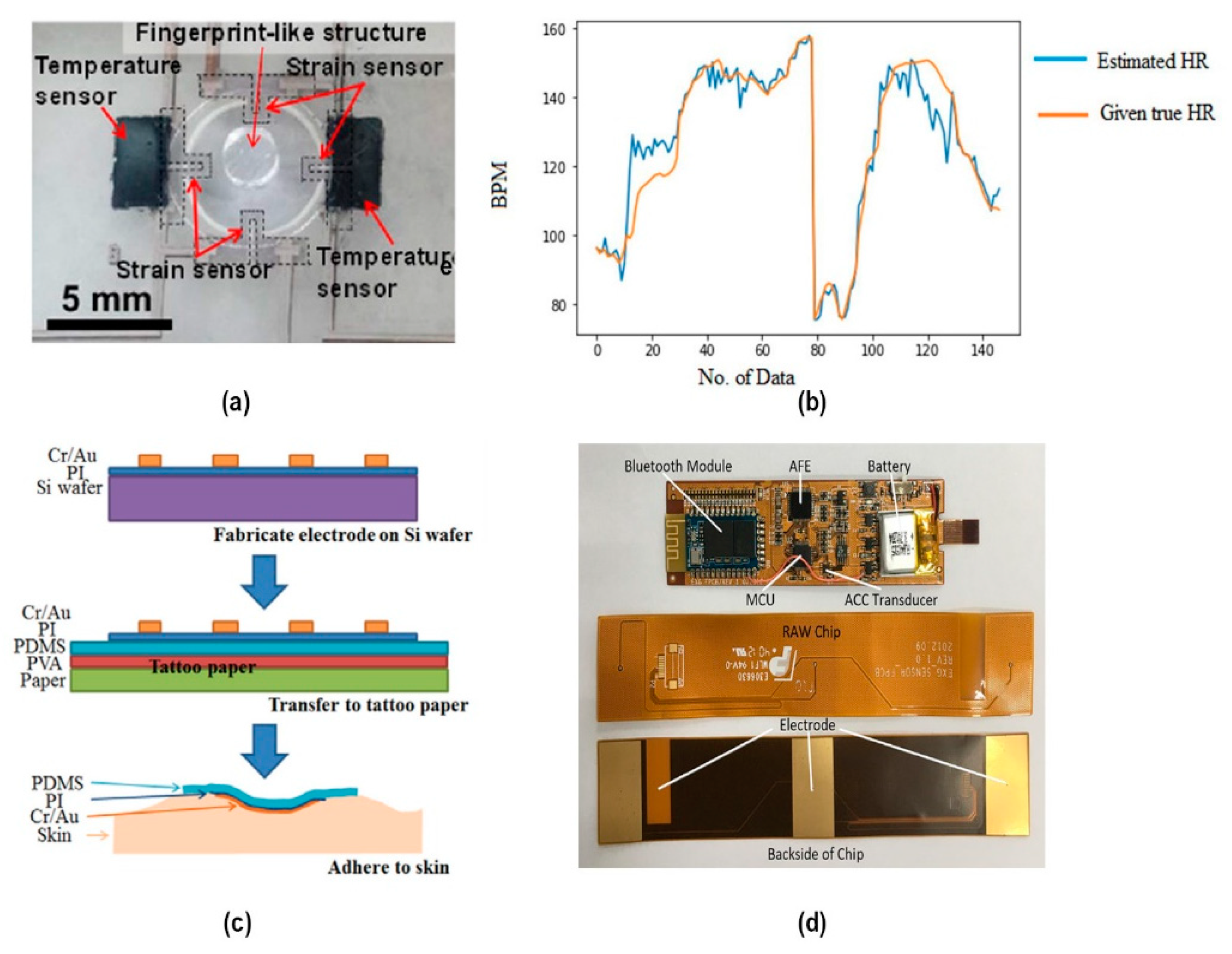
3. Respiratory Rate Monitoring
4. Temperature Monitoring
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shirer, M. Available online: https://www.idc.com/getdoc.jsp?containerId=prUS46122120 (accessed on 11 May 2020).
- Hua, Q.; Sun, J.; Liu, H.; Bao, R.; Yu, R.; Zhai, J.; Pan, C.; Wang, Z.L. Skin-inspired highly stretchable and conformable matrix networks for multifunctional sensing. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz Shahandashti, P.; Pourkheyrollah, H.; Jahanshahi, A.; Ghafoorifard, H. Highly conformable stretchable dry electrodes based on inexpensive flex substrate for long-term biopotential (EMG/ECG) monitoring. Sens. Actuators Phys. 2019, 295, 678–686. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.B. Capacitive biopotential measurement for electrophysiological signal acquisition: A review. IEEE Sens. J. 2016, 16, 2832–2853. [Google Scholar] [CrossRef]
- Zhang, L. Design, Fabrication and Performance Study of the Flexible Supercapacitor Electrode. Ph.D. Thesis, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2020. [Google Scholar]
- Myers, A.C.; Huang, H.; Zhu, Y. Wearable silver nanowire dry electrodes for electrophysiological sensing. RSC Adv. 2015, 5, 11627–11632. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Luo, M.; Xu, X.; Dai, G.; Yang, J. Highly stretchable polymer/silver nanowires composite sensor for human health monitoring. Nano Res. 2020, 13, 919–926. [Google Scholar] [CrossRef]
- Lam, C.L.; Saleh, S.M.; Yudin, M.B.M.; Harun, F.K.; Sriprachuabwong, C.; Tuantranont, A.; Wicaksono, D.H. Graphene Ink-Coated Cotton Fabric-Based Flexible Electrode for Electrocardiography. In Proceedings of the 2017 5th International Conference on Instrumentation, Communications, Information Technology, and Biomedical Engineering (ICICI-BME), Bandung, Indonesia, 6–7 November 2017; pp. 73–75. [Google Scholar]
- Zahed, M.A.; Das, P.S.; Maharjan, P.; Barman, S.C.; Sharifuzzaman, M.; Yoon, S.H.; Park, J.Y. Flexible and robust dry electrodes based on electroconductive polymer spray-coated 3D porous graphene for long-term electrocardiogram signal monitoring system. Carbon 2020, 165, 26–36. [Google Scholar] [CrossRef]
- Villegas, A.; McEneaney, D.; Escalona, O. Arm-ECG Wireless Sensor System for Wearable Long-Term Surveillance of Heart Arrhythmias. Electronics 2019, 8, 1300. [Google Scholar] [CrossRef] [Green Version]
- Beach, C.; Krachunov, S.; Pope, J.; Fafoutis, X.; Piechocki, R.J.; Craddock, I.; Casson, A.J. An Ultra Low Power Personalizable Wrist Worn ECG Monitor Integrated With IoT Infrastructure. IEEE Access 2018, 6, 44010–44021. [Google Scholar] [CrossRef]
- Kosmyna, N.; Morris, C.; Sarawgi, U.; Nguyen, T.; Maes, P. AttentivU: A Wearable Pair of EEG and EOG Glasses for Real-Time Physiological Processing. In Proceedings of the 2019 IEEE 16th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Chicago, IL, USA, 19–22 May 2019; pp. 1–4. [Google Scholar]
- Li, X.; Sun, Y. NCMB-Button: A Wearable Non-contact System for Long-Term Multiple Biopotential Monitoring. In Proceedings of the 2017 IEEE/ACM International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), Philadelphia, PA, USA, 17–19 July 2017; pp. 348–355. [Google Scholar]
- Liu, S.; Liu, X.; Jiang, Y.; Wang, X.; Huang, P.; Wang, H.; Zhu, M.; Tan, J.; Li, P.; Lin, C.; et al. Flexible Non-contact Electrodes for Bioelectrical Signal Monitoring. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4305–4308. [Google Scholar]
- Wang, X.; Liu, S.; Zhu, M.; Liu, Z.; Jiang, Y.; Wang, D.; Li, P.; Samuel, O.W.; Wu, W.; Chen, S.; et al. Performance of Flexible Non-contact Electrodes in Bioelectrical Signal Measurements. In Proceedings of the 2019 IEEE International Conference on Real-time Computing and Robotics (RCAR), Irkutsk, Russia, 4–9 August 2019; pp. 175–179. [Google Scholar]
- Gao, Y.; Soman, V.V.; Lombardi, J.P.; Rajbhandari, P.P.; Dhakal, T.P.; Wilson, D.; Poliks, M.; Ghose, K.; Turner, J.N.; Jin, Z. Heart Monitor Using Flexible Capacitive ECG Electrodes. IEEE Trans. Instrum. Meas. 2019. [Google Scholar] [CrossRef]
- Christian, S.; François, P.; Marina, S.; Pascal, F.-P.; Gilles, O.H.; Franck, M.; Jean-François, S.; Isabelle, N.; Louis, B.; Karine, R.; et al. A Novel Wearable Device for Continuous Ambulatory ECG Recording: Proof of Concept and Assessment of Signal Quality. Biosensors 2019, 9, 17. [Google Scholar]
- Wang, L.-H.; Zhang, W.; Guan, M.-H.; Jiang, S.-Y.; Fan, M.-H.; Abu, P.A.R.; Chen, C.-A.; Chen, S.-L. A Low-Power High-Data-Transmission Multi-Lead ECG Acquisition Sensor System. Sensors 2019, 19, 4996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Bao, S.; Lu, C.; Wang, L.; Ma, J.; Wang, P.; Lu, H.; Shu, F.; Oetomo, S.B.; Chen, W. Design of an Integrated Wearable Multi-Sensor Platform Based on Flexible Materials for Neonatal Monitoring. IEEE Access 2020, 8, 23732–23747. [Google Scholar] [CrossRef]
- Yapici, M.K.; Alkhidir, T.E. Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors. Sensors 2017, 17, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Othman, A.; Alatoom, A.; Farooq, A.; Al-Sayah, M.; Al-Nashash, H. Novel flexible implantable electrodes based on conductive polymers and Titanium dioxide. In Proceedings of the 2018 IEEE 4th Middle East Conference on Biomedical Engineering (MECBME), Gammarth, Tunis, 28–30 March 2018; pp. 30–33. [Google Scholar]
- Lee, S.M.; Byeon, H.J.; Kim, B.H.; Lee, J.; Jeong, J.Y.; Lee, J.H.; Moon, J.-H.; Park, C.; Choi, H.; Lee, S.-H.; et al. Flexible and implantable capacitive microelectrode for bio-potential acquisition. Biochip. J. 2017, 11, 153–163. [Google Scholar] [CrossRef]
- Lee, J.-H. Miniaturized Human Insertable Cardiac Monitoring System with Wireless Power Transmission Technique. J. Sens. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Jiuwei, G.; Qianbo, L.; Lu, Z.; Xuewen, W.; Wei, H. Flexible Sensing Technology for Bioelectricity. Mater. Rep. 2020, 34, 1095–1106. [Google Scholar]
- Smith, W.M.; Riddell, F.; Madon, M.; Gleva, M.J. Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours. Am. Heart J. 2017, 185, 67–73. [Google Scholar] [CrossRef]
- Yang, H.; Ye, H.; Zhou, J.; Zhang, H.; Chen, D. The causes and suppression methods of motion artifacts of wearable textile electrocardio-electrodes. Basic Sci. J. Text. Univ. 2019, 32, 126–132. [Google Scholar]
- Wang, Z.Z.; Yi, P.Y.; Peng, L.F.; Lai, X.M.; Ni, J. Continuous Fabrication of Highly Conductive and Transparent Ag Mesh Electrodes for Flexible Electronics. IEEE Trans. Nanotechnol. 2017, 16, 687–694. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Nguyen, V.H. Promising applications of graphene and graphene-based nanostructures. Adv. Nat. Sci.-Nanosci. Nanotechnol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Kalita, G.; Wakita, K.; Umeno, M.; Hayashi, Y.; Tanemura, M. Synthesis of Continuous Graphene on Metal Foil for Flexible Transparent Electrode Application. In Proceedings of the 2013 IEEE 5th International Nanoelectronics Conference, New York, NY, USA, 2–4 January 2013; pp. 281–284. [Google Scholar]
- Kondo, M.; Fujita, T.; Kanda, K.; Maenaka, K. Nanocarbon Electrode for Wearable Device with Flexible Material. In Proceedings of the 2019 International Conference on Machine Learning and Cybernetics (ICMLC), Kobe, Japan, 7–10 July 2019; pp. 1–6. [Google Scholar]
- Jung, H.; Kwon, D.; Lee, S.; Ahn, J.; Kim, A.; Kim, Y.; Moon, J. Carbon Based Electrode for Wearable Biosignal Monitoring Patch. In Proceedings of the 2018 International Flexible Electronics Technology Conference (IFETC), Ottawa, ON, Canada, 7–9 August 2018; pp. 1–2. [Google Scholar]
- Zhang, Y.; Liang, B.; Jiang, Q.F.; Li, Y.; Feng, Y.; Zhang, L.Q.; Zhao, Y.M.; Xiong, X.L. Flexible and wearable sensor based on graphene nanocomposite hydrogels. Smart Mater. Struct. 2020, 29. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-F.; Liu, J.-Q.; Yang, B.; Yang, C.-S. PDMS-based low cost flexible dry electrode for long-term EEG measurement. IEEE Sens. J. 2012, 12, 2898–2904. [Google Scholar] [CrossRef]
- Jung, H.-C.; Moon, J.-H.; Baek, D.-H.; Lee, J.-H.; Choi, Y.-Y.; Hong, J.-S.; Lee, S.-H. CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Li, Z.B.; Chen, X.; Chen, J.P. A flexible dry micro-dome electrode for ECG monitoring. Microsyst. Technol. 2015, 21, 1241–1248. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, X.; Wang, W.; Li, Z. A microneedle electrode array on flexible substrate for long-term EEG monitoring. Sens. Actuators B Chem. 2017, 244, 750–758. [Google Scholar] [CrossRef]
- Bai, J.X.; Liu, Y.P.; Liu, X.; Cui, R.; Cao, H.L.; Shan, Y.H.; Yu, F.; Yuan, Y.H.; Wang, R.X. Design of Electromyography Wireless Acquisition System Based on Flexible Dry Electrodes. Micronanoelectron. Technol. 2019, 56, 556–563. [Google Scholar]
- Yang, B. Study on Capacitive Coupling Human Vital Signs Measurement. Ph.D. Thesis, Tsinghua University, Beijing, China, 2017. [Google Scholar]
- Li, X.; Sun, Y. Design and evaluation of a non-contact wireless biopotential monitoring system with motion artifacts. In Proceedings of the 2017 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Orland, FL, USA, 16–19 February 2017; pp. 69–72. [Google Scholar]
- Fuketa, H.; Yoshioka, K.; Shinozuka, Y.; Ishida, K.; Yokota, T.; Matsuhisa, N.; Inoue, Y.; Sekino, M.; Sekitani, T.; Takamiya, M.; et al. 1 μm-Thickness Ultra-Flexible and High Electrode-Density Surface Electromyogram Measurement Sheet with 2 V Organic Transistors for Prosthetic Hand Control. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 824–833. [Google Scholar] [CrossRef]
- Arzbaecher, R.; Hampton, D.R.; Burke, M.C.; Garrett, M.C. Subcutaneous electrocardiogram monitors and their field of view. J. Electrocardiol. 2010, 43, 601–605. [Google Scholar] [CrossRef] [Green Version]
- Charvet, G.; Foerster, M.; Chatalic, G.; Michea, A.; Porcherot, J.; Bonnet, S.; Filipe, S.; Audebert, P.; Robinet, S.; Josselin, V. A wireless 64-channel ECoG recording electronic for implantable monitoring and BCI applications: WIMAGINE. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 783–786. [Google Scholar]
- Dong, S.; Chen, W.; Wang, X.; Zhang, S.; Xu, K.; Zheng, X. Flexible ECoG electrode for implantation and neural signal recording applications. Vacuum 2017, 140, 96–100. [Google Scholar] [CrossRef]
- Lu, Y.; Lyu, H.; Richardson, A.G.; Lucas, T.H.; Kuzum, D. Flexible neural electrode array based-on porous graphene for cortical microstimulation and sensing. Sci. Rep. 2016, 6, 33526. [Google Scholar] [CrossRef]
- Wu, P.; Xiao, A.; Zhao, Y.A.; Chen, F.X.; Ke, M.F.; Zhang, Q.; Zhang, J.W.; Shi, X.W.; He, X.H.; Chen, Y. An implantable and versatile piezoresistive sensor for the monitoring of human-machine interface interactions and the dynamical process of nerve repair. Nanoscale 2019, 11, 21103–21118. [Google Scholar] [CrossRef] [PubMed]
- Chamanzar, M.; Borysov, M.; Maharbiz, M.M.; Blanche, T.J. High-density optrodes for multi-scale electrophysiology and optogenetic stimulation. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 6838–6841. [Google Scholar]
- Shin, J.; Liu, Z.; Bai, W.; Liu, Y.; Yan, Y.; Xue, Y.; Kandela, I.; Pezhouh, M.; MacEwan, M.R.; Huang, Y.; et al. Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Sci. Adv. 2019, 5, eaaw1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, H.P.; Zhong, Y.S.; Nguyen, T.K.; Park, Y.; Dinh, T.; Song, E.M.; Vadivelu, R.K.; Masud, M.K.; Li, J.H.; Shiddiky, M.J.A.; et al. Long-Lived, Transferred Crystalline Silicon Carbide Nanomembranes for Implantable Flexible Electronics. ACS Nano 2019, 13, 11572–11581. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.T.; Rod, P. Current and Emerging Uses of Insertable Cardiac Monitors: Evaluation of Syncope and Monitoring for Atrial Fibrillation. Cardiol. Rev. 2017, 25, 22–29. [Google Scholar] [CrossRef]
- Williams, I.; Rapeaux, A.; Pearson, J.; Nazarpour, K.; Brunton, E.; Luan, S.; Liu, Y.; Constandinou, T.G. SenseBack–Implant considerations for an implantable neural stimulation and recording device. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; pp. 1–4. [Google Scholar]
- Laiwalla, F.; Lee, J.; Lee, A.; Mok, E.; Leung, V.; Shellhammer, S.; Song, Y.; Larson, L.; Nurmikko, A. A Distributed Wireless Network of Implantable Sub-mm Cortical Microstimulators for Brain-Computer Interfaces. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6876–6879. [Google Scholar]
- Kim, A.; Ochoa, M.; Rahimi, R.; Ziaie, B. New and Emerging Energy Sources for Implantable Wireless Microdevices. IEEE Access 2015, 3, 89–98. [Google Scholar] [CrossRef]
- del Agua, I.; Mantione, D.; Ismailov, U.; Sanchez-Sanchez, A.; Aramburu, N.; Malliaras, G.G.; Mecerreyes, D.; Ismailova, E. DVS-Crosslinked PEDOT:PSS Free-Standing and Textile Electrodes toward Wearable Health Monitoring. Adv. Mater. Technol. 2018, 3. [Google Scholar] [CrossRef]
- Vizcaya, P.R.; Perpiñan, G.I.; McEneaney, D.J.; Escalona, O.J. Standard ECG lead I prospective estimation study from far-field bipolar leads on the left upper arm: A neural network approach. Biomed. Signal. Process. Control. 2019, 51, 171–180. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Ambra, E.; Maddiona, L.; Romeo, M.; Mazzillo, M.; Rundo, F.; Fallica, G.; di Pompeo, F.; Chiarelli, A.M.; Zappasodi, F.; et al. PPG/ECG Multisite Combo System Based on SiPM Technology. In Convegno Nazionale Sensori; Springer: Cham, Switzerland, 2018; pp. 353–360. [Google Scholar]
- Akter Shathi, M.; Minzhi, C.; Khoso, N.A.; Deb, H.; Ahmed, A.; Sai Sai, W. All organic graphene oxide and Poly (3, 4-ethylene dioxythiophene)-Poly (styrene sulfonate) coated knitted textile fabrics for wearable electrocardiography (ECG) monitoring. Synth. Met. 2020, 263. [Google Scholar] [CrossRef]
- Celik, N.; Balachandran, W.; Manivannan, N.; Winter, E.; Schnalzer, B.; Burgsteiner, H. Wearable mobile ear-based ECG monitoring device using graphene-coated sensors. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Wilhelm, V.R.; Theerasak, C.; Valentin, G.; David, L.; David, S.; Mandic, D.P. Smart Helmet: Wearable Multichannel ECG and EEG. IEEE J. Transl. Eng. Health Med. 2016, 4, 2700111. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.; Khan, M.U.G.; Iqbal, R.; Riaz, O. Robust Multi-sensor Fusion for the Development of EEG Controlled Vehicle. IEEE Sens. J. 2020. [Google Scholar] [CrossRef]
- Szafir, D.; Mutlu, B. Pay attention! Designing adaptive agents that monitor and improve user engagement. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Austin, TX, USA, 5–10 May 2012; 2012; pp. 11–20. [Google Scholar] [CrossRef]
- Gao, K.; Shen, G.; Zhao, N.; Jiang, C.; Yang, B.; Liu, J. Wearable multifunction sensor for the detection of forehead EEG signal and sweat rate on skin simultaneously. IEEE Sens. J. 2020. [Google Scholar] [CrossRef]
- Tang, T.; Yan, L.; Park, J.H.; Wu, H.; Zhang, L.; Lee, H.Y.B.; Yoo, J. 34.6 EEG Dust: A BCC-Based Wireless Concurrent Recording/Transmitting Concentric Electrode. In Proceedings of the 2020 IEEE International Solid- State Circuits Conference (ISSCC), San Francisco, CA, USA, 16–20 February 2020; pp. 516–518. [Google Scholar]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, N.; Ding, J.; Zhao, N.; Leung, B.H.K.; Poon, C.C.Y. Mobile Health: Design of Flexible and Stretchable Electrophysiological Sensors for Wearable Healthcare Systems. In Proceedings of the 2014 11th International Conference on Wearable and Implantable Body Sensor Networks, Zurich, Switzerland, 16–19 June 2014; pp. 87–91. [Google Scholar]
- Lee, H.; Lee, S.; Lee, W.; Yokota, T.; Fukuda, K.; Someya, T. Ultrathin Organic Electrochemical Transistor with Nonvolatile and Thin Gel Electrolyte for Long-Term Electrophysiological Monitoring. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Koo, J.H.; Jeong, S.; Shim, H.J.; Son, D.; Kim, J.; Kim, D.C.; Choi, S.; Hong, J.I.; Kim, D.H. Wearable Electrocardiogram Monitor Using Carbon Nanotube Electronics and Color-Tunable Organic Light-Emitting Diodes. ACS Nano 2017, 11, 10032–10041. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Yokota, T.; Lee, S.; Fukuda, K. Electronic Skins for Robotics and Wearables. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 66–71. [Google Scholar]
- Choi, M.; Kim, S.W. Driver’s movement monitoring system using capacitive ECG sensors. In Proceedings of the 2017 IEEE 6th Global Conference on Consumer Electronics (GCCE), Nagoya, Japan, 24–27 October 2017; pp. 1–2. [Google Scholar]
- Seo, M.; Choi, M.; Lee, J.S.; Kim, S.W. Adaptive Noise Reduction Algorithm to Improve R Peak Detection in ECG Measured by Capacitive ECG Sensors. Sensors 2018, 18, 2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasato, Y.; Izumi, S.; Kawaguchi, H.; Yoshimoto, M. Capacitively coupled ECG sensor system with digitally assisted noise cancellation for wearable application. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Turin, Italy, 19–21 October 2017; pp. 1–4. [Google Scholar]
- Spanò, E.; Pascoli, S.D.; Iannaccone, G. Low-Power Wearable ECG Monitoring System for Multiple-Patient Remote Monitoring. IEEE Sens. J. 2016, 16, 5452–5462. [Google Scholar] [CrossRef]
- Movesense Sports Bra. Available online: https://www.movesense.com/product/movesense-sports-bra/ (accessed on 10 May 2020).
- Soh, P.J.; Vandenbosch, G.A.E.; Mercuri, M.; Schreurs, D.M.M. Wearable Wireless Health Monitoring: Current Developments, Challenges, and Future Trends. IEEE Microw. Mag. 2015, 16, 55–70. [Google Scholar] [CrossRef]
- Vavrinsky, E.; Subjak, J.; Donoval, M.; Wagner, A.; Zavodnik, T.; Svobodova, H. Application of Modern Multi-Sensor Holter in Diagnosis and Treatment. Sensors 2020, 20, 2663. [Google Scholar] [CrossRef]
- Chung, H.U.; Kim, B.H.; Lee, J.Y.; Lee, J.; Xie, Z.; Ibler, E.M.; Lee, K.; Banks, A.; Jeong, J.Y.; Kim, J.; et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 2019, 363. [Google Scholar] [CrossRef] [Green Version]
- Tiankui, L. Perinatal Fetus Real-Time Monitoring and Pregnant Women Health Care Wearable Equipment Design Research. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2017. [Google Scholar]
- Li, S.; Zhang, W.; Bu, S.; Huang, M. Development of wearable ECG and PCG acquisition device. Pop. Sci. Technol. 2015, 17, 15–17, 28. [Google Scholar]
- Zhu, Y.F.; Wang, W.J.; Ma, Y.L.; Lv, Y.; Ma, L.K. Application and Management of Wearable Devices for Pregnant Women. Med. J. Peking Union Med. Coll. Hosp. 2018, 9, 25–30. [Google Scholar]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.F.; Wang, J.; Mercier, P.P. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Gao, W.; Wang, C.; Javey, A. Physical and Chemical Sensing With Electronic Skin. IEEE 2019, 107, 2155–2167. [Google Scholar] [CrossRef]
- Xiong, F.; Chen, D.Y.; Chen, Z.H.; Dai, S.M. Cancellation of motion artifacts in ambulatory ECG signals using TD-LMS adaptive filtering techniques. J. Vis. Commun. Image Represent. 2019, 58, 606–618. [Google Scholar] [CrossRef]
- Bashar, S.S.; Miah, M.S.; Karim, A.Z.; Al Mahmud, M.A.; Hasan, Z. A Machine Learning Approach for Heart Rate Estimation from PPG Signal using Random Forest Regression Algorithm. In Proceedings of the 2019 International Conference on Electrical, Computer and Communication Engineering (ECCE), Cox’s Bazar, Bangladesh, 7–9 February 2019. [Google Scholar] [CrossRef]
- Zou, C.; Qin, Y.J.; Sun, C.L.; Li, W.; Chen, W. Motion artifact removal based on periodical property for ECG monitoring with wearable systems. Pervasive Mob. Comput. 2017, 40, 267–278. [Google Scholar] [CrossRef]
- Liu, G.Z.; Huang, B.Y.; Wang, L. A wearable respiratory biofeedback system based on generalized body sensor network. Telemed. J. E-Health Off. J. Am. Telemed. Assoc. 2011, 17, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.-Z.; Wu, D.; Mei, Z.-Y.; Zhu, Q.-S.; Wang, L. Automatic detection of respiratory rate from electrocardiogram, respiration induced plethysmography and 3D acceleration signals. J. Cent. South. Univ. 2013, 20, 2423–2431. [Google Scholar] [CrossRef]
- Chen, H.; Bao, S.; Ma, J.; Wang, P.; Lu, H.; Oetomo, S.B.; Chen, W. A Wearable Daily Respiration Monitoring System Using PDMS-graphene Compound Tensile Sensor for Adult. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1269–1273. [Google Scholar]
- Hung, P.D.; Bonnet, S.; Guillemaud, R.; Castelli, E.; Yen, P.T.N. Estimation of respiratory waveform using an accelerometer. In Proceedings of the 2008 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Paris, France, 14–17 May 2008; pp. 1493–1496. [Google Scholar]
- Bates, A.; Ling, M.J.; Mann, J.; Arvind, D.K. Respiratory Rate and Flow Waveform Estimation from Tri-axial Accelerometer Data. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 144–150. [Google Scholar]
- Vertens, J.; Fischer, F.; Heyde, C.; Höflinger, F.; Zhang, R.; Reindl, L.; Gollhofer, A. Measuring Respiration and Heart Rate Using Two Acceleration Sensors on a Fully Embedded Platform. In Proceedings of the 3rd International Congress on Sport Sciences Research and Technology Support (icSPORTS 2015), Lisbon, Portugal, 15–17 November 2015. [Google Scholar] [CrossRef] [Green Version]
- Mack, D.C.; Patrie, J.T.; Suratt, P.M.; Felder, R.A.; Alwan, M. Development and Preliminary Validation of Heart Rate and Breathing Rate Detection Using a Passive, Ballistocardiography-Based Sleep Monitoring System. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 111–120. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Wang, B.; Wu, H.; Liu, H.; Zhang, Y. A Prototype of Wearable Respiration Biofeedback Platform and Its Preliminary Evaluation on Cardiovascular Variability. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–4. [Google Scholar]
- Fan, W.; He, Q.; Meng, K.; Tan, X.; Zhou, Z.; Zhang, G.; Yang, J.; Wang, Z.L. Machine-knitted washable sensor array textile for precise epidermal physiological signal monitoring. Sci. Adv. 2020, 6, eaay2840. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lu, B.; Feng, X. Biocompatible and Ultra-Flexible Inorganic Strain Sensors Attached to Skin for Long-Term Vital Signs Monitoring. IEEE Electron. Device Lett. 2016, 37, 496–499. [Google Scholar] [CrossRef]
- Yamamoto, A.; Nakamoto, H.; Bessho, Y.; Watanabe, Y.; Oki, Y.; Ono, K.; Fujimoto, Y.; Terada, T.; Ishikawa, A. Monitoring respiratory rates with a wearable system using a stretchable strain sensor during moderate exercise. Med. Biol. Eng. Comput. 2019, 57, 2741–2756. [Google Scholar] [CrossRef] [PubMed]
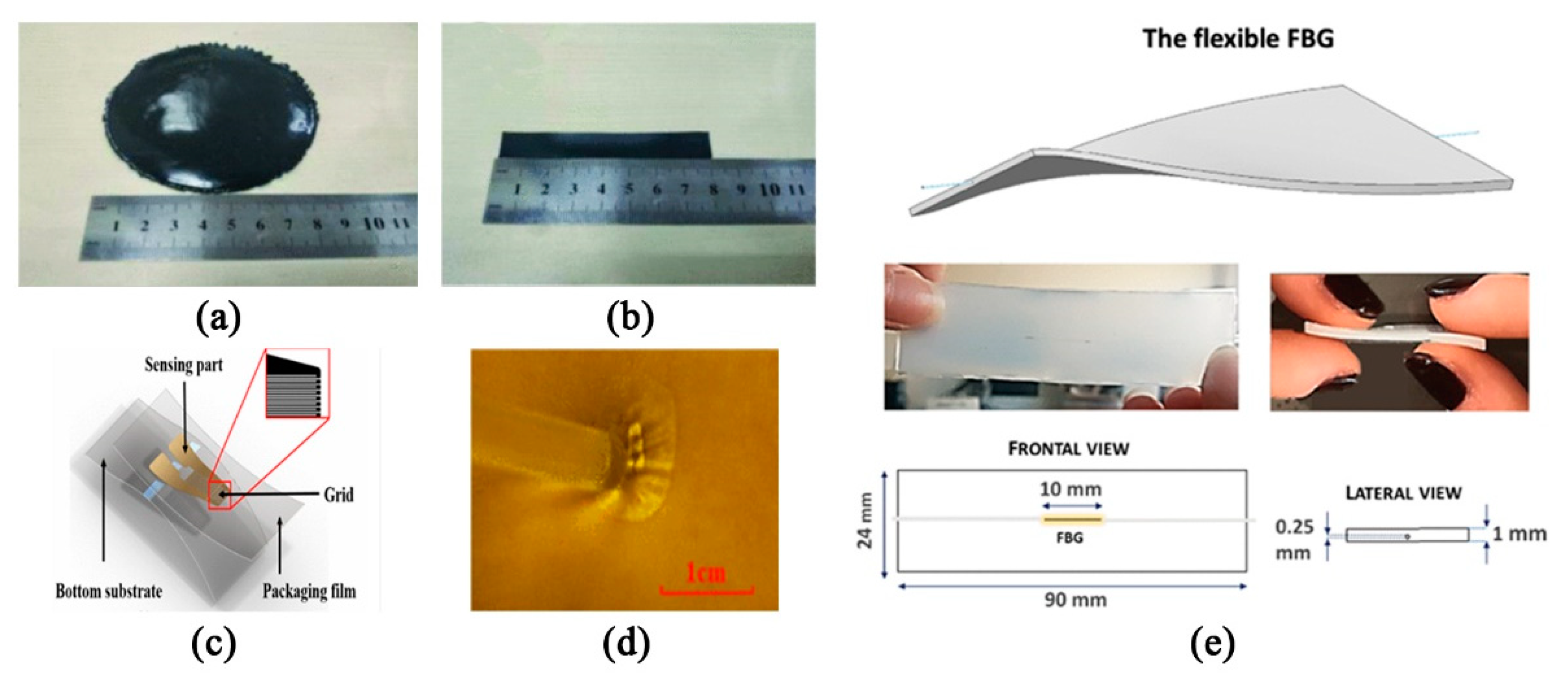
- Kawamura, M.; Ishizawa, H.; Sato, S.; Koyama, S. Application to vital signs by Fiber Bragg Grating sensing. In Proceedings of the SICE Annual Conference 2011, Tokyo, Japan, 13–18 September 2011; pp. 2702–2704. [Google Scholar]
- Dziuda, Ł.; Krej, M.; Skibniewski, F.W. Fiber Bragg Grating Strain Sensor Incorporated to Monitor Patient Vital Signs During MRI. IEEE Sens. J. 2013, 13, 4986–4991. [Google Scholar] [CrossRef]
- Fajkus, M.; Nedoma, J.; Martinek, R.; Brablik, J.; Vanus, J.; Novak, M.; Zabka, S.; Vasinek, V.; Hanzlikova, P.; Vojtisek, L. MR Fully Compatible and Safe FBG Breathing Sensor: A Practical Solution for Respiratory Triggering. IEEE Access 2019, 7, 123013–123025. [Google Scholar] [CrossRef]
- Presti, D.L.; Massaroni, C.; D’Abbraccio, J.; Massari, L.; Caponero, M.; Longo, U.G.; Formica, D.; Oddo, C.M.; Schena, E. Wearable System Based on Flexible FBG for Respiratory and Cardiac Monitoring. IEEE Sens. J. 2019, 19, 7391–7398. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, T.; Chen, H.; Wang, F.; Pu, Y.; Gao, C.; Li, S. Mini Review on Flexible and Wearable Electronics for Monitoring Human Health Information. Nanoscale Res. Lett. 2019, 14, 263. [Google Scholar] [CrossRef] [Green Version]
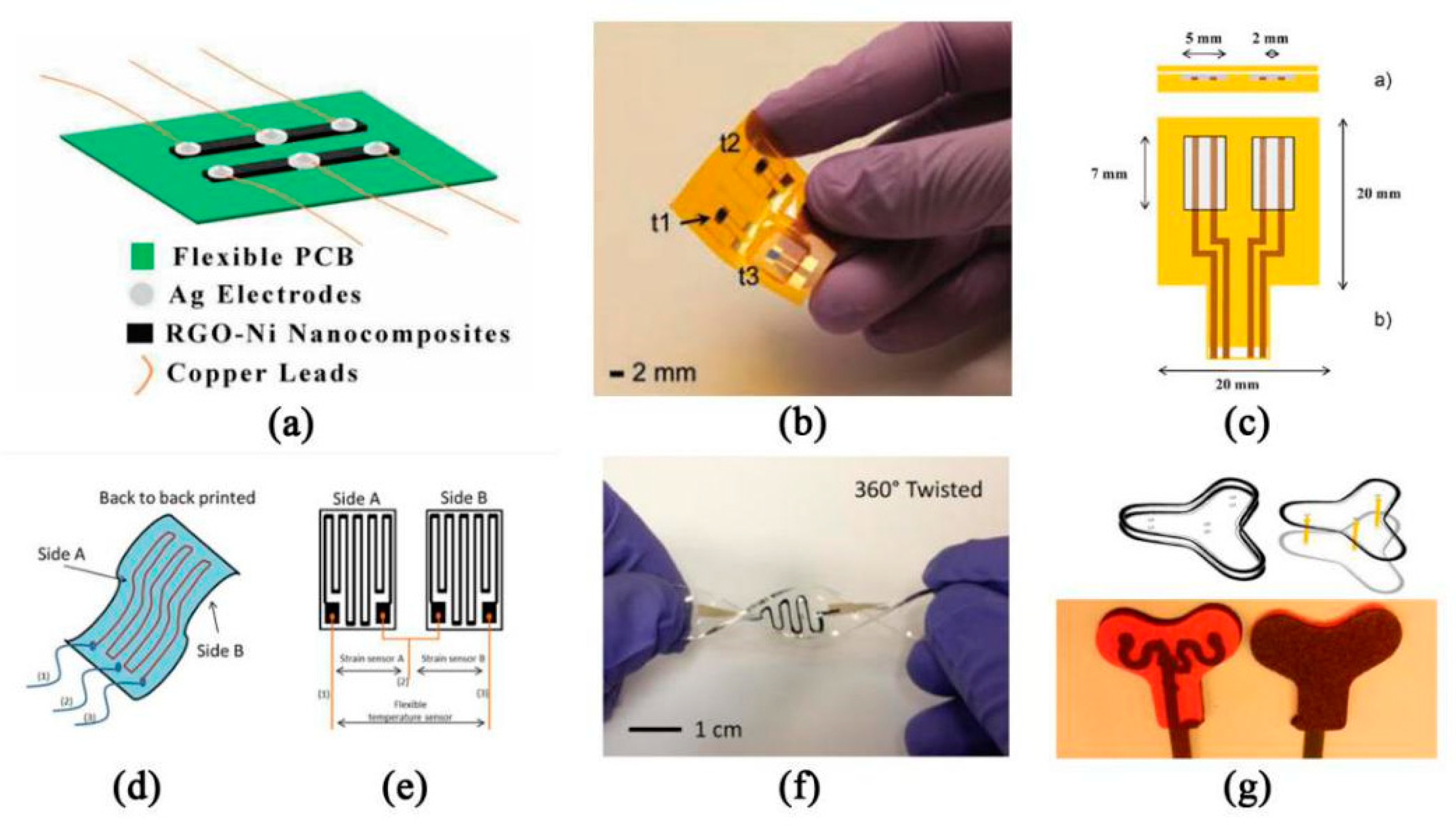
- Kedambaimoole, V.; Neella, N.; Gaddam, V.; Rajanna, K.; Nayak, M.M. Graphene-Nickel composite films on flexible PCB for temperature monitoring. In Proceedings of the 2017 IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Los Angeles, CA, USA, 9–12 April 2017; pp. 173–176. [Google Scholar]
- Khan, Y.; Garg, M.; Gui, Q.; Schadt, M.; Gaikwad, A.; Han, D.; Yamamoto, N.A.D.; Hart, P.; Welte, R.; Wilson, W. Flexible Hybrid Electronics: Direct Interfacing of Soft and Hard Electronics for Wearable Health Monitoring. Adv. Funct. Mater. 2016, 26, 8764–8775. [Google Scholar] [CrossRef]
- Giuliani, A.; Placidi, M.; Francesco, F.D.; Pucci, A. A new polystyrene-based ionomer/MWCNT nanocomposite for wearable skin temperature sensors. React. Funct. Polym. 2014, 76, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Jiang, J.; Sun, F.; Sun, L.; Wang, T.; Liu, Y.; Li, M. Flexible wearable graphene/alginate composite non-woven fabric temperature sensor with high sensitivity and anti-interference. Cellulose 2020, 27, 2369–2380. [Google Scholar] [CrossRef]
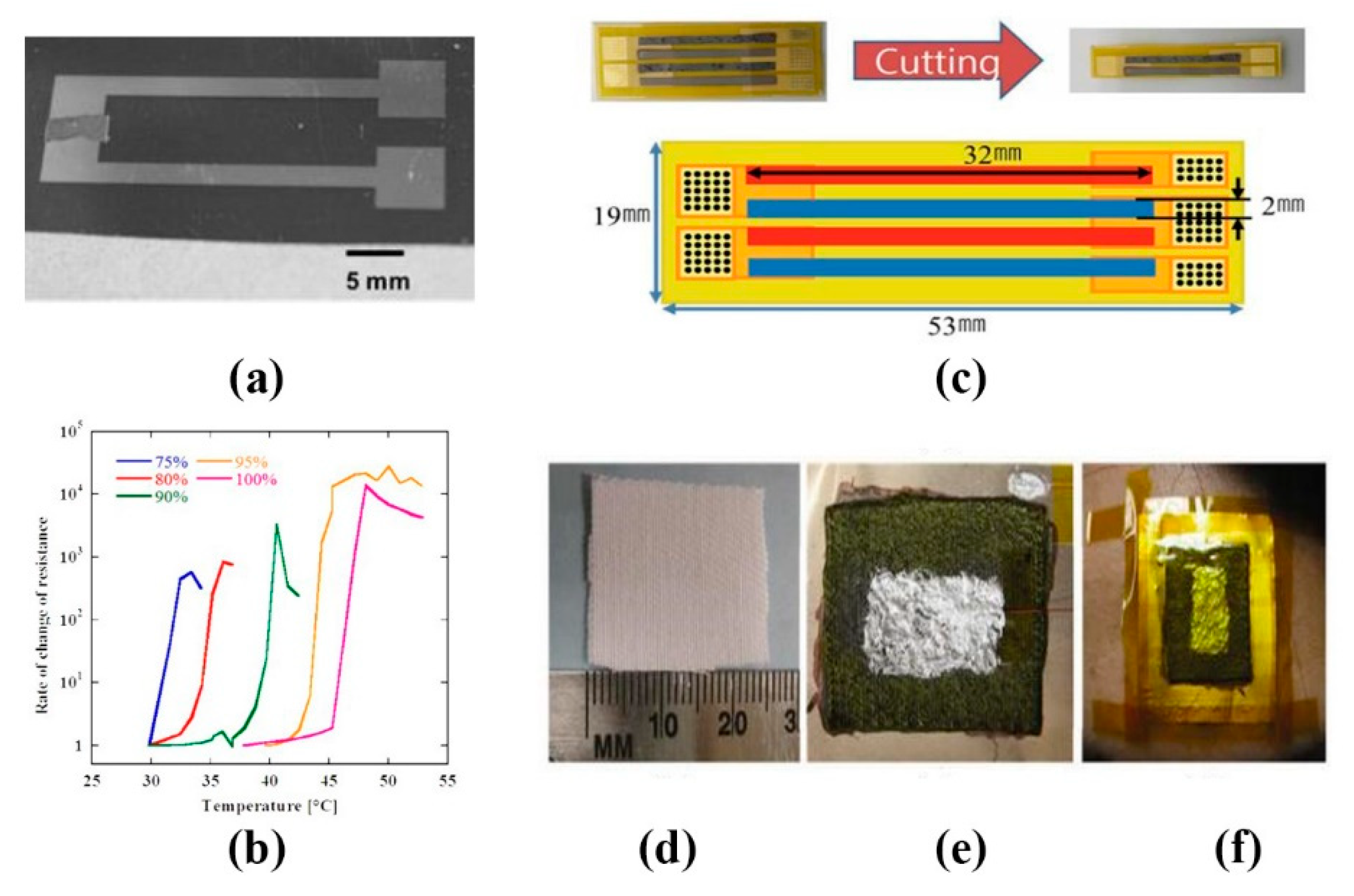
- Ali, S.; Bae, J.; Bermak, A. A flexible differential temperature sensor for wearable electronics applications. In Proceedings of the 2019 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Glasgow, UK, 8–10 July 2019; pp. 1–3. [Google Scholar]
- Yan, C.; Wang, J.; Lee, P.S. Stretchable Graphene Thermistor with Tunable Thermal Index. ACS Nano 2015, 9, 2130–2137. [Google Scholar] [CrossRef]
- Bonzi, M.; Fiorelli, E.M.; Solbiati, M.; Montano, N. Accuracy of Peripheral Thermometers for Estimating Temperature. Ann. Intern. Med. 2016, 165, 73. [Google Scholar] [CrossRef]
- Atallah, L.; Ciuhu, C.; Wang, C.; Bongers, E.; Blom, T.; Paulussen, I.; Noordergraaf, G. An ergonomic wearable core body temperature sensor. In Proceedings of the 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Las Vegas, NV, USA, 4–7 March 2018; pp. 70–73. [Google Scholar]
- Tien, N.T.; Jeon, S.; Kim, D.-I.; Trung, T.Q.; Jang, M.; Hwang, B.-U.; Byun, K.-E.; Bae, J.; Lee, E.; Tok, J.B.H.; et al. A Flexible Bimodal Sensor Array for Simultaneous Sensing of Pressure and Temperature. Adv. Mater. 2014, 26, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shen, H.; Han, S. Flexible Thermoelectric Module Using Bi-Te and Sb-Te Thin Films for Temperature Sensors. J. Electron. Mater. 2019, 48, 5464–5470. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.-H.; Hou, T.; Zhang, F.; Wang, Z.L. Nanowire-composite based flexible thermoelectric nanogenerators and self-powered temperature sensors. Nano Res. 2012, 5, 888–895. [Google Scholar] [CrossRef]
- Nakamura, T.; Yokota, T.; Terakawa, Y.; Reeder, J.; Voit, W.; Someya, T.; Sekino, M. Development of flexible and wide-range polymer-based temperature sensor for human bodies. In Proceedings of the 2016 IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Las Vegas, NV, USA, 24–27 February 2016; pp. 485–488. [Google Scholar]


| Vital Signs | Materials | Key Features | Limitations | Reference | |
|---|---|---|---|---|---|
| Contact sensor | ECG/EMG | Ag NW/PDMS | Anti-microbial, Eliminated motion artifacts | Material oxidation | [6] |
| ECG | Polymer/Ag NWs electrode | Highly stretchable, low sensing limit, and good durability | Requires tight contact | [7] | |
| ECG | Graphene, textile | Easy to make | High noise | [8] | |
| ECG | PEDOT: PSS, LIG | Prolonged stability, High waveform quality | Prone to motion artifact | [9] | |
| ECG/EMG | PDMS | Scalable, less skin irritation | Prone to motion artifact | [3] | |
| ECG | Ag/AgCl | Wi-Fi wireless transmission | High power consumption, short lifespan | [10] | |
| ECG | Ag/AgCl | Low power consumption, dry 3D printed electrodes | Short battery lifespan | [11] | |
| EMG | Ag, nylon plastic | Convenient, real time processed | Data accuracy | [12] | |
| Non-contact sensor | ECG/EMG/EEG | PS25255 EPIC | Portability, long-term monitoring | Poor tight contact, prone to motion | [13] |
| ECG/EMG/EEG | Flexible printed circuits (FPC) | Flexible, no obvious power frequency noise | Baseline drift exists | [14,15] | |
| ECG | ASOPA4002 | Completely flexible and ultra-thin | High power consumption | [16] | |
| ECG | Silicone-based sensors | Comfortable, noise immunization | Short monitoring period | [17] | |
| ECG | Silicone dry electrode | Reliable, low power consumption, low cost, | Irregular waveforms, low CR | [18] | |
| ECG | PDMS-Graphene | Textile based, high quality | Limited stability | [19] | |
| ECG | Graphene | Soft, low cost, scalable | Contact impedance exists | [20] | |
| Implant-able sensor | Peripheral neural signals | TiO2, silicone | Good biocompatibility | Unknown mechanical properties | [21] |
| ECG | PI, AU/Ti | Flexible, robust performance | High impedance | [22] | |
| ECG | Ag/AgCl | Low noise, good biocompatibility | High power consumption | [23] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, M.; Bai, Y.; Zhang, J.; Liu, H.; Zhu, W. Recent Progress in Flexible Wearable Sensors for Vital Sign Monitoring. Sensors 2020, 20, 4009. https://doi.org/10.3390/s20144009
Liu J, Liu M, Bai Y, Zhang J, Liu H, Zhu W. Recent Progress in Flexible Wearable Sensors for Vital Sign Monitoring. Sensors. 2020; 20(14):4009. https://doi.org/10.3390/s20144009
Chicago/Turabian StyleLiu, Jihong, Meilin Liu, Yu Bai, Jiahao Zhang, Hongwei Liu, and Wenbin Zhu. 2020. "Recent Progress in Flexible Wearable Sensors for Vital Sign Monitoring" Sensors 20, no. 14: 4009. https://doi.org/10.3390/s20144009
APA StyleLiu, J., Liu, M., Bai, Y., Zhang, J., Liu, H., & Zhu, W. (2020). Recent Progress in Flexible Wearable Sensors for Vital Sign Monitoring. Sensors, 20(14), 4009. https://doi.org/10.3390/s20144009





