3D Hydrogen Titanate Nanotubes on Ti Foil: A Carrier for Enzymatic Glucose Biosensor
Abstract
:1. Introduction
2. Experiments
2.1. Reagents
2.2. Preparation of the Glucose Biosensor Based on the HTNTs
2.3. Characterization Techniques
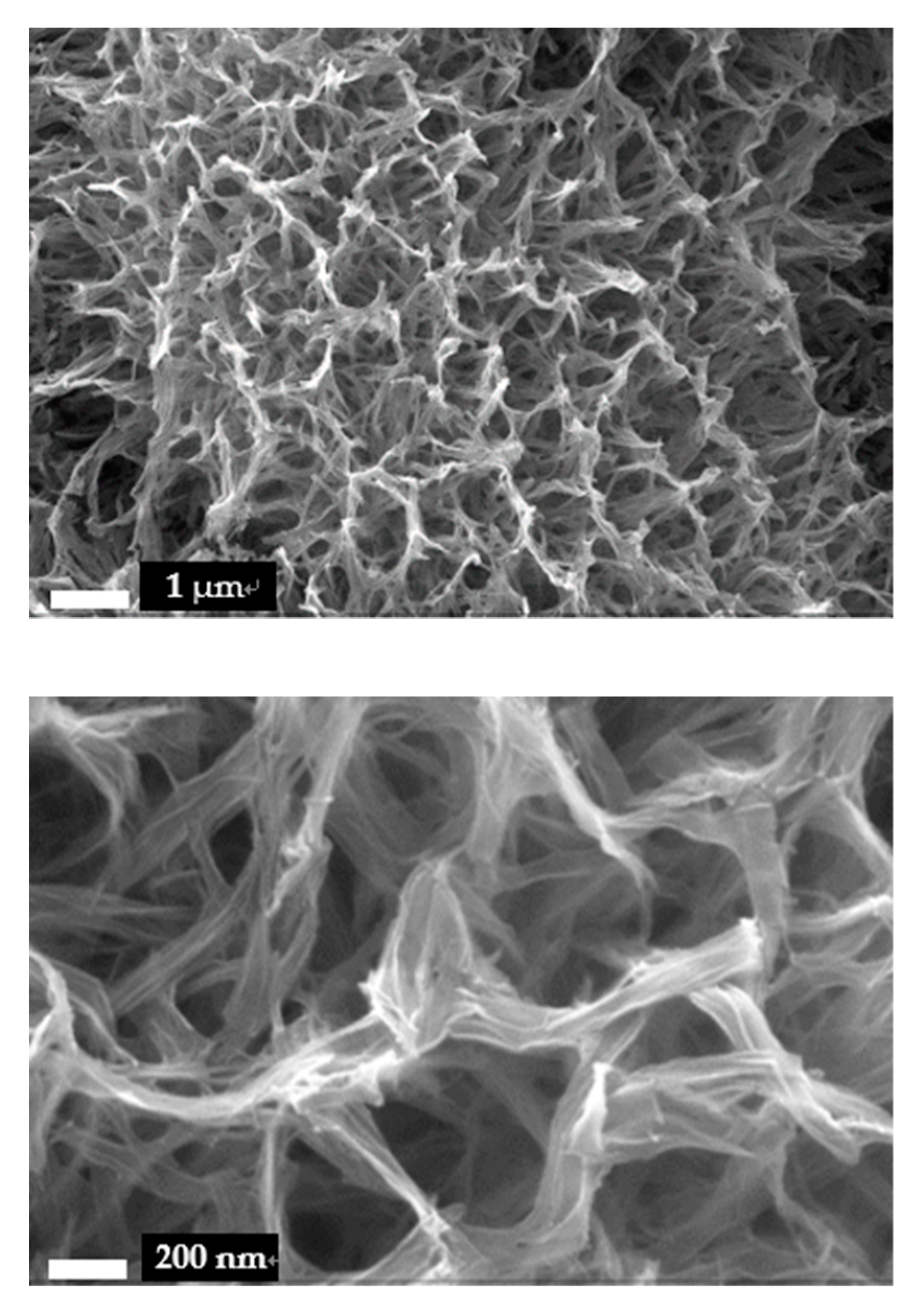
3. Results and Discussion
3.1. Surface Morphology
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Lopez-Gallego, F.; Schmidt-Dannert, C. Multi-enzymatic synthesis. Curr. Opin. Chem. Biol. 2010, 14, 174–183. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotec. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Lee, S. Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, G. Target-Specific Covalent Immobilization of Enzymes from Cell Lysate on SiO2 Nanoparticles for Biomass Saccharification. ACS Appl. Nano Mater. 2020, 3, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, D.; Niroui, F.; Leung, K.T. High-performance, flexible enzymatic glucose biosensor based on ZnO nanowires supported on a gold-coated polyester substrate. ACS Appl. Mater. Inter. 2010, 2, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Stenzel, M.H. All wrapped up: Stabilization of enzymes within single enzyme nanoparticles. J. Am. Chem. Soc. 2019, 141, 2754–2769. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Soldatkin, O.O.; Kucherenko, D.Y.; Soldatkina, O.V.; Dzyadevych, S.V. Advances in nanomaterial application in enzyme-based electrochemical biosensors: A review. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, X. Nanotechnology and nanomaterial-based no-wash electrochemical biosensors: From design to application. Nanoscale 2019, 11, 19105–19118. [Google Scholar] [CrossRef]
- El Harrad, L.; Bourais, I.; Mohammadi, H.; Amine, A. Recent Advances in Electrochemical Biosensors Based on Enzyme Inhibition for Clinical and Pharmaceutical Applications. Sensors 2018, 18, 164. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, S.R.; Vilela, R.S.; Camargo, H.S.; Guedes, M.I.; Fernandes, K.F.; Colmati, F. Enzymatic Electrochemical Biosensor Based on Multiwall Carbon Nanotubes and Cerium Dioxide Nanoparticles for Rutin Detection. Int. J. Electrochem. Sci. 2018, 13, 563–586. [Google Scholar] [CrossRef]
- Hondred, J.A.; Breger, J.C.; Alves, N.J.; Trammell, S.A.; Walper, S.A.; Medintz, I.L.; Claussen, J.C. Printed graphene electrochemical biosensors fabricated by inkjet maskless lithography for rapid and sensitive detection of organophosphates. ACS Appl. Mater. Interfaces 2018, 10, 11125–11134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, W.; Zhang, X.; Chai, H.; Huang, Y. FeNPs@ Co3O4 hollow nanocages hybrids as effective peroxidase mimics for glucose biosensing. Sens. Actuators B Chem. 2018, 263, 575–584. [Google Scholar] [CrossRef]
- Guo, S.; Wang, E. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Christwardana, M.; Kwon, Y. Yeast and carbon nanotube based biocatalyst developed by synergetic effects of covalent bonding and hydrophobic interaction for performance enhancement of membraneless microbial fuel cell. Bioresour. Technol. 2017, 225, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, X.; Baimanov, D.; Wang, L.; Xiao, Y.; Liu, H.; Li, Y.; Gao, X.; Zhao, Y.; Chen, C. Immobilized ferrous ion and glucose oxidase on graphdiyne and its application on one-step glucose detection. ACS Appl. Mater. Interfaces 2019, 11, 2647–2654. [Google Scholar] [CrossRef]
- Smecca, E.; Sanzaro, S.; Grosso, D.; Bottein, T.; Mannino, G.; Condorelli, G.G.; La Magna, A.; Alberti, A. Nitrogen doped spongy TiO2 layers for sensors application. Mat. Sci. Semicon. Proc. 2019, 98, 44–48. [Google Scholar] [CrossRef]
- Xue, J.; Gao, C.; Zhang, L.; Cui, K.; He, W.; Yu, J. A single-interface photoelectrochemical sensor based on branched TiO2 nanorods@ strontium titanate for two biomarkers detection. J. Mater. Chem. B 2018, 6, 4697–4703. [Google Scholar] [CrossRef]
- Tian, X.; Liu, L.; Li, Y.; Yang, C.; Zhou, Z.; Nie, Y.; Wang, Y. Nonenzymatic electrochemical sensor based on CuO-TiO2 for sensitive and selective detection of methyl parathion pesticide in ground water. Sens. Actuators B Chem. 2018, 256, 135–142. [Google Scholar] [CrossRef]
- Yao, P.; Yu, S.; Shen, H.; Yang, J.; Min, L.; Yang, Z.; Zhu, X. A TiO2–SnS2 nanocomposite as a novel matrix for the development of an enzymatic electrochemical glucose biosensor. New J. Chem. 2019, 43, 16748–16752. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Liu, S.; Zhang, Q.; Liu, X.; Wong, D.K. Gold nanoparticle encapsulated-tubular TiO2 nanocluster as a scaffold for development of thiolated enzyme biosensors. Anal. Chem. 2013, 85, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Huo, K.; Chen, R.; Gao, B.; Fu, J.; Chu, P.K. Recyclable and high-sensitivity electrochemical biosensing platform composed of carbon-doped TiO2 nanotube arrays. Anal. Chem. 2011, 83, 8138–8144. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Nosaka, A.Y.; Nosaka, Y. Adsorption and photocatalytic decomposition of amino acids in TiO2 photocatalytic systems. J. Phys. Chem. B 2006, 110, 25525–25531. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Zhu, B.; Li, J.; Zhou, J.; Wang, S.; Zhang, S.; Wu, S.; Huang, W. Synthesis and characterization of thermally stable nanotubular TiO2 and its photocatalytic activity. J. Phys. Chem. C 2008, 112, 18772–18775. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, Q.; Huang, X.; Wang, S.; Zhang, S.; Wu, S.; Huang, W. Characterization and catalytic performance of TiO2 nanotubes-supported gold and copper particles. J. Mol. Catal. A Chem. 2006, 249, 211–217. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.L.; Guo, D.; Ma, L.L.; Zhu, B.L.; Wang, P.; Wang, G.C.; Zhang, S.M.; Huang, W.P. Fabrication and photocatalytic performance of C, N, F-tridoped TiO2 nanotubes. Catal. Today 2019, 327, 182–189. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, B.L.; Wang, G.C.; Liu, Z.F.; Huang, W.P.; Zhang, S.M. Enhanced CO catalytic oxidation over an Au–Pt alloy supported on TiO2 nanotubes: Investigation of the hydroxyl and Au/Pt ratio influences. Catal. Sci. Technol. 2018, 8, 6109–6122. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xie, Y.; Wang, Y.; Du, H.; Xia, C.; Tian, F. Glucose biosensor based on glucose oxidase immobilized on unhybridized titanium dioxide nanotube arrays. Microchim. Acta 2014, 181, 381–387. [Google Scholar] [CrossRef]
- Xiao, F.X. Construction of highly ordered ZnO–TiO2 nanotube arrays (ZnO/TNTs) heterostructure for photocatalytic application. ACS Appl. Mater. Interfaces 2012, 4, 7055–7063. [Google Scholar] [CrossRef]
- Guo, C.X.; Li, C.M. Direct electron transfer of glucose oxidase and biosensing of glucose on hollow sphere-nanostructured conducting polymer/metal oxide composite. Phys. Chem. Chem. Phys. 2010, 12, 12153–12159. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ren, Y.; Zhang, Y.; Li, J.; Li, H.; Huang, X.; Hu, X.; Xu, Q. Nanoflake-like SnS2 matrix for glucose biosensing based on direct electrochemistry of glucose oxidase. Biosens. Bioelectron. 2011, 26, 4337–4341. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, Y.; Li, J.; Zhang, Y.; Hu, X. Facile synthesis of tetragonal columnar-shaped TiO2 nanorods for the construction of sensitive electrochemical glucose biosensor. Biosens. Bioelectron. 2014, 54, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Devasenathipathy, R.; Chen, S.M.; Wang, S.F.; Devi, P.; Tai, Y. Electrodeposition of copper nanoparticles using pectin scaffold and hydrogen peroxide. Electrochim. Acta 2015, 176, 804–810. [Google Scholar] [CrossRef]
- Zheng, B.; Xie, S.; Qian, L.; Yuan, H.; Xiao, D.; Choi, M.M. Gold nanoparticles-coated eggshell membrane with immobilized glucose oxidase for fabrication of glucose biosensor. Sens. Actuators B Chem. 2011, 152, 49–55. [Google Scholar] [CrossRef]
- Brouzgou, A.; Tsiakaras, P. Electrocatalysts for glucose electrooxidation reaction: A review. Top. Catal. 2015, 58, 1311–1327. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors–a review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]







| Configuration of Biosensor | Linear Range (Mm) | Sensitivity (μA·mM−1·cm−2) | Detection Limit (mM) | Response Time (s) | Ref. |
|---|---|---|---|---|---|
| GOx/TiO2-SnS2/Nafion/GCE | 0.008–1.13 1.13–5.53 | 18.9 | 0.0018 | <8 | [20] |
| GOx/SnS2/Nafion | 0.025–1.1 | 7.6 | 0.01 | 8 | [32] |
| GOx/TiO2 | 0.005–1.32 | 23.2 | 0.002 | <3 | [33] |
| GOx/ZnO-NWs/Au/PET | 0.2–2.0 | 19.5 | <0.05 | <5 | [6] |
| Graphene/pectin-CuNPs | 0.01–5.5 | 0.0457 | 0.0021 | <5 | [34] |
| GOx-AuNPs/ESM GOx/HTNTs/Ti (this work) | 0.008–0.966 1–10 | \ 1.541 | 0.0035 0.059 | <3 <1.5 | [35] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Yue, Z.; Huo, G.; Zhang, S.; Zhu, B.; Zhang, S.; Huang, W. 3D Hydrogen Titanate Nanotubes on Ti Foil: A Carrier for Enzymatic Glucose Biosensor. Sensors 2020, 20, 1024. https://doi.org/10.3390/s20041024
Ma L, Yue Z, Huo G, Zhang S, Zhu B, Zhang S, Huang W. 3D Hydrogen Titanate Nanotubes on Ti Foil: A Carrier for Enzymatic Glucose Biosensor. Sensors. 2020; 20(4):1024. https://doi.org/10.3390/s20041024
Chicago/Turabian StyleMa, Lulu, Zhao Yue, Guona Huo, Shasha Zhang, Baolin Zhu, Shoumin Zhang, and Weiping Huang. 2020. "3D Hydrogen Titanate Nanotubes on Ti Foil: A Carrier for Enzymatic Glucose Biosensor" Sensors 20, no. 4: 1024. https://doi.org/10.3390/s20041024
APA StyleMa, L., Yue, Z., Huo, G., Zhang, S., Zhu, B., Zhang, S., & Huang, W. (2020). 3D Hydrogen Titanate Nanotubes on Ti Foil: A Carrier for Enzymatic Glucose Biosensor. Sensors, 20(4), 1024. https://doi.org/10.3390/s20041024




