Vital Block and Vital Sign Server for ECG and Vital Sign Monitoring in a Portable u-Vital System
Abstract
:1. Introduction
2. Vital Sign Sensing Device Trend and Data Transmission
3. Proposed Portable u-Vital System
3.1. Configuration of VB and VSS
3.2. Flow Chart of VB and VSS
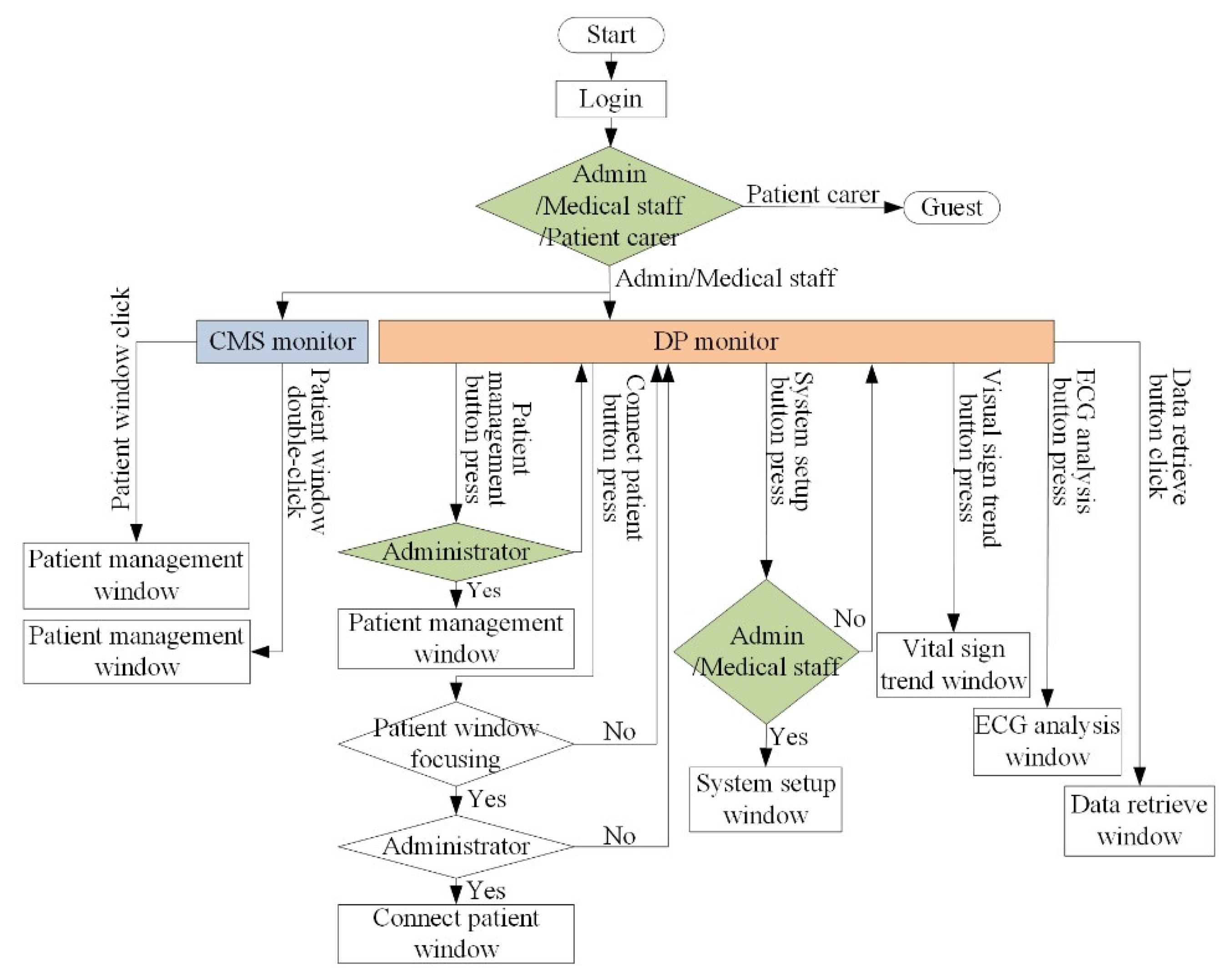
3.3. GUI of VB and VSS
3.4. Usage Scenario between VB and VSS
3.5. Efficient R-Point Detector for Real-Time ECG Processing
4. Results
4.1. Developed VB and VSS
4.2. ECG Experiment for Proposed R-Point Detection Method
4.3. Operation Experiment between VSS and VB
4.4. Field Test
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- A Simple Explanation of the Internet of Things. Available online: http://www.forbes.com/sites/jacobmorgan/2014/05/13/simple-explanation-internet-things-that-anyone-can-understand/#6c37c7b66828/ (accessed on 1 January 2020).
- Mehta, D. Internet of medical things: Note on futuristic healthcare. Inventi Impact Telemed. 2016, 4, 244–245. [Google Scholar]
- Zanella, A.; Bui, N.; Castellani, A.; Vangelista, L.; Zorzi, M. Internet of things for smart cities. IEEE Internet Things J. 2014, 1, 22–32. [Google Scholar] [CrossRef]
- Dimiter, V.D. Medical internet of things and big data in healthcare. Healthc. Inform. Res. 2016, 22, 156–163. [Google Scholar] [CrossRef] [PubMed]
- IoT Healthcare Market Worth 158.07 Billion USD by 2022. Available online: https://www.marketsandmarkets.com/PressReleases/iot-healthcare.asp/ (accessed on 1 January 2020).
- Medtech and the Internet of Medical Things. Available online: https://www2.deloitte.com/global/en/pages/life-sciences-and-healthcare/articles/medtech-internet-of-medical-things.html/ (accessed on 1 January 2020).
- Madias, J.E. A proposal for monitoring patients with heart failure via “smart phone technology”-based electrocardiograms. J. Electrocardiol. 2016, 49, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Android App for Patient Monitoring. Available online: https://www.amrita.edu/center/awna/research/patient-monitoring-app/ (accessed on 1 January 2020).
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.S.; Gaydecki, P.A.; Zaidi, A.; Fitzpatrick, A.P. A new QRS detection algorithm Based on the Hilbert transform. In Proceedings of the Computers in Cardiology 2000, Cambridge, MA, USA, 24–27 September 2000; pp. 379–382. [Google Scholar]
- Christov, I.I. Real time electrocardiogram QRS detection using combined adaptive threshold. Biomed. Eng. Online 2004, 3, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Lian, Y. Effective ECG QRS detection based on multiscale mathematical morphology filtering. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Castells-Rufas, D.; Carrabina, J. Simple real-time QRS detector with the MaMeMi filter. Biomed. Signal Process. Control 2015, 21, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Meraha, M.; Abdelmalika, T.A.; Larbi, B.H. R-peaks detection based on stationary wavelet transform. Comput. Methods Programs Biomed. 2015, 121, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, H. Simple and robust realtime QRS detection algorithm based on spatiotemporal characteristic of the QRS complex. PLoS ONE 2016, 11, e0150144. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Cunha, J.P.S. Wearable health devices-vital sign monitoring, systems and technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhayajneh, A.; Baccarini, A.N.; Weiss, G.M.; Hayajneh, T.; Farajidavar, A. Biometric authentication and verification for medical cyber physical systems. Electronics 2018, 7, 436. [Google Scholar] [CrossRef] [Green Version]
- MySignals SW Technical Guide. Available online: http://www.libelium.com/development/mysignals/ (accessed on 1 January 2020).
- Shobha, G.; Chittal, R.R.; Kumar, K. Medical applications of wireless networks. In Proceedings of the 2nd International Conference on Systems and Networks Communications, Cap Esterel, France, 25–31 August 2007. [Google Scholar]
- Lai, D.T.H.; Begg, R.K.; Palaniswami, M. Sensor Networks in healthcare: A new paradigm for improving future global health. In Healthcare Sensor Networks: Challenges Toward Practical Implementation, 1st ed.; Lai, D.T.H., Begg, R.K., Palaniswami, M., Eds.; CRC Press: New York, NY, USA, 2011; pp. 9–11. [Google Scholar]
- Schobel, J.; Schickler, M.; Pryss, R.; Nienhaus, H. Using vital sensors in mobile healthcare business applications: Challenges, examples, lessons learned. In Proceedings of the 9th International Conference on Web Information Systems and Technologies, Aachen, Germany, 8–10 May 2013. [Google Scholar]
- Cornet, V.P.; Holden, R.J. Systematic review of smartphone-based passive sensing for health and wellbeing. J. Biomed. Inform. 2018, 77, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Wireless B Scan. Available online: http://www.sonoptek.com/wireless.html/ (accessed on 1 January 2020).
- L7 Linear Handheld Ultrasound Scanner. Available online: https://clarius.com/products/linear-handheld-ultrasound/ (accessed on 1 January 2020).
- Ultrasound Made Easy. Available online: https://biimultrasound.us/biim-technology/ (accessed on 1 January 2020).
- Breathe Easier, Sleep More Naturally. Available online: http://www.respironics.com/ (accessed on 1 January 2020).
- Khalifa, M.; Alswailem, O. Hospital information systems (HIS) acceptance and satisfaction: A case study of a tertiary care hospital. Procedia Comput. Sci. 2015, 63, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Kuo, K.M.; Liu, C.F.; Talley, P.C.; Pan, S.Y. Strategic improvement for quality and satisfaction of hospital information systems. J. Healthc. Eng. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OEM/ODM. Available online: https://micromaxhealth.com/products/oem-odm/ (accessed on 30 January 2020).
- Health Information Systems. Available online: http://phinnetwork.org/resources/health-information-systems-his/ (accessed on 1 January 2020).
- Feldman, K.; Johnson, R.A.; Chawla, N.V. The State of data in healthcare: Path towards standardization. J. Healthc. Inform. Res. 2018, 2, 248–271. [Google Scholar] [CrossRef]
- Grannis, S.J.; Xu, H.; Vest, J.R.; Kasthurirathne, S.; Bo, N.; Moscovitch, B.; Torkzadeh, R.; Rising, J. Evaluating the effect of data standardization and validation on patient matching accuracy. J. Am. Med. Inform. Assoc. 2019, 26, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.; Hoka, S.; Farag, E. BiSpectral Index (BIS) monitoring may detect critical hypotension before automated non-invasive blood pressure (NIBP) measurement during general anaesthesia; a case report. F1000Research 2014, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patient Monitor. Available online: http://www.bistosamerica.com/modules/catalogue_eng2/cg_view.html?cc=12&PHPSESSID=93cb2bdaeed208f40b3bbd8ddef32a8d&p=1&no=21&PHPSESSID=93cb2bdaeed208f40b3bbd8ddef32a8d/ (accessed on 30 January 2020).
- Respiratory Rate Patient Monitor/ETCO2/SPO2/Intensive Care. Available online: https://www.medicalexpo.com/prod/meditech-equipment/product-80924-760194.html/ (accessed on 30 January 2020).
- Vital Signs Monitor ECG, SPO2, NIBP, RESP, TEMP and CO2 Capnography. Available online: https://www.quirumed.com/en/vital-signs-monitor-ecg-spo2-nibp-resp-temp-and-co2-capnography.html/ (accessed on 30 January 2020).
- Global Multiparameter Patient Monitoring Market-Pipeline Analysis, Competitive Landscape and Forecast. Available online: https://www.prnewswire.com/news-releases/multiparameter-patient-monitoring---global-pipeline-analysis-competitive-landscape-and-market-forecasts-to-2017-137405708.html/ (accessed on 1 January 2020).
- Luhf-Luxembourg Heart Failure. Available online: http://santec.tudor.lu/project/luhf/ (accessed on 1 January 2020).
- MySQL. Available online: https://www.mysql.com/ (accessed on 30 January 2020).
- MIT-BIH Arrhythmia Database Directory. Available online: https://physionet.org/physiobank/database/html/mitdbdir/mitdbdir.htm/ (accessed on 1 January 2020).
- MS400 Multiparameter Simulator. Available online: https://contechealth.com/products/ms400-multiparameter-simulator/ (accessed on 13 January 2020).
- B40 Patent Monitor. Available online: https://www.gehealthcare.com/products/patient-monitoring/patient-monitors/b40-patient-monitor/ (accessed on 6 February 2020).
- Multi-Meter Resp Monitor. Available online: https://www.usa.philips.com/healthcare/product/HC865350/intellivue-mx40-wearable-patient-monitor/ (accessed on 6 February 2020).
- ECG Multi-Parameter Monitor. Available online: https://eu.nihonkohden.com/en/products/patientmonitoring/life_scope_tr.html/ (accessed on 6 February 2020).
- Multi-Parameter SPO2 Monitor. Available online: https://www.draeger.com/en-us_us/Hospital/Products/Patient-Monitoring/Telemetry/Infinity-M300/ (accessed on 6 February 2020).
- Seliem, M.; Elgazzar, K. BIoMT: Blockchain for the internet of medical things. In Proceedings of the IEEE International Black Sea Conference on Communications and Networking, Sochi, Russia, 3–6 June 2019. [Google Scholar]
- Vazirani, A.A.; Donoghue, O.; Brindley, D.; Meinert, E. Blockchain vehicles for efficient medical record management. Digit. Med. 2020, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D. Blockchain applications for healthcare data management. Healthc. Inform. Res. 2019, 25, 51–56. [Google Scholar] [CrossRef] [PubMed]



















| Tape | Total | FN | FP | Se [%] | +P [%] | DER [%] | Tape | Total | FN | FP | Se [%] | +P [%] | DER [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 2273 | 0 | 0 | 100 | 100 | 0 | 201 | 1963 | 9 | 2 | 99.54 | 99.90 | 0.56 |
| 101 | 1865 | 3 | 5 | 99.84 | 99.73 | 0.43 | 202 | 2136 | 4 | 3 | 99.81 | 99.86 | 0.33 |
| 102 | 2187 | 0 | 0 | 100 | 100 | 0 | 203 | 2980 | 25 | 16 | 99.16 | 99.46 | 1.38 |
| 103 | 2084 | 0 | 0 | 100 | 100 | 0 | 205 | 2656 | 5 | 2 | 99.81 | 99.92 | 0.26 |
| 104 | 2229 | 6 | 16 | 99.73 | 99.29 | 0.99 | 207 | 1862 | 6 | 9 | 99.68 | 99.52 | 0.81 |
| 105 | 2572 | 13 | 21 | 99.49 | 99.19 | 1.32 | 208 | 2955 | 14 | 6 | 99.53 | 99.80 | 0.68 |
| 106 | 2027 | 2 | 4 | 99.90 | 99.80 | 0.30 | 209 | 3004 | 2 | 3 | 99.93 | 99.90 | 0.17 |
| 107 | 2137 | 3 | 0 | 99.86 | 100 | 0.14 | 210 | 2650 | 22 | 8 | 99.17 | 99.70 | 1.13 |
| 108 | 1774 | 9 | 25 | 99.49 | 98.60 | 1.92 | 212 | 2748 | 2 | 3 | 99.93 | 99.89 | 0.18 |
| 109 | 2532 | 3 | 0 | 99.88 | 100 | 0.12 | 213 | 3251 | 2 | 7 | 99.94 | 99.79 | 0.28 |
| 111 | 2124 | 2 | 2 | 99.91 | 99.91 | 0.19 | 214 | 2265 | 1 | 2 | 99.96 | 99.91 | 0.13 |
| 112 | 2539 | 0 | 1 | 100 | 99.96 | 0.04 | 215 | 3363 | 2 | 2 | 99.94 | 99.94 | 0.12 |
| 113 | 1795 | 1 | 0 | 99.94 | 100 | 0.06 | 217 | 2209 | 7 | 3 | 99.68 | 99.86 | 0.45 |
| 114 | 1879 | 2 | 3 | 99.89 | 99.84 | 0.27 | 219 | 2154 | 1 | 3 | 99.95 | 99.86 | 0.19 |
| 115 | 1953 | 0 | 0 | 100 | 100 | 0 | 220 | 2048 | 1 | 0 | 99.95 | 100 | 0.05 |
| 116 | 2412 | 12 | 6 | 99.50 | 99.75 | 0.75 | 221 | 2427 | 4 | 1 | 99.84 | 99.96 | 0.21 |
| 117 | 1535 | 0 | 0 | 100 | 100 | 0 | 222 | 2483 | 6 | 1 | 99.76 | 99.96 | 0.28 |
| 118 | 2278 | 1 | 1 | 99.96 | 99.96 | 0.09 | 223 | 2605 | 4 | 2 | 99.85 | 99.92 | 0.23 |
| 119 | 1987 | 0 | 0 | 100 | 100 | 0 | 228 | 2053 | 9 | 12 | 99.56 | 99.42 | 1.02 |
| 121 | 1863 | 5 | 1 | 99.73 | 99.95 | 0.32 | 230 | 2256 | 2 | 2 | 99.91 | 99.91 | 0.18 |
| 122 | 2476 | 0 | 0 | 100 | 100 | 0 | 231 | 1571 | 2 | 1 | 99.87 | 99.94 | 0.19 |
| 123 | 1518 | 2 | 1 | 99.87 | 99.93 | 0.20 | 232 | 1780 | 2 | 3 | 99.89 | 99.83 | 0.28 |
| 124 | 1619 | 1 | 0 | 99.94 | 100 | 0.06 | 233 | 3079 | 4 | 2 | 99.87 | 99.94 | 0.19 |
| 200 | 2601 | 3 | 11 | 99.88 | 99.58 | 0.54 | 234 | 2753 | 2 | 1 | 99.93 | 99.96 | 0.11 |
| Total | 109510 | 206 | 191 | 99.81 | 99.83 | 0.36 |
| Method | TB | FN | FP | Se [%] | +P [%] | DER [%] |
|---|---|---|---|---|---|---|
| Pan et al. [9] | 116,137 | 277 | 507 | 99.76 | 99.56 | 0.68 |
| Benitez et al. [10] | - | 203 | 187 | 99.81 | 99.83 | 0.36 |
| Cristov [11] | 110,050 | 240 | 239 | 99.78 | 99.78 | 0.44 |
| Zhang et al. [12] | 109,510 | 213 | 201 | 99.81 | 99.80 | 0.39 |
| Castells-Rufas et al. [13] | 109,494 | 614 | 353 | 99.43 | 99.67 | 0.88 |
| Merah et al. [14] | 109,494 | 178 | 126 | 99.84 | 99.88 | 0.28 |
| Kim et al. [15] | 109,494 | 107 | 97 | 99.90 | 99.91 | 0.19 |
| Proposed | 109,510 | 206 | 191 | 99.81 | 99.83 | 0.36 |
| Functions | B40 (GE) | IntelliVue MX40 (Philips) | Life Scope TR (Nihon Kohden) | Infinity M300 (Draeger) | Proposed u-Vital System |
|---|---|---|---|---|---|
| ECG | O | O | O | O | O |
| SpO2 | O | O | O | O | O |
| NiBP | O | O | O | X | O |
| BT | O | X | O | X | O |
| BIS | O | X | O | X | X |
| Connection with modules | wire | wire | wire | wire | wireless |
| Wireless network (Gateway) | O | X | X | O | O |
| Num. of connected patients | - | 1 | 16 | 1 | 48 |
| Arrhythmia alarm | O | X | O | O | O |
| HRV | X | X | X | X | O |
| Storage of full-waveforms | X | X | X | X | O |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, T.W.; Kwon, K.K.; Kim, K.H. Vital Block and Vital Sign Server for ECG and Vital Sign Monitoring in a Portable u-Vital System. Sensors 2020, 20, 1089. https://doi.org/10.3390/s20041089
Bae TW, Kwon KK, Kim KH. Vital Block and Vital Sign Server for ECG and Vital Sign Monitoring in a Portable u-Vital System. Sensors. 2020; 20(4):1089. https://doi.org/10.3390/s20041089
Chicago/Turabian StyleBae, Tae Wuk, Kee Koo Kwon, and Kyu Hyung Kim. 2020. "Vital Block and Vital Sign Server for ECG and Vital Sign Monitoring in a Portable u-Vital System" Sensors 20, no. 4: 1089. https://doi.org/10.3390/s20041089





