Electrochemical Immunosensor for the Early Detection of Rheumatoid Arthritis Biomarker: Anti-Cyclic Citrullinated Peptide Antibody in Human Serum Based on Avidin-Biotin System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Electrochemical Measurements
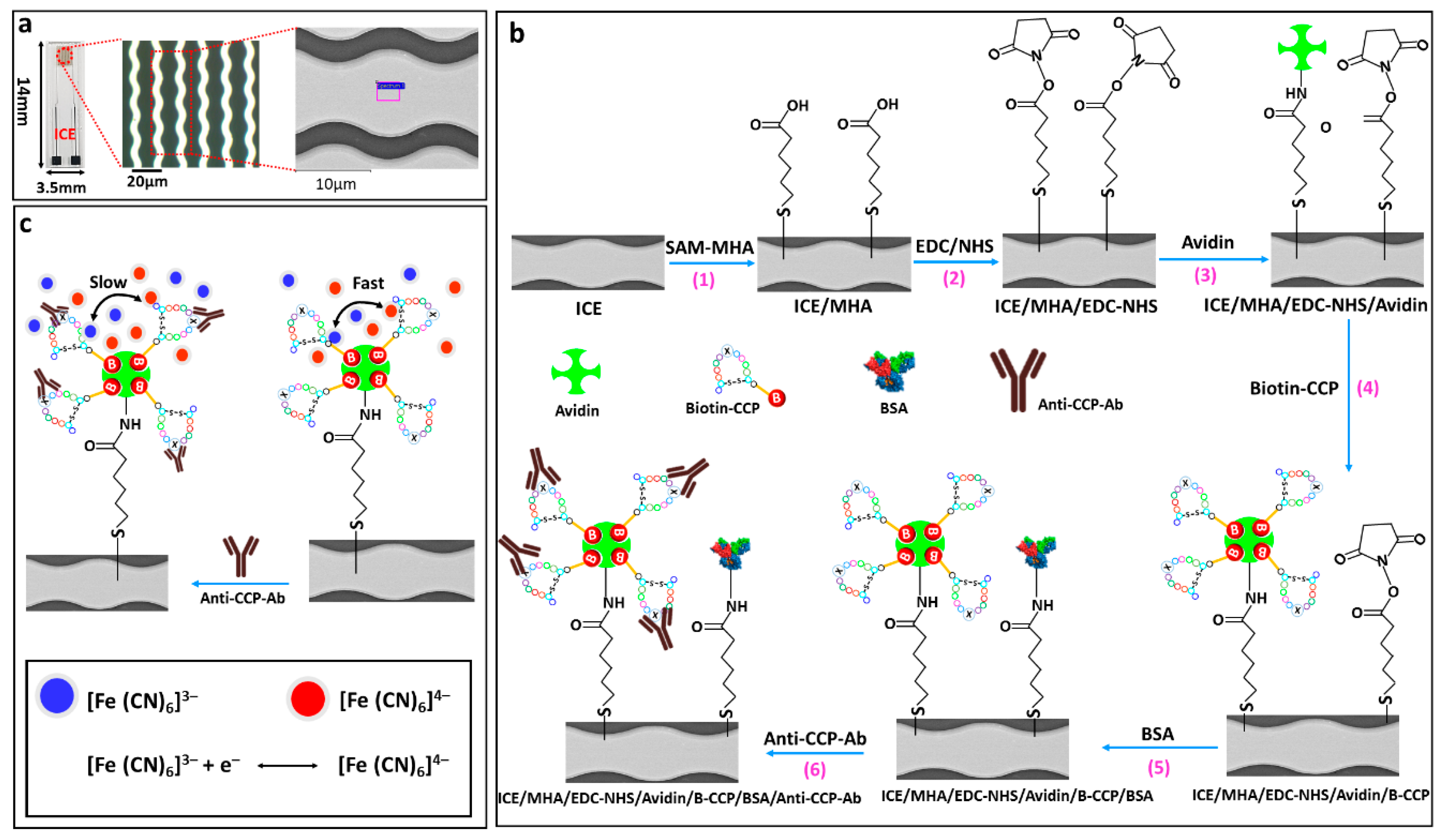
2.4. Fabrication of the ICE Arrays
2.5. Preparation of the Anti-CCP-ab Bioelectrode
3. Results
3.1. Characterization of SAM-MHA Functionalization on ICE
3.2. Optimization of the Immunosesnor
3.3. Electrochemical Characterization of the ICE Modified Electrode
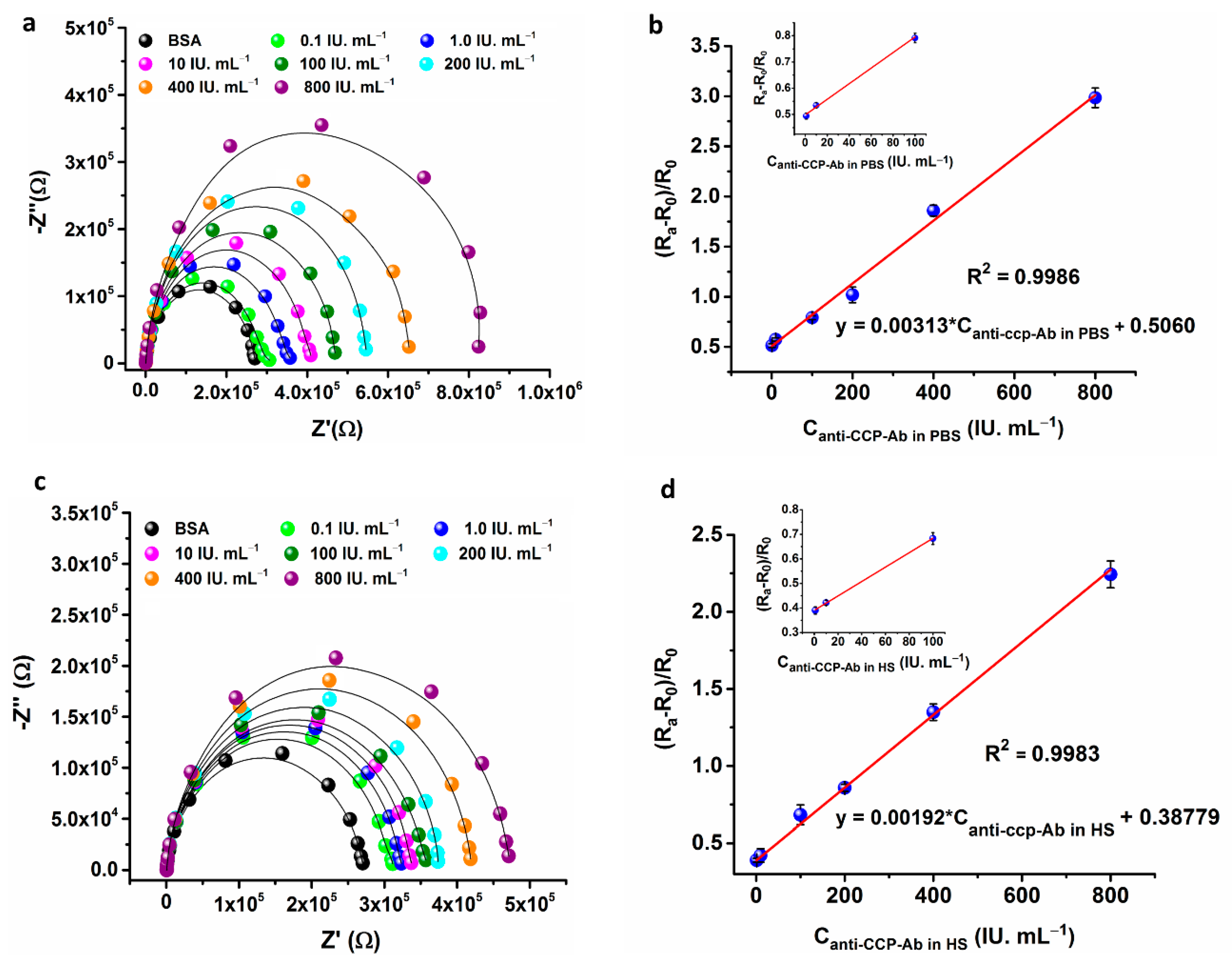
3.3.1. Electrochemical Impedance Spectroscopy (EIS)
3.3.2. Cyclic Voltammetry (CV)
3.4. Electrochemical Response Studies of the Modified ICE Bioelectrode
3.5. Application of the Bioelectrode
3.5.1. Interference Study
3.5.2. Stability Study
3.5.3. Reproducibility
3.6. Real-Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinnadayyala, S.R.; Park, J.; Abbasi, M.A.; Cho, S. Label-free electrochemical impedimetric immunosensor for sensitive detection of IgM rheumatoid factor in human serum. Biosens. Bioelectron. 2019, 143, 111642. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Therneau, T.M.; Gabriel, S.E. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010, 62, 1576–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, R.; Shoukrey, K.; Griffiths, R. Rheumatoid arthritis and anaesthesia. Anaesthesia 2011, 66, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Fugger, L.; Svejgaard, A. Association of MHC and rheumatoid arthritis HLA-DR4 and rheumatoid arthritis: Studies in mice and men. Arthritis Res. 2000, 2, 208–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregersen, P.K.; Silver, J.; Winchester, R.J. The Shared Epitope Hypothesis. Arthritis Rheum. 1987, 30, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.T.; Petersen, J.; Ramarathinam, S.H.; Scally, S.W.; Loh, K.L.; Thomas, R.; Suri, A.; Baker, D.G.; Purcell, A.W.; Reid, H.H.; et al. The interplay between citrullination and HLA-DRB1 polymorphism in shaping peptide binding hierarchies in rheumatoid arthritis. J. Biol. Chem. 2018, 293, 3236–3251. [Google Scholar] [CrossRef] [Green Version]
- Nielen, M.M.J.; Van Schaardenburg, D.; Reesink, H.W.; Van De Stadt, R.J.; Van Der Horst-Bruinsma, I.E.; De Koning, M.H.M.T.; Habibuw, M.R.; Vandenbroucke, J.P.; Dijkmans, B.A.C. Specific Autoantibodies Precede the Symptoms of Rheumatoid Arthritis: A Study of Serial Measurements in Blood Donors. Arthritis Rheum. 2004, 50, 380–386. [Google Scholar] [CrossRef]
- Enriconi dos Anjos, L.M.; Pereira, I.A.; d ’Orsi, E.; Seaman, A.P.; Burlingame, R.W.; Morato, E.F. A comparative study of IgG second- and third-generation anti-cyclic citrullinated peptide (CCP) ELISAs and their combination with IgA third-generation CCP ELISA for the diagnosis of rheumatoid arthritis. Clin. Rheumatol. 2009, 28, 153–158. [Google Scholar] [CrossRef]
- Alexiou, I.; Germenis, A.; Ziogas, A.; Theodoridou, K.; Sakkas, L.I. Diagnostic value of anti-cyclic citrullinated peptide antibodies in Greek patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2007, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pei, F.; Wang, X.; Sun, Z.; Hu, C.; Dou, H. Meta-analysis: Diagnostic accuracy of anti-cyclic citrullinated peptide antibody for juvenile idiopathic arthritis. J. Immunol. Res. 2015, 2015, 915276. [Google Scholar] [CrossRef]
- Aggarwal, R.; Liao, K.; Nair, R.; Ringold, S.; Costenbader, K.H. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Care Res. 2009, 61, 1472–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshafey, R.; Siaj, M.; Tavares, A.C. Au nanoparticle decorated graphene nanosheets for electrochemical immunosensing of p53 antibodies for cancer prognosis. Analyst 2016, 141, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Jaskowski, T.D.; Hill, H.R.; Russo, K.L.; Lakos, G.; Szekanecz, Z.; Teodorescu, M. Relationship between rheumatoid factor isotypes and IgG anti-cyclic citrullinated peptide antibodies. J. Rheumatol. 2010, 37, 1582–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chon, H.; Lee, S.; Wang, R.; Bang, S.Y.; Lee, H.S.; Bae, S.C.; Lee, H.; Kim, B.; Choo, J. SERS-based immunoassay of anti-cyclic citrullinated peptide for early diagnosis of rheumatoid arthritis. RSC Adv. 2014, 4, 32924–32927. [Google Scholar] [CrossRef]
- Chon, H.; Lee, S.; Wang, R.; Bang, S.-Y.; Lee, H.-S.; Bae, S.-C.; Hong, S.H.; Yoon, Y.H.; Lim, D.; Choo, J. Highly sensitive immunoassay of anti-cyclic citrullinated peptide marker using surface-enhanced Raman scattering detection. Int. Conf. Nano-Bio Sensing. Imaging Spectrosc. 2015, 9523, 95230J1–95230J8. [Google Scholar]
- Tanaka, R.; Takemura, M.; Sato, M.; Yamada, Y.; Nakagawa, T.; Horibe, T.; Hoshi, M.; Otaki, H.; Ito, H.; Seishima, M.; et al. Comparison of chemiluminescence enzyme immunoassay (CLEIA) with ELISA for the determination of anti-cyclic citrullinated peptide antibodies. Clin. Chim. Acta 2010, 411, 22–25. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, J.O.; Yoo, Y.M.; Shin, S.; Kim, J.Q.; Park, M.H.; Song, E.Y. Performance analysis of the ARCHITECT anti-cyclic citrullinated peptide antibody in the diagnosis of rheumatoid arthritis. Clin. Chem. Lab. Med. 2010, 48, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.; Lee, G.Y.; Jeon, B.J.; Pyun, J.C. Fluorescence immunoassay of anti-cyclic citrulinated peptide (CCP) autoantibodies by using parylene-H film. Biochip J. 2011, 5, 242–245. [Google Scholar] [CrossRef]
- Funari, R.; Chu, K.-Y.; Shen, A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens. Bioelectron. 2020, 169, 112578. [Google Scholar]
- Cabral-Miranda, G.; Cardoso, A.R.; Ferreira, L.C.S.; Sales, M.G.F.; Bachmann, M.F. Biosensor-based selective detection of Zika virus specific antibodies in infected individuals. Biosens. Bioelectron. 2018, 113, 101–107. [Google Scholar] [CrossRef]
- Randriantsilefisoa, R.; Cuellar-Camacho, J.L.; Chowdhury, M.S.; Dey, P.; Schedler, U.; Haag, R. Highly sensitive detection of antibodies in a soft bioactive three-dimensional biorthogonal hydrogel. J. Mater. Chem. B 2019, 7, 3220–3231. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.-Y.; Wang, B.-T. Highly Sensitive SPR Biosensor Based on Graphene Oxide and Staphylococcal Protein A Co-Modified TFBG for Human IgG Detection. IEEE Trans Instrum. Meas. 2019, 68, 3350–3357. [Google Scholar] [CrossRef]
- Zeni, L.; Perri, C.; Cennamo, N.; Arcadio, F.; D’Agostino, G.; Salmona, M.; Beeg, M.; Gobbi, M. A portable optical-fibre-based surface plasmon resonance biosensor for the detection of therapeutic antibodies in human serum. Sci. Rep. 2020, 10, 11154. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, S.; Sánchez-Tirado, E.; Martínez-García, G.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical biosensor for the simultaneous determination of rheumatoid factor and anti-cyclic citrullinated peptide antibodies in human serum. Analyst 2020, 145, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- de Gracia Villa, M.; Jiménez-Jorquera, C.; Haro, I.; José Gomara, M.; Sanmartí, R.; Fernández-Sánchez, C.; Mendoza, E. Carbon nanotube composite peptide-based biosensors as putative diagnostic tools for rheumatoid arthritis. Biosens. Bioelectron. 2011, 27, 113–118. [Google Scholar] [CrossRef]
- Soares, A.C.; Soares, J.C.; Shimizu, F.M.; Rodrigues, V.D.C.; Awan, I.T.; Melendez, M.E.; Piazzetta, M.H.O.; Gobbi, R.M.; Reis, A.L.; Fregnani, J.H.T.G.; et al. A simple architecture with self-assembled monolayers to build immunosensors for detecting the pancreatic cancer biomarker CA19-9. Analyst 2018, 143, 3302–3308. [Google Scholar] [CrossRef] [Green Version]
- Muharemagic, D.; Labib, M.; Ghobadloo, S.M.; Zamay, A.S.; Bell, J.C.; Berezovski, M.V. Anti-Fab Aptamers for Shielding Virus from Neutralizing Antibodies. J. Am. Chem. Soc. 2012, 134, 17168–17177. [Google Scholar] [CrossRef]
- Limbut, W.; Kanatharana, P.; Mattiasson, B.; Asawatreratanakul, P.; Thavarungkul, P.A. comparative study of capacitive immunosensors based on self-assembled monolayers formed from thiourea, thioctic acid, and 3-mercaptopropionic acid. Biosens. Bioelectron. 2006, 22, 233–240. [Google Scholar] [CrossRef]
- Nguyen, K.C. Quantitative analysis of COOH-terminated alkanethiol SAMs on gold nanoparticle surfaces. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 045008. [Google Scholar] [CrossRef] [Green Version]
- Yam, C.M.; Deluge, M.; Tang, D.; Kumar, A.; Cai, C.Z. Preparation, characterization, resistance to protein adsorption, and specific avidin–biotin binding of poly(amidoamine) dendrimers functionalized with oligo(ethylene glycol) on gold. J. Colloid Interface Sci. 2006, 296, 118–130. [Google Scholar] [CrossRef]
- Xu, F.; Zhen, G.L.; Yu, F.; Kuennemann, E.; Textor, M.; Knoll, W. Combined Affinity and Catalytic Biosensor: In Situ Enzymatic Activity Monitoring of Surface-Bound Enzymes. J. Am. Chem. Soc. 2005, 127, 13084–13085. [Google Scholar] [CrossRef] [PubMed]
- Boozer, C.; Ladd, J.; Chen, S.F.; Jiang, S.T. DNA-Directed Protein Immobilization for Simultaneous Detection of Multiple Analytes by Surface Plasmon Resonance Biosensor. Anal. Chem. 2006, 78, 1515–1519. [Google Scholar]
- Zhang, J.; Pourceau, G.; Meyer, A.; Vidal, S.; Praly, J.P.; Souteyrand, E.; Vasseur, J.J.; Morvan, F.; Chevolot, Y. DNA-directed immobilisation of glycomimetics for glycoarrays application: Comparison with covalent immobilisation, and development of an on-chip IC50 measurement assay. Biosens. Bioelectron. 2009, 24, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, A.; Blomstergren, A.; Nord, O.; Lukacs, M.; Lundeberg, J.; Uhlen, M. The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis 2005, 26, 501–510. [Google Scholar] [CrossRef]
- Yagati, A.K.; Park, J.; Kim, J.; Ju, H.; Chang, K.-A.; Cho, S. Sensitivity enhancement of capacitive tumor necrosis factor-α detection. Jpn. J. Appl. Phys. 2016, 55, 1–6. [Google Scholar] [CrossRef]
- Yagati, A.K.; Park, J.; Cho, S. Reduced Graphene Oxide Modified the Interdigitated Chain Electrode for an Insulin Sensor. Sensors 2016, 16, 109. [Google Scholar] [CrossRef]
- Yagati, A.K.; Pyun, J.C.; Min, J.; Cho, S. Label-free and direct detection of C-reactive protein using reduced graphene oxide-nanoparticle hybrid impedimetric sensor. Bioelectrochemistry 2016, 107, 37–44. [Google Scholar] [CrossRef]
- Huang, M.; Li, H.; He, H.; Zhang, X.; Wang, S. An electrochemical impedance sensor for simple and specific recognition of G–G mismatches in DNA. Anal. Methods 2016, 8, 7413–7419. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Park, J.; Kim, Y.H.; Choi, S.H.; Lee, S.-M.; Cho, W.W.; Lee, G.-Y.; Pyun, J.-C.; Cho, S. Electrochemical Detection of C-Reactive Protein in Human Serum Based on Self-Assembled Monolayer-Modified Interdigitated Wave-Shaped Electrode. Sensors 2019, 19, 5560. [Google Scholar] [CrossRef] [Green Version]
- Ngoc Le, H.T.; Park, J.; Chinnadayyala, S.R.; Cho, S. Sensitive electrochemical detection of amyloid beta peptide in human serum using an interdigitated chain-shaped electrode. Biosens. Bioelectron. 2019, 144, 111694. [Google Scholar] [CrossRef]
- Longo, E.; Wright, K.; Caruso, M.; Gatto, E.; Palleschi, A.; Scarselli, M.; De Crescenzi, M.; Crisma, M.; Formaggio, F.; Toniolo, C.; et al. Peptide flatlandia: A new-concept peptide for positioning of electroactive probes in proximity to a metal surface. Nanoscale 2015, 7, 15495–15506. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.R.; Lim, J.; Oh, J.; Kim, D.; Lee, D.; Kim, J.-W.; Shin, H.S.; Kim, J.H.; Jun, S.C. Substrate and buffer layer effect on the structural and optical properties of graphene oxide thin films. RSC Adv. 2013, 3, 5926–5936. [Google Scholar] [CrossRef]
- Pasha, S.K.; Kaushik, A.; Vasudev, A.; Snipes, S.A.; Bhansali, S. Electrochemical immunosensing of saliva cortisol. J. Electrochem. Soci. 2014, 161, 3077–3082. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection. A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar]
- Zhao, Y.; Liu, Y.; Li, X.; Wang, H.; Zhang, Y.; Ma, H.; Wei, Q. Label-free ECL immunosensor for the early diagnosis of rheumatoid arthritis based on asymmetric heterogeneous polyaniline-gold nanomaterial. Sens. Actuators B Chem. 2018, 257, 354–361. [Google Scholar] [CrossRef]




| Electrode | RS [Ω] | CPE | Rct [Ω] | Chi Square | |
|---|---|---|---|---|---|
| T [Ω−1. sp] | P | ||||
| Bare ICE | 575.1 | 2.450 × 10−7 | 0.85416 | 15,640 | 0.0545 |
| MHA | 550.6 | 1.594 × 10−7 | 0.90534 | 978,900 | 0.0330 |
| EDC-NHS | 552.8 | 1.266 × 10−7 | 0.92107 | 86,520 | 0.0343 |
| Avidin | 486.0 | 9.487 × 10−8 | 0.93138 | 120,400 | 0.0291 |
| B-CCP | 562.3 | 1.361 × 10−7 | 0.92019 | 197,400 | 0.0300 |
| BSA | 568.6 | 1.577 × 10−7 | 0.92658 | 265,500 | 0.0291 |
| Anti-CCP-ab | 527.1 | 9.478 × 10−8 | 0.93961 | 421,700 | 0.0276 |
| Detection Assay | Linear Range (IU mL−1) | LOD (IU mL−1) | Sample Type | Reference |
|---|---|---|---|---|
| Electrochemical Amperometric | 10–1000 | 2.5 | PBS | [19] |
| Fluorescence immunoassay (BioPlexTM 2200) | 3–300.0 | 0.2 | HS | [14] |
| ELISA (ImmunLisaTM CCP) | 25–3200.0 | 1.6 | HS | [15] |
| Electrochemiluminescence | 0.041–6.26 | 0.008 | PBS | [45] |
| EIS | 1–800 | 0.60 | PBS | This work |
| EIS | 1–800 | 0.82 | HS | This work |
| Test Sample | Conc. Of Anti-CCP-ab in Diluted HS (IU mL−1) | Spiked (IU mL−1) | Found (IU mL−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| A | 0 | 1 | 0.98 | 98.0 | 1.09 |
| B | 0 | 20 | 19.98 | 99.9 | 1.15 |
| C | 0 | 10 | 9.95 | 99.5 | 1.27 |
| D | 0 | 50 | 50.59 | 101.18 | 1.92 |
| E | 0 | 500 | 499.02 | 99.80 | 1.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinnadayyala, S.R.; Cho, S. Electrochemical Immunosensor for the Early Detection of Rheumatoid Arthritis Biomarker: Anti-Cyclic Citrullinated Peptide Antibody in Human Serum Based on Avidin-Biotin System. Sensors 2021, 21, 124. https://doi.org/10.3390/s21010124
Chinnadayyala SR, Cho S. Electrochemical Immunosensor for the Early Detection of Rheumatoid Arthritis Biomarker: Anti-Cyclic Citrullinated Peptide Antibody in Human Serum Based on Avidin-Biotin System. Sensors. 2021; 21(1):124. https://doi.org/10.3390/s21010124
Chicago/Turabian StyleChinnadayyala, Somasekhar R., and Sungbo Cho. 2021. "Electrochemical Immunosensor for the Early Detection of Rheumatoid Arthritis Biomarker: Anti-Cyclic Citrullinated Peptide Antibody in Human Serum Based on Avidin-Biotin System" Sensors 21, no. 1: 124. https://doi.org/10.3390/s21010124





