Flexible Recruitments of Fundamental Muscle Synergies in the Trunk and Lower Limbs for Highly Variable Movements and Postures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Protocol
2.2. Data Collection
2.3. EMG Processing
2.4. Muscle Synergy Analysis
2.5. Clustering the Modules across Participants
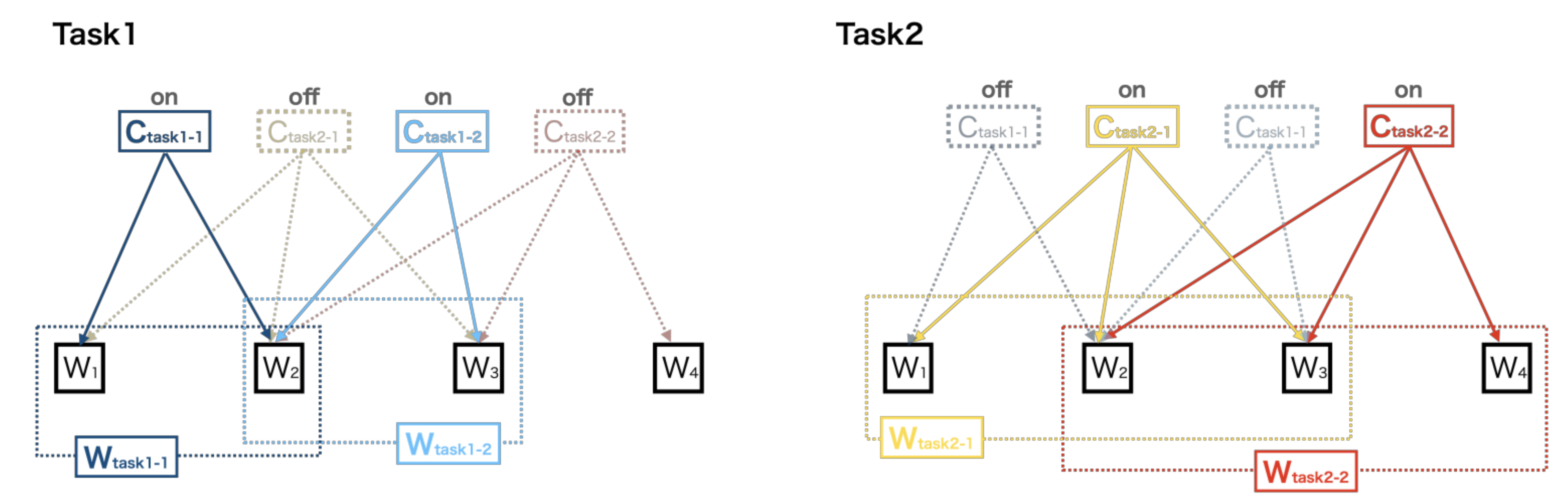
2.6. Contributions of the Muscle Synergy of All Tasks to the Execution of Each Task
2.7. Contributions of Merging Muscle Synergy of All Tasks towards Single-Task Execution
3. Results
3.1. Muscle Synergies Extracted from All-Task EMG Matrices
3.2. Relationship between Muscle Synergies Extracted from All-Task EMG Matrices and Those Extracted from Single-Task Matrices
4. Discussion
4.1. Characteristics of Muscle Synergies across 24 Tasks
4.2. Hypothetical Neural Mechanisms Underlying Muscle-Synergy Controlling Diverse Behavior
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernstein, N.A. The Co-Ordination and Regulation of Movements; Pergamon Press: Oxford, UK, 1967. [Google Scholar]
- Bizzi, E.; Cheung, V.C.K.; d’Avella, A.; Saltiel, P.; Tresch, M. Combining modules for movement. Brain Res. Rev. 2008, 57, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Tresch, M.C.; Jarc, A. The case for and against muscle synergies. Curr. Opin. Neurobiol. 2009, 19, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Kutch, J.J.; Valero-Cuevas, F.J. Challenges and New Approaches to Proving the Existence of Muscle Synergies of Neural Origin. PLOS Comput. Biol. 2012, 8, e1002434. [Google Scholar] [CrossRef]
- Bizzi, E.; Cheung, V.C.K. The neural origin of muscle synergies. Front. Comput. Neurosci. 2013, 7, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.J.; Eskinazi, I.; Jackson, J.N.; Rao, A.V.; Patten, C.; Fregly, B.J. Muscle Synergies Facilitate Computational Prediction of {Subject-Specific} Walking Motions. Front. Bioeng. Biotechnol. 2016, 4, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappellini, G.; Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Motor Patterns in Human Walking and Running. J. Neurophysiol. 2006, 95, 3426–3437. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, H.; Ogawa, T.; Kawashima, N.; Shinya, M.; Nakazawa, K. Distinct sets of locomotor modules control the speed and modes of human locomotion. Sci. Rep. 2016, 6, 36275. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Poppele, R.E.; Lacquaniti, F. Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 2004, 556, 267–282. [Google Scholar] [CrossRef]
- Scano, A.; Dardari, L.; Molteni, F.; Giberti, H.; Tosatti, L.M.; d’Avella, A. A Comprehensive Spatial Mapping of Muscle Synergies in Highly Variable {Upper-Limb} Movements of Healthy Subjects. Front. Physiol. 2019, 10, 1231. [Google Scholar] [CrossRef]
- D’Avella, A.; Fernandez, L.; Portone, A.; Lacquaniti, F. Modulation of phasic and tonic muscle synergies with reaching direction and speed. J. Neurophysiol. 2008, 100, 1433–1454. [Google Scholar] [CrossRef] [PubMed]
- Shaharudin, S.; Agrawal, S. Muscle synergies during incremental rowing {VO2max} test of collegiate rowers and untrained subjects. J. Sports Med. Phys. Fit. 2016, 56, 980–989. [Google Scholar]
- Kristiansen, M.; Samani, A.; Madeleine, P.; Hansen, E.A. Muscle synergies during bench press are reliable across days. J. Electromyogr. Kinesiol. 2016, 30, 81–88. [Google Scholar] [CrossRef]
- Vaz, J.R.; Olstad, B.H.; Cabri, J.; Kjendlie, P.-L.; Pezarat-Correia, P.; Hug, F. Muscle coordination during breaststroke swimming: Comparison between elite swimmers and beginners. J. Sports Sci. 2016, 34, 1941–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazifi, M.M.; Yoon, H.U.; Beschorner, K.; Hur, P. Shared and {Task-Specific} Muscle Synergies during Normal Walking and Slipping. Front. Hum. Neurosci. 2017, 11, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santuz, A.; Ekizos, A.; Eckardt, N.; Kibele, A.; Arampatzis, A. Challenging human locomotion: Stability and modular organisation in unsteady conditions. Sci. Rep. 2018, 8, 2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Logan, D.; Giszter, S.F. Motor primitives are determined in early development and are then robustly conserved into adulthood. Proc. Natl. Acad. Sci. USA 2019, 116, 12025–12034. [Google Scholar] [CrossRef] [Green Version]
- Cheung, V.C.K.; Cheung, B.M.F.; Zhang, J.H.; Chan, Z.Y.S.; Ha, S.C.W.; Chen, C.-Y.; Cheung, R.T.H. Plasticity of muscle synergies through fractionation and merging during development and training of human runners. Nat. Commun. 2020, 11, 4356. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, S.A.; Ting, L.H. Common muscle synergies for balance and walking. Front. Comput. Neurosci. 2013, 7, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, G.; Ivanenko, Y.P.; d’Avella, A.; Serrao, M.; Ranavolo, A.; Draicchio, F.; Cappellini, G.; Casali, C.; Lacquaniti, F. Neuromuscular adjustments of gait associated with unstable conditions. J. Neurophysiol. 2015, 114, 2867–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Avella, A.; Bizzi, E. Shared and specific muscle synergies in natural motor behaviors. Proc. Natl. Acad. Sci. USA 2005, 102, 3076–3081. [Google Scholar] [CrossRef] [Green Version]
- Hagio, S.; Kouzaki, M. The flexible recruitment of muscle synergies depends on the required force-generating capability. J. Neurophysiol. 2014, 112, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Barroso, F.O.; Torricelli, D.; Moreno, J.C.; Taylor, J.; Gomez-Soriano, J.; Bravo-Esteban, E.; Piazza, S.; Santos, C.; Pons, J.L. Shared muscle synergies in human walking and cycling. J. Neurophysiol. 2014, 112, 1984–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartori, M.; Gizzi, L.; Lloyd, D.G.; Farina, D. A musculoskeletal model of human locomotion driven by a low dimensional set of impulsive excitation primitives. Front. Comput. Neurosci. 2013, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- David, L.; Gallahue, O.; Goodway, J.C.; Jacqueline, D. Understanding Motor Development: Infants, Children, Adolescents, Adults; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Huang, B.; Xiong, C.; Chen, W.; Liang, J.; Sun, B.-Y.; Gong, X. Common kinematic synergies of various human locomotor behaviours. R. Soc. Open Sci. 2021, 8, 210161. [Google Scholar] [CrossRef]
- Cook, G.; Burton, L.; Hoogenboom, B.J.; Voight, M. Functional movement screening: The use of fundamental movements as an assessment of function—Part 2. Int. J. Sports Phys. Ther. 2014, 9, 549–563. [Google Scholar] [PubMed]
- Cook, G.; Burton, L.; Hoogenboom, B.J.; Voight, M. Functional movement screening: The use of fundamental movements as an assessment of function—Part 1. Int. J. Sports Phys. Ther. 2014, 9, 396–409. [Google Scholar] [PubMed]
- Hug, F. Can muscle coordination be precisely studied by surface electromyography? J. Electromyogr. Kinesiol. 2011, 21, 1–12. [Google Scholar] [CrossRef]
- Ranaldi, S.; De Marchis, C.; Conforto, S. An automatic, adaptive, information-based algorithm for the extraction of the {sEMG} envelope. J. Electromyogr. Kinesiol. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- De Marchis, C.; Ranaldi, S.; Serrao, M.; Ranavolo, A.; Draicchio, F.; Lacquaniti, F.; Conforto, S. Modular motor control of the sound limb in gait of people with trans-femoral amputation. J. Neuroeng. Rehabil. 2019, 16, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Y.; Deng, H.; Samuel, O.W.; Cheung, V.; Xu, L.; Li, G. Modulation of muscle synergies for multiple forearm movements under variant force and arm position constraints. J. Neural Eng. 2020, 17, 026015. [Google Scholar] [CrossRef]
- Tresch, M.C.; Cheung, V.C.K.; d’Avella, A. Matrix factorization algorithms for the identification of muscle synergies: Evaluation on simulated and experimental data sets. J. Neurophysiol. 2006, 95, 2199–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, S.A.; Torres-Oviedo, G.; Safavynia, S.A.; Ting, L.H. Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J. Neurophysiol. 2011, 106, 999–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Oviedo, G.; Macpherson, J.M.; Ting, L.H. Muscle synergy organization is robust across a variety of postural perturbations. J. Neurophysiol. 2006, 96, 1530–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zar, J.H. Biostatistical Analysis; Pearson Education: Karnataka, India, 1999. [Google Scholar]
- Frère, J.; Hug, F. Between-subject variability of muscle synergies during a complex motor skill. Front. Comput. Neurosci. 2012, 6, 99. [Google Scholar] [CrossRef] [Green Version]
- Delis, I.; Hilt, P.M.; Pozzo, T.; Panzeri, S.; Berret, B. Deciphering the functional role of spatial and temporal muscle synergies in whole-body movements. Sci. Rep. 2018, 8, 8391. [Google Scholar] [CrossRef]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Ser. B Stat. Methodol. 2001, 63, 411–423. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Turolla, A.; Agostini, M.; Silvoni, S.; Bennis, C.; Kasi, P.; Paganoni, S.; Bonato, P.; Bizzi, E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. USA 2012, 109, 14652–14656. [Google Scholar] [CrossRef] [Green Version]
- Hagio, S.; Fukuda, M.; Kouzaki, M. Identification of muscle synergies associated with gait transition in humans. Front. Hum. Neurosci. 2015, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Takei, T.; Confais, J.; Tomatsu, S.; Oya, T.; Seki, K. Neural basis for hand muscle synergies in the primate spinal cord. Proc. Natl. Acad. Sci. USA 2017, 114, 8643–8648. [Google Scholar] [CrossRef] [Green Version]
- Overduin, S.A.; d’Avella, A.; Carmena, J.M.; Bizzi, E. Microstimulation Activates a Handful of Muscle Synergies. Neuron 2012, 76, 1071–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, V.C.K.; Seki, K. Approaches to Revealing the Neural Basis of Muscle Synergies: A Review and A Critique. J. Neurophysiol. 2021, 125, 1580–1597. [Google Scholar] [CrossRef]
- Hodges, P.W.; Richardson, C.A. Contraction of the Abdominal Muscles Associated With Movement of the Lower Limb. Phys. Ther. 1997, 77, 132–142. [Google Scholar] [CrossRef]
- Ting, L.H.; Chiel, H.J.; Trumbower, R.D.; Allen, J.L.; McKay, J.L.; Hackney, M.E.; Kesar, T.M. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 2015, 86, 38–54. [Google Scholar] [CrossRef] [Green Version]
- Jacquelin Perry, J.M.B. Gait Analysis: Normal and Pathological Function, 2nd ed.; SLACK Incorporated: West Deptford, NJ, USA, 2010; ISBN 9781617114304. [Google Scholar]
- McCrea, D.A.; Rybak, I.A. Organization of mammalian locomotor rhythm and pattern generation. Brain Res. Rev. 2008, 57, 134–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, B.X.W.; Del Vecchio, A.; Falla, D. The influence of musculoskeletal pain disorders on muscle synergies—A systematic review. PLoS ONE 2018, 13, e0206885. [Google Scholar] [CrossRef]
- Bekius, A.; Bach, M.M.; van der Krogt, M.M.; de Vries, R.; Buizer, A.I.; Dominici, N. Muscle Synergies During Walking in Children With Cerebral Palsy: A Systematic Review. Front. Physiol. 2020, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Van Criekinge, T.; Vermeulen, J.; Wagemans, K.; Schröder, J.; Embrechts, E.; Truijen, S.; Hallemans, A.; Saeys, W. Lower limb muscle synergies during walking after stroke: A systematic review. Disabil. Rehabil. 2020, 42, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of muscle synergy outcomes in clinics, robotics, and sports: A systematic review. Appl. Bionics Biomech. 2018, 2018, 3934698. [Google Scholar] [CrossRef]
- Irastorza-Landa, N.; García-Cossio, E.; Sarasola-Sanz, A.; Brötz, D.; Birbaumer, N.; Ramos-Murguialday, A. Functional synergy recruitment index as a reliable biomarker of motor function and recovery in chronic stroke patients. J. Neural Eng. 2021, 18, 046061. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.-C.; Leung, W.-C.; Cheung, V.C.K.; Zhou, P.; Tong, K.-Y. Pathway-specific modulatory effects of neuromuscular electrical stimulation during pedaling in chronic stroke survivors. J. NeuroEng. Rehabil. 2019, 16, 1–15. [Google Scholar] [CrossRef]
- Niu, C.M.; Bao, Y.; Zhuang, C.; Li, S.; Wang, T.; Cui, L.; Xie, Q.; Lan, N. Synergy-Based FES for Post-Stroke Rehabilitation of Upper-Limb Motor Functions. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 256–264. [Google Scholar] [CrossRef]
- Ambrosini, E.; Parati, M.; Peri, E.; De Marchis, C.; Nava, C.; Pedrocchi, A.; Ferriero, G.; Ferrante, S. Changes in leg cycling muscle synergies after training augmented by functional electrical stimulation in subacute stroke survivors: A pilot study. J. Neuroeng. Rehabil. 2020, 17, 35. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Bao, Y.; Zhuang, C.; Li, S.; Wang, T.; Zhang, X.; Ma, Y.; Xuan, Z.; Gu, L.; Lan, N.; et al. Effectiveness of short-term training with a synergy-based FES paradigm on motor function recovery post-stroke. Ann. Phys. Rehabil. Med. 2018, 61, e33. [Google Scholar] [CrossRef]
- Kantak, S.S.; Zahedi, N.; McGrath, R. Complex Skill Training Transfers to Improved Performance and Control of Simpler Tasks After Stroke. Phys. Ther. 2017, 97, 718–728. [Google Scholar] [CrossRef]
- De Marchis, C.; Di Somma, J.; Zych, M.; Conforto, S.; Severini, G. Consistent visuomotor adaptations and generalizations can be achieved through different rotations of robust motor modules. Sci. Rep. 2018, 8, 12657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, K.M.; Tresch, M.C.; Perreault, E.J. The number and choice of muscles impact the results of muscle synergy analyses. Front. Comput. Neurosci. 2013, 7, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, V.C.K.; d’Avella, A.; Bizzi, E. Adjustments of motor pattern for load compensation via modulated activations of muscle synergies during natural behaviors. J. Neurophysiol. 2009, 101, 1235–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turpin, N.A.; Uriac, S.; Dalleau, G. How to improve the muscle synergy analysis methodology? Eur. J. Appl. Physiol. 2021, 121, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- Lemay, M.A.; Galagan, J.E.; Hogan, N.; Bizzi, E. Modulation and vectorial summation of the spinalized frog’s hindlimb end-point force produced by intraspinal electrical stimulation of the cord. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Yaron, A.; Kowalski, D.; Yaguchi, H.; Takei, T.; Seki, K. Forelimb force direction and magnitude independently controlled by spinal modules in the macaque. Proc. Natl. Acad. Sci. USA 2020, 117, 27655–27666. [Google Scholar] [CrossRef] [PubMed]



| Locomotion | 1 | Walk (1.5 m/s) | |
| 2 | Run (2.7 m/s) | ||
| 3 | Bilateral jump | ||
| 4 | Rt single leg jump | ||
| 5 | Lt single leg jump | ||
| 6 | Sit to stand to sit | ||
| Stability | Static Postures | 7 | Rt single leg stance |
| 8 | Lt single leg stance | ||
| Dynamic Postures | 9 | Deep squat | |
| 10 | Rt single leg squat | ||
| 11 | Lt single leg squat | ||
| 12 | Rt lunge | ||
| 13 | Lt lunge | ||
| 14 | Rocking backward | ||
| 15 | Rocking forward | ||
| 16 | Rt cross extension | ||
| 17 | Lt cross extension | ||
| 18 | Cat-and-dog | ||
| Axial | 19 | Forward bend | |
| 20 | Rt side bend | ||
| 21 | Lt side bend | ||
| 22 | Backward bend | ||
| 23 | Rt rotation | ||
| 24 | Lt rotation | ||
| Similarity (Normalized) | The Number of Samples | |
|---|---|---|
| W1 | 0.77 (±0.13) | 8 |
| W2 | 0.70 (±0.18) | 8 |
| W3 | 0.74 (±0.12) | 8 |
| W4 | 0.76 (±0.14) | 7 |
| W5 | 0.62 (±0.16) | 13 |
| W6 | 0.77 (±0.12) | 10 |
| W7 | 0.81 (±0.08) | 6 |
| W8 | 0.74 (±0.13) | 7 |
| W9 | 0.84 (±0.08) | 4 |
| W10 | 0.58 (±0.17) | 8 |
| W11 | 0.86 (±0.08) | 5 |
| W12 | 0.68 (±0.15) | 11 |
| W13 | 0.82 (±0.17) | 11 |
| Unilateral Patterns | Major Muscles | Minor Muscles | |
|---|---|---|---|
| Right Patterns | Left Patterns | ||
| W1 | W6 | ispTA, ispRF, ispVM | (ispESL2, ispEST9, ispEST1, conTA, conESL2, conEST9, conLD) |
| W2 | W7 | ispVM, ispRF, ispGM, ispGmed | (ispMG, ispOE, conBF, conOE, conESL2) |
| W3 | W8 | ispMG, ispGmed | (ispRF, ispVM, ispBF, ispGM, ispEST1, ispLD, conTA, conBF, contOE, conESL2) |
| W4 | W9 | ispBF | (ispMG, ispGM, ispOE, ispESL2, conESL2, conEST9) |
| W5 | W10 | ispEST9, ispLD | (ispOE, ispESL2, ispEST1, conBF, conGM, conGmed, conOE, conESL2, conEST9, conEST1, conLD) |
| Bilateral patterns | |||
| M11 | bilESL2 | (bilEST9, bilEST1) | |
| M12 | bilEST1 | (bilLD) | |
| M13 | bilRAS, bilOE | ||
| Movement and Postural Tasks | Walk | Run | BilJP SLS | RtSJP | Lt SJP | STS | Rt SLS | Lt SLS | DS | Rt SS | Lt SS | Rt LG | Lt LG | RB | RF | Rt CE | Lt CE | CD | FB | Rt SB | Lt SB | BB | Rt RT | Lt RT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of total synergy clusters | 4 | 4 | 4 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| Number of synergy clusters that are well matched by a single synergy cluster of all tasks | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Number of synergy clusters that are well matched by merging synergy clusters of all tasks | 4 | 4 | 4 | 3 | 3 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 |
| Number of synergy clusters that are unmatched by synergies of all tasks | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, H.; Yokoyama, H.; Sasaki, A.; Kato, T.; Nakazawa, K. Flexible Recruitments of Fundamental Muscle Synergies in the Trunk and Lower Limbs for Highly Variable Movements and Postures. Sensors 2021, 21, 6186. https://doi.org/10.3390/s21186186
Saito H, Yokoyama H, Sasaki A, Kato T, Nakazawa K. Flexible Recruitments of Fundamental Muscle Synergies in the Trunk and Lower Limbs for Highly Variable Movements and Postures. Sensors. 2021; 21(18):6186. https://doi.org/10.3390/s21186186
Chicago/Turabian StyleSaito, Hiroki, Hikaru Yokoyama, Atsushi Sasaki, Tatsuya Kato, and Kimitaka Nakazawa. 2021. "Flexible Recruitments of Fundamental Muscle Synergies in the Trunk and Lower Limbs for Highly Variable Movements and Postures" Sensors 21, no. 18: 6186. https://doi.org/10.3390/s21186186






