1. Introduction
Bone is the basic unit of the human skeletal system that provides the framework and bears the weight of the body, protects the vital organs and supports mechanical movement of human body. Bone consists of the protein collagen which is the fiber link with a calcium phosphate salt, a substance that makes bones strong. Calcium salts in bone is accounted for 99 percent with 1 percent is in the blood. Osteoporosis is a condition that causes weakening of the bones because the mineral calcium in the bones decreases. Osteoporosis is a cause of humpback and bone fragility. Osteoporosis is called the “deadly silence” because the symptoms of the disease will move forward all the time without warning before. The patient will know when the bone has broken already. Osteoporosis was found in women than men because women have bone mass less than men. When women are menopausal and hormone estrogen decrease, which helps calcium to hold the bone down.
Diagnosis of osteoporosis in general requires bone mineral density (BMD) and bone mineral content (BMC) measurement. There exist three different well-known modalities that can be used for bone mineral density and content measurement including X-ray, ultrasonic and microwave modality. X-ray modality based on absorptiometry is the most widely used modality that has captured researcher attention during the last decade. Ligesh et al. [
1] estimated bone mineral density using radiographic absorptiometry by taking a step-wedge bone phantom with digital X-ray. The measured gray-level values of all the steps as well as the corresponding known BMD values were used to create the calibration curve and later used for BMD estimation of the unknown gray level of the bone area. The disadvantage of the proposed technics of Ligesh et al. is that the technic is bone thickness and X-ray exposure parameter-setting dependent as only single energy X-ray is used. The calibration curve is then must be computed for each subject. Lee et al. [
2] estimated bone mineral density by analyzing trabecular pattern on the distal radial bone. They defined trabecular pattern parameter (TTP) in which ROI of distal radial image is divided into many sub-blocs. The gray level in each sub-bloc is then normalized to scale 0 to 1. An averaged intensity in each sub-bloc is computed. TTP is then defined by the average of the averaged intensity of all sub-bloc. R
2 of 0.92 is claimed for the linear relation between TTP and BMD. The proposed method of Lee et al. also suffered from bone thickness dependent and X-ray exposure parameter setting dependent. To solve the drawbacks of thickness dependent and X-ray exposure parameter setting dependent, modified Beer-Lambert law using two energy levels of X-ray are used to derive bone-mineral density [
3]. The technics is called dual-energy X-ray absorptiometry or DEXA. In general, to acquire two energy levels, a high and low source tube voltages is alternately supplied to X-ray generator. Dendere et al. [
4] proposed the system that includes an additional detector above the existing one in a slot-scanning radiography system to capture dual-energy images. The system successfully measured bone mineral density (BMD) of the middle phalanx of the middle finger with small error when compared with those from a commercial DEXA machine. Chiang et al. [
5] used cone beam computed tomography (CBCT) to acquire the gray level distribution of trabecular jawbone of patients in different ages and determines the threshold of loose bone mineral density. The technic is used as screening propose, i.e., if the average gray value of patient’s CBCT image is less than some threshold, the dentist may need to examine the patient with DEXA. Even though bone mineral measurement based on dual-energy X-ray absorptiometry is the gold standard recommended by World Health Organization, it only provides a 2-D projected measurement of the bone mineral density. For improved accuracy of bone mineral measurement, clinical computed tomography (CT) imaging can be used for measurement of volumetric bone mineral density. 3D bone mineral density measurement uses a series of K2HPO4 solution phantoms with various concentrations as a reference standard [
6]. The equivalent volumetric BMDs of the solution phantoms can be calculated by their known percent weights of K2HPO4. Regression analysis is then used to map the CT number with the volumetric BMD. Even though computed tomography imaging can provide more accurate estimation for bone mineral density, the technics suffered from extra radiation dosage delivered to the patient. More research on bone mineral density based on X-ray modalities can be found in [
7,
8,
9,
10].
Quantitative ultrasound (QUS) has become increasingly potential methods for measuring bone mineral density because of its advantages of low-cost, portable, easy to access, non-radiation-based modality and high precision. Moreover, as ultrasonic is mechanical wave, its propagation through the bone is affected by its physical structure especially bone composition and bone mass and hence can be used to assess bone. Normally, the investigations are performed on the heel bone of ulna bone as it mainly composed of a trabecular bone, which is easy to access. Lei et al. [
11] developed a high-speed synchronous acquisition of ultrasonic waves to acquire broadband ultrasonic attenuation (BUA) and speed of sound (SOS) and used them to diagnose and forecast the bone mass loss, osteoporosis. Samir et al. [
12] developed a prototype of ultrasound forearm bone densitometer to measure BMD. The prototype of the device was constructed using a pair of wideband, flat, composite ultrasonic transducers operating at a central frequency of 0.5 MHz. Ultrasonic transmit time and ultrasonic attenuation are used as the key parameters for bone mineral density estimation. The proposed technic demonstrated high sensitivity to bone mass as compared with peripheral DEXA bone densitometer and high accuracy to estimate the BMD at ulna bone region. Kaufman et al. [
13] developed a quantitative ultrasound device that measures two time-delay parameters. The first time-delay parameter is defined as the difference between the transit time of an ultrasound pulse to travel through soft-tissue, cortex and medullary cavity, and the transit time through soft tissue only of equal overall distance; where the second time-delay parameter is defined as the difference between the transit time of an ultrasound pulse to travel through soft-tissue and cortex only, and the transit time through soft tissue only again of equal overall distance. The square root of the product of these two time-delay parameters is then used to estimate bone mineral density of the radial bone. Validated with clinical DEXA UltraScan 650 model, the plot between the BMD measured from DEXA and the square root of the product of these two time-delay parameters demonstrates a linear relation with
R2 of 0.92. More research on QUS for bone density measurement can referred to [
14,
15,
16].
Microwave modality is an alternative promising technic that can be used for bone mineral density assessment. Microwave is an electromagnetic wave of which the energy is between ultrasound and X-ray. As microwave is a non-ionization radiation, it is hence healthier than X-ray and yet more penetrable than the ultrasound. The microwave modality has gain increasing attention recently due to the development of receiving microwave antenna and microwave source technology. Augustine et al. [
17] developed an ultra-wideband (UWB) measurement system for bone mineral evaluation. UWB frequencies from 1–8 GHz has been used and received reflected signal from the bone phantom. The resonant frequencies of the UWB Vivaldi antenna used in the research are highly dependent on dielectric properties of bone and hence can be used to evaluate bone mineral density. Kerketta and Ghosh [
18] developed a monopole UVB microwave antenna ranging from 3 GHz to 10 GHz to study on a level of bone mineral content. A change in relative attenuation of the microwave signal when passed through the bone is related bone mineral density. The experiment is conducted on animal bone phantom with various mass density. Cruz et al. [
19] designed a 2.4 GHz antenna to measure the relative attenuation of the transmitted microwave signal traversing through several samples of bovine bone, silica, and bone powder. Support vector machine is further applied to classify a sample as having low or high bone density. Barros et al. [
20] designed the 2.4 GHz microwave antenna to detect the levels of attenuation of the transmitted microwave through a phantom of various bone density. Wavelet Transform was applied to the receiving electromagnetic signals, and it can be used to extract relevant features of these signals for bone mineral classification. Even though microwave has gained increasing momentum for bone mineral assessment, the research is mostly conducted in the bone phantom. Furthermore, under current microwave antenna technology, the antenna with smaller size still cannot be developed. The limitation hampers the development of microwave imaging that can be used in in-vivo clinical application.
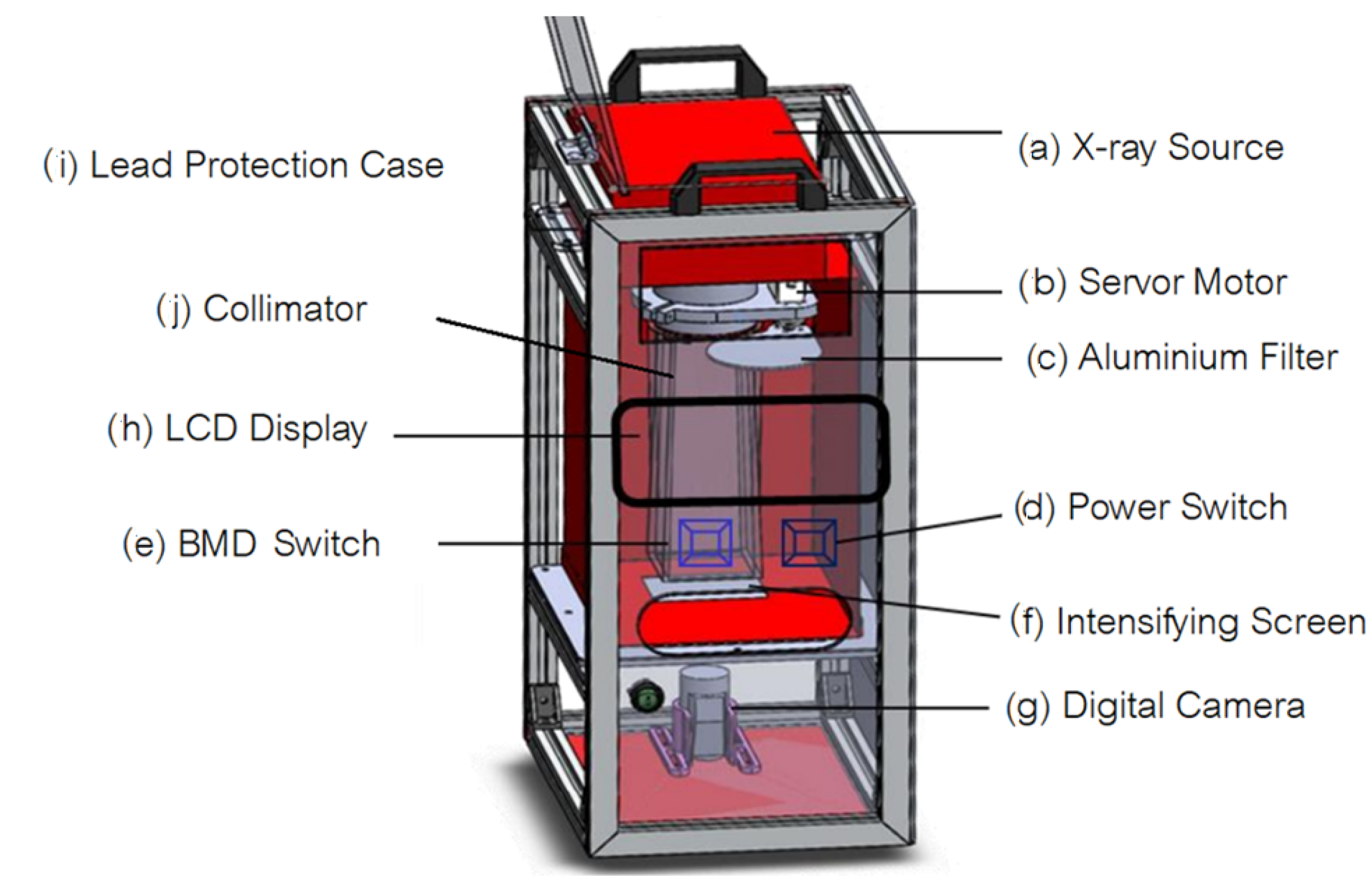
In our research, we developed a bone mineral density measurement system based on dual energy X-ray. The salient aspects and/or contributions of this paper are enumerated as follows.
- (1)
We develop a dedicated microcontroller digital X-ray apparatus that can be interfaced with personal computer to control the bone mineral density measurement process.
- (2)
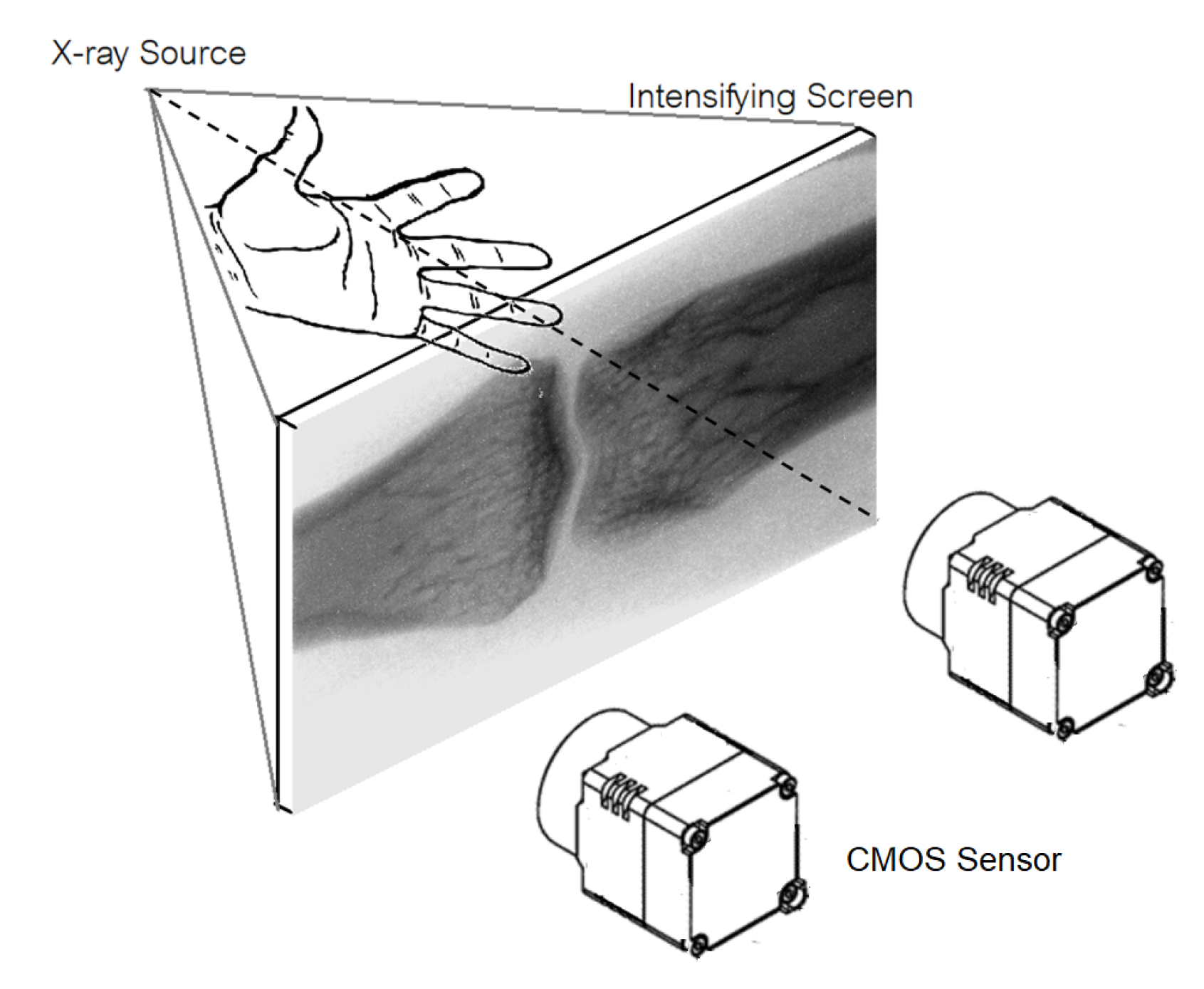
We design a low-cost upgradable indirect X-ray detector by using CMOS sensor mounted in a light-protective case to capture image on the X-ray phosphor intensify screen. By concatenating multiple CMOS sensors, resolution of our X-ray digital system can be increased comparable the commercial X-ray detector panel.
- (3)
When compared the commercial, our bone mineral measurement system is low cost and can be used as screening examination of bone mineral deficiency at district hospital.
- (4)
We optimized the setting X-ray exposure parameters such that the proposed bone mineral density measurement system based on dual energy X-ray delivers low dose to the subject. The effective dose is as low as that of the commercial.
- (5)
The portable bone mineral density measurement system based on dual energy X-ray is re-designed and constructed with the same concept proposed in this research.