VOC Detections by p-Type Semiconducting Sensors Using Nano-Sized SmFeO3 Particles
Abstract
:1. Introduction
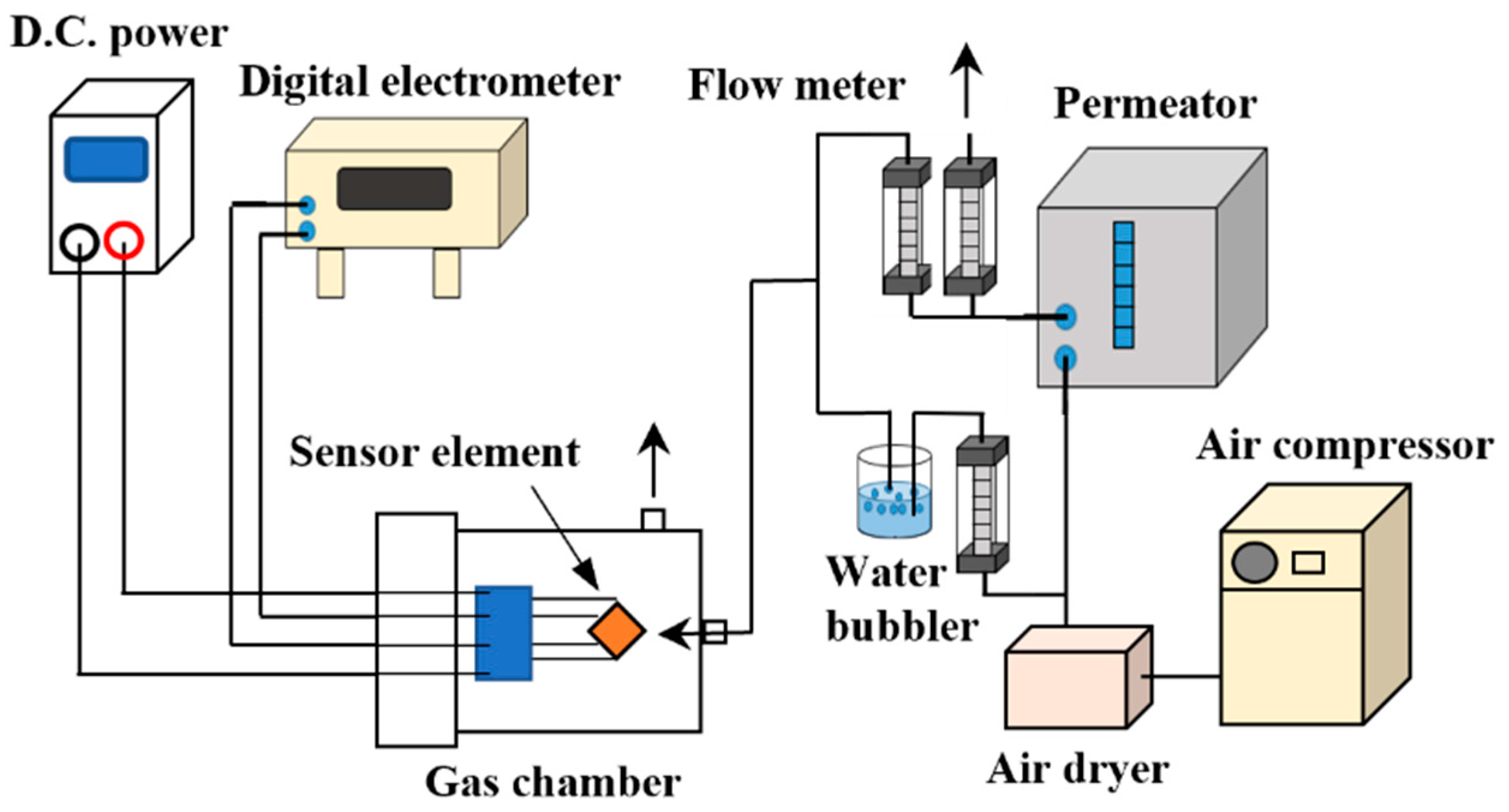
2. Materials and Methods
3. Results
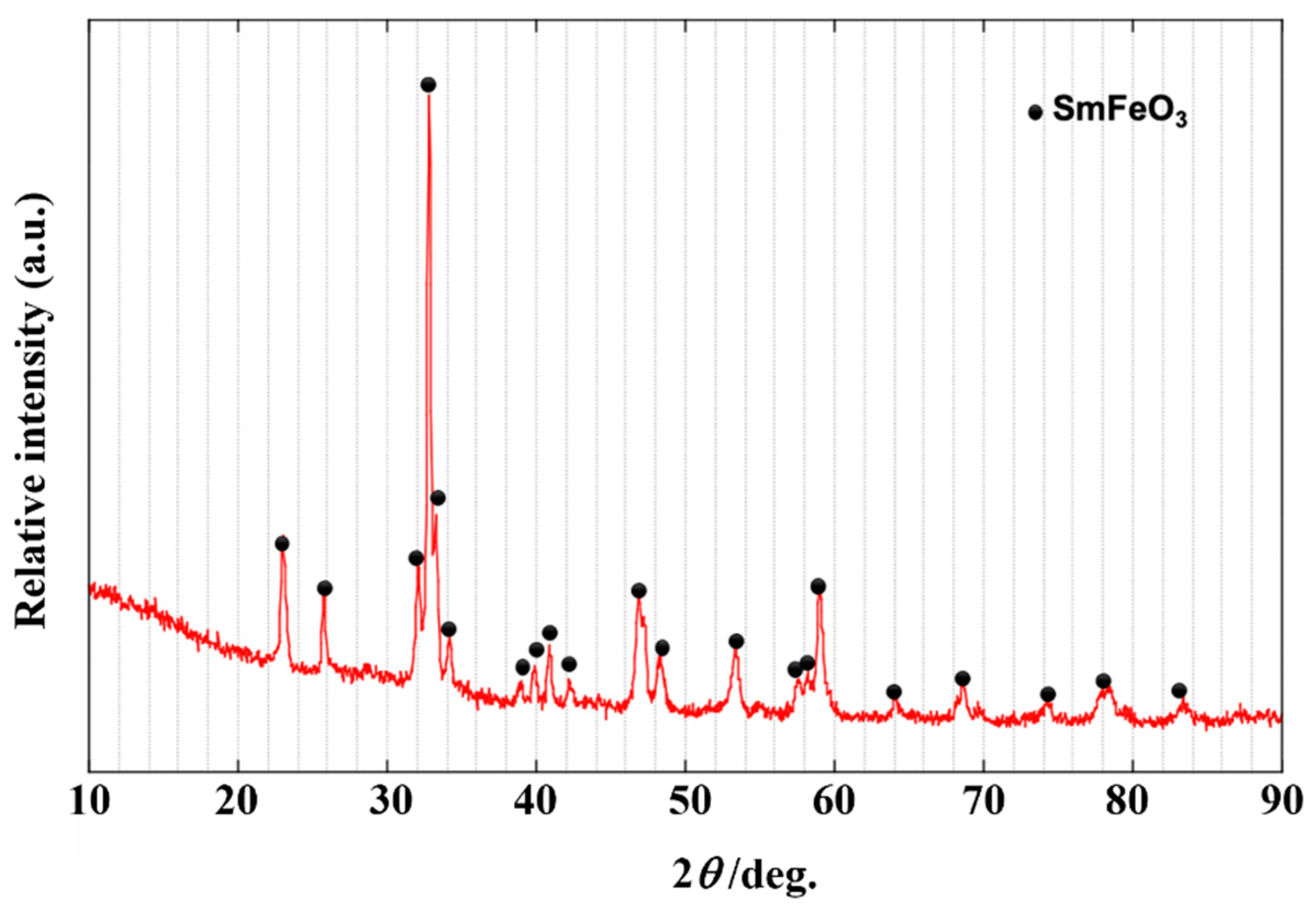
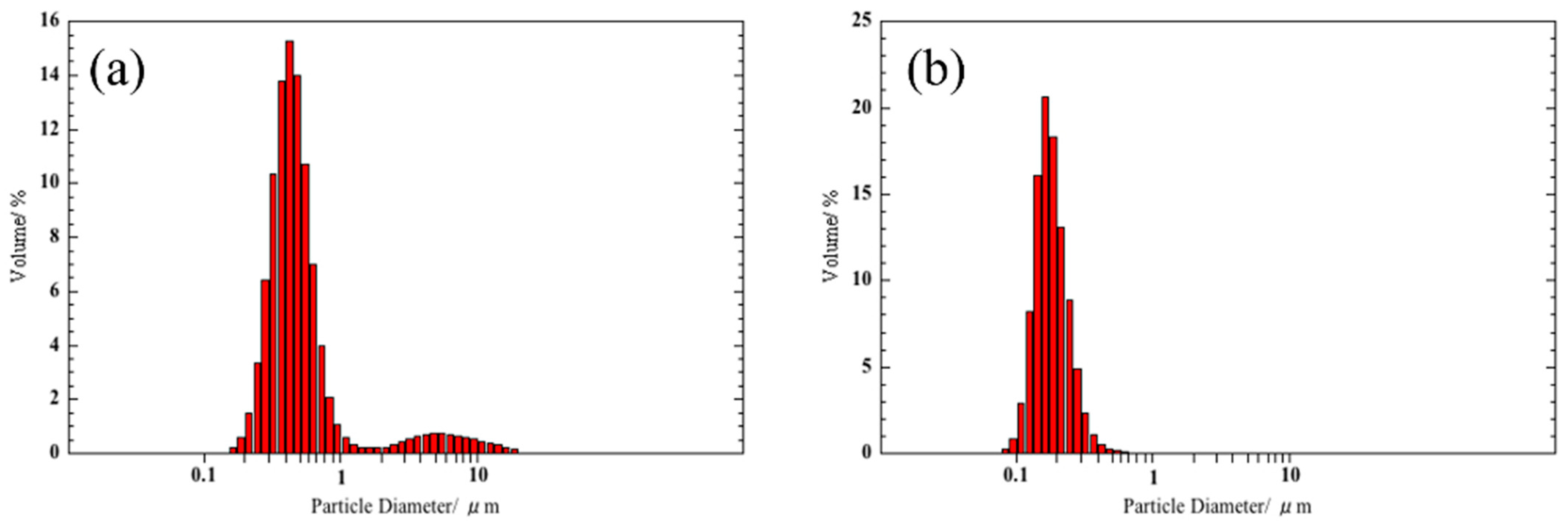
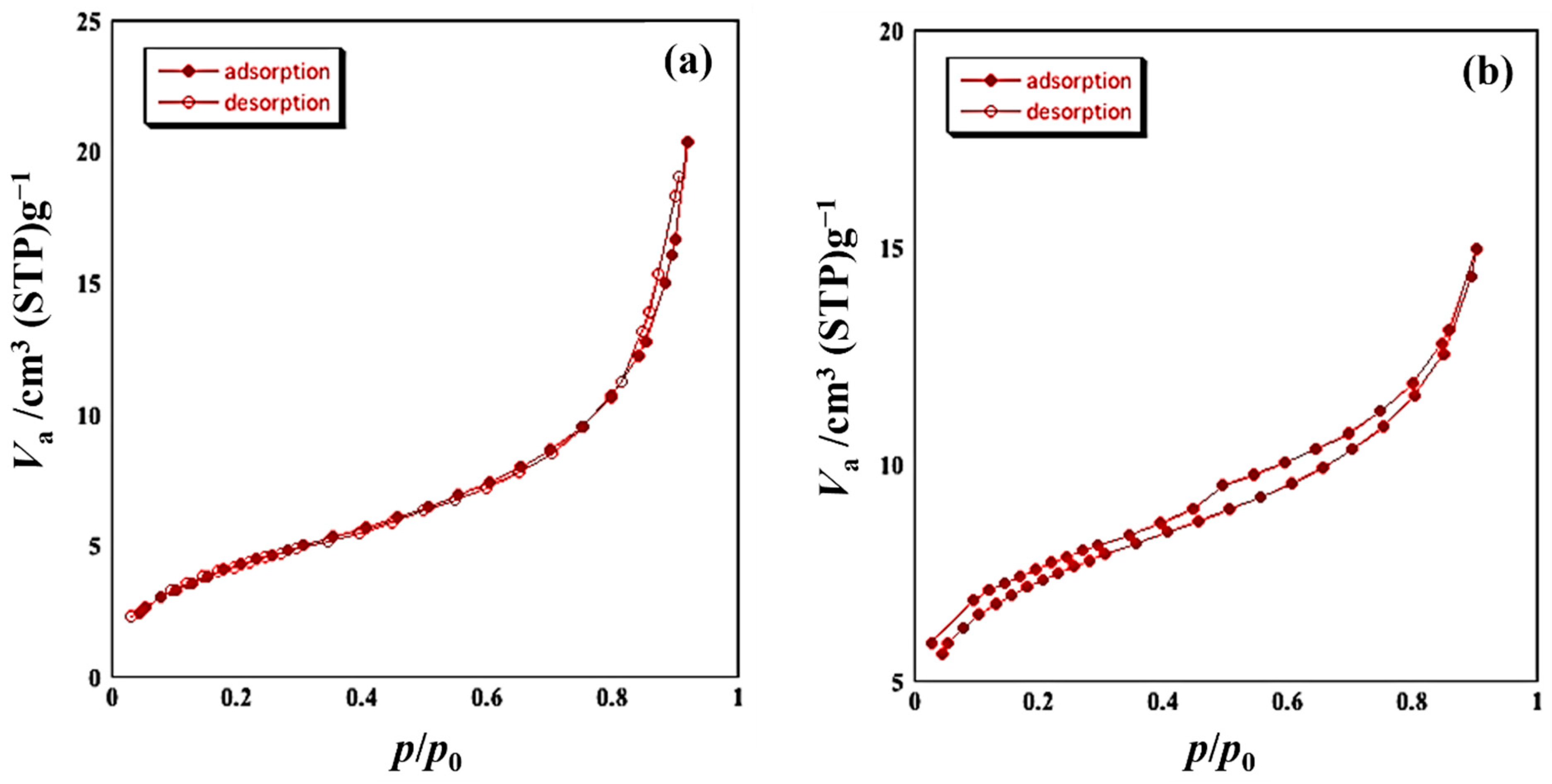
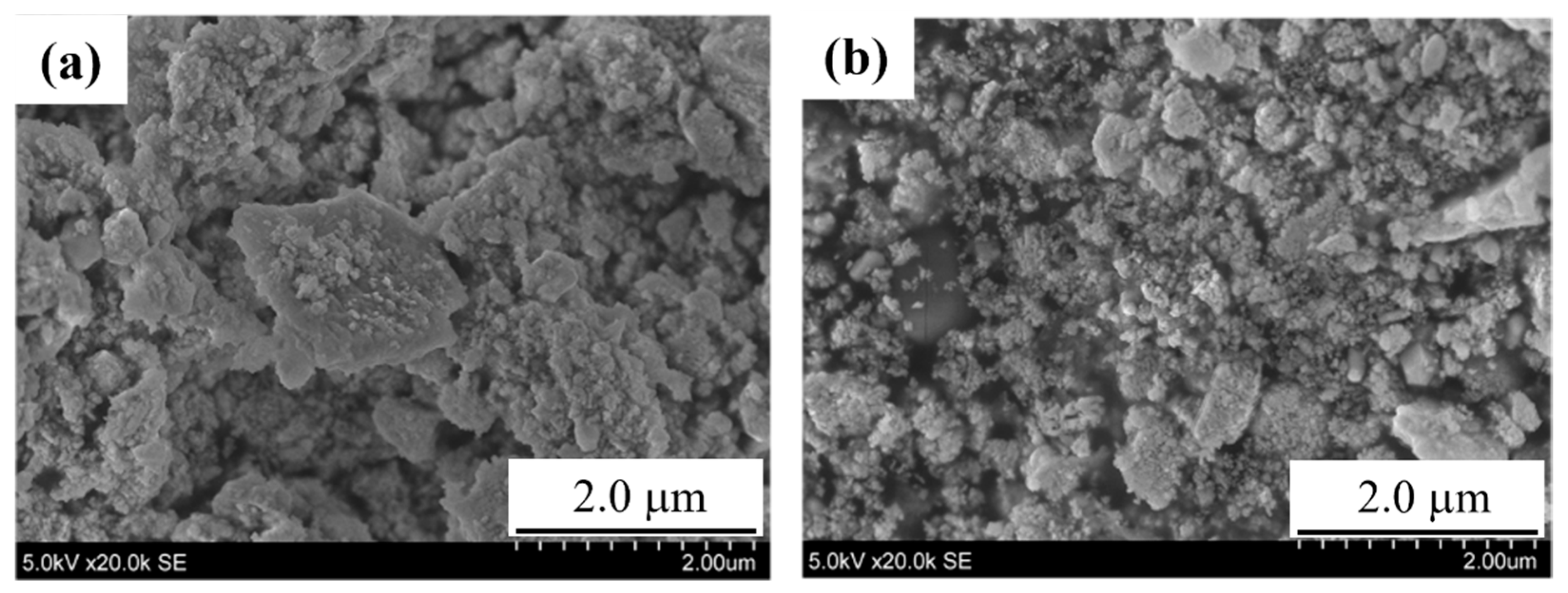
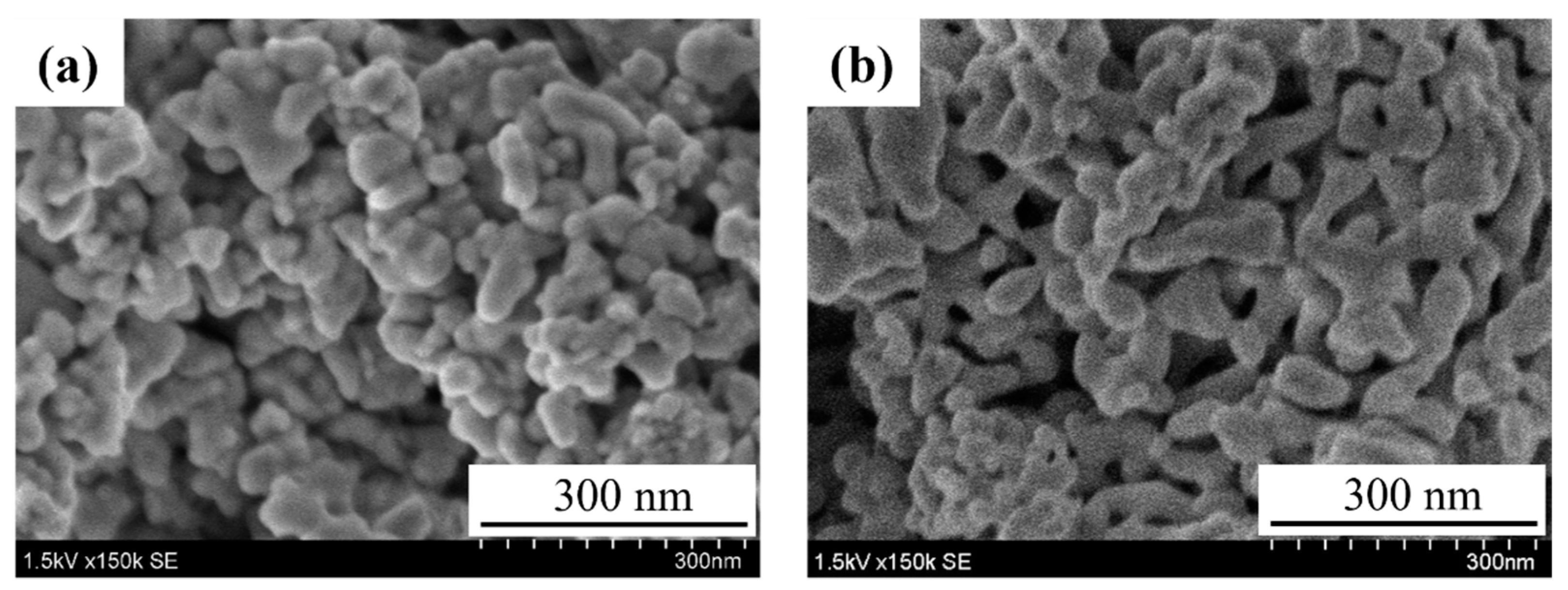
3.1. Preparation of SmFeO3 Nanoparticles
3.2. Evaluation of Sensor Response Characteristics
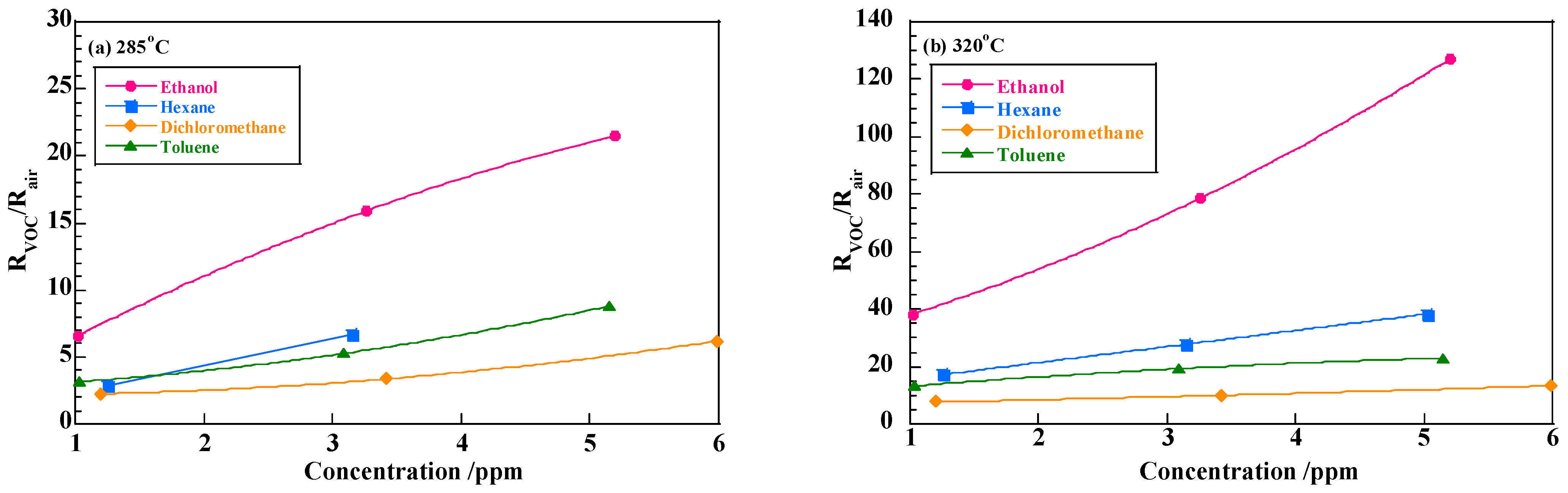
3.3. VOC Detection Using p-Type Semiconducting Oxides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A New Detector for Gaseous Components Using Semiconductive Thin Films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Matthias, B. Surface science studies of gas sensing materials: SnO2. Sensors 2006, 6, 1345–1366. [Google Scholar]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Mori, M.; Iwamoto, Y.; Asamoto, M.; Itagaki, Y.; Yahiro, H.; Sadaoka, Y.; Takase, S.; Shimizu, Y.; Yuasa, M.; Shimanoe, K.; et al. Effect of preparation routes on the catalytic activity over SmFeO3 oxide. Catal. Today 2008, 139, 125–129. [Google Scholar] [CrossRef]
- Hui, L.; Futai, M.; Zuzhu, M.; Haiyang, L.; Yabo, X. Study of the reactivity of surface oxygen species on SmMnO3 and SmFeO3 catalysts. J. Alloys Compd. 1993, 193, 68–69. [Google Scholar] [CrossRef]
- Toan, N.; Saukko, S.; Lantto, V. Gas sensing with semiconducting perovskite oxide LaFeO3. Phys. B Condens. Matter 2003, 327, 279–282. [Google Scholar] [CrossRef]
- Aono, H.; Ohmori, J.; Sadaoka, Y. Effects of sintering atmosphere on surface structure and electrical properties of LaFeO3 prepared by thermal decomposition of La[Fe(CN)6]⋅4H2O. J. Ceram. Soc. Jpn. 2000, 108, 892. [Google Scholar] [CrossRef]
- Aono, H.; Traversa, E.; Sakamoto, M.; Sadaoka, Y. Crystallographic characterization and NO2 gas sensing property of LaFeO3 prepared by thermal decomposition of Ln-Fe hexacyanocomplexes, Ln[Fe(CN)6]⋅4H2O, La, Ln = Nd, Sm, Gd, and Dy. Sens. Actuators B 2003, 94, 132. [Google Scholar] [CrossRef]
- Martinelli, G.; Carotta, M.C.; Ferroni, M.; Sadaoka, Y.; Traversa, E. Screen-printed perovskite-type thick films as gas sensors for environmental monitoring. Sens. Actuators B Chem. 1999, 55, 99–110. [Google Scholar] [CrossRef]
- Traversa, E.; Sadaoka, Y.; Carotta, M.C.; Martinelli, G. Environmental monitoring field tests using screen-printed thick-film sensors based on semiconducting oxides. Sens. Actuators B Chem. 2000, 65, 181. [Google Scholar] [CrossRef]
- Aono, H.; Sato, M.; Traversa, E.; Sakamoto, M.; Sadaoka, Y. Design of Ceramic Materials for Chemical Sensors: Effect of SmFeO3 Processing on Surface and Electrical Properties. J. Am. Ceram. Soc. 2004, 84, 341–347. [Google Scholar] [CrossRef]
- Carotta, M.C.; Martinelli, G.; Sadaoka, Y.; Nunziante, P.; Traversa, E. Gas-sensitive electrical properties of perovskite-type SmFeO3 thick films. Sens. Actuators B Chem. 1998, 48, 270–276. [Google Scholar] [CrossRef]
- Tomoda, M.; Okano, S.; Itagaki, Y.; Aono, H.; Sadaoka, Y. Air quality prediction by using semiconducting gas sensor with newly fabricated SmFeO3 film. Sens. Actuators B Chem. 2003, 97, 190–197. [Google Scholar] [CrossRef]
- Mori, M.; Itagaki, Y.; Iseda, J.; Sadaoka, Y.; Ueda, T.; Mitsuhashi, H.; Nakatani, M. Influence of VOC structures on sensing property of SmFeO3 semiconductive gas sensor. Sens. Actuators B Chem. 2014, 202, 873–877. [Google Scholar] [CrossRef]
- Hosoya, Y.; Itagaki, Y.; Aono, H.; Sadaoka, Y. Ozone detection in air using SmFeO3 gas sensor. Sens. Actuators B Chem. 2005, 108, 198–201. [Google Scholar] [CrossRef]
- Mathe, V.L. Structural, dielectric and electrical properties of SmxBi1−xFeO3 ceramics. J. Magn. Magn. Mater. 2003, 263, 344–352. [Google Scholar] [CrossRef]
- Ishihara, T.; Sato, K.; Takita, Y. Electrophoretic Deposition of Y2O3-Stabilized ZrO2 Electrolyte Films in Solid Oxide Fuel Cells. J. Am. Ceram. Soc. 1996, 79, 913–919. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Li, H.; Yu, K.; Lei, X.; Guo, B.; Li, C.; Fu, H.; Zhu, Z. Synthesis of the MoS2@CuO heterogeneous structure with improved photocatalysis performance and H2O adsorption analysis. Dalton Trans. 2015, 44, 10438–10447. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B Chem. 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Itagaki, Y.; Fujihashi, K.; Aono, H.; Mori, M.; Sadaoka, Y. VOC sensing behavior of semiconducting Sm2O3/SmFeO3 mixtures. J. Ceram. Soc. Jpn. 2015, 123, 961–966. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Tagliatesta, P. NiO Grained-Flowers and Nanoparticles for Ethanol Sensing. Materials 2020, 13, 1880. [Google Scholar] [CrossRef] [Green Version]
- Kruefu, V.; Wisitsoraat, A.; Phokharatkul, D.; Tuantranont, A.; Phanichphant, S. Enhancement of p-type gas-sensing performance of NiO nanoparticles prepared by precipitation with RiO2 impregnation. Sens. Actuators B Chem. 2016, 236, 466–473. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Liu, H.; Zhao, W.; Ming, A.; Wei, F. Preparation of porous Co3O4 and its response to ethanol with low energy consumption. RSC Adv. 2020, 10, 2191–2197. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Lee, C.-S.; Kim, B.-Y.; Kwak, C.-H.; Yoon, J.-W.; Jeong, H.-M.; Lee, J.-H. Honeycomb-like Periodic Porous LaFeO3 Thin Film Chemiresistors with Enhanced Gas-Sensing Performances. ACS Appl. Mater. Interfaces 2014, 6, 16217–16226. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Yuan, Q.; Yu, X.; Wang, D.; Chen, Y. Ag-Modified Porous Perovskite-Type LaFeO3 for Efficient Ethanol Detection. Nanomaterials 2020, 12, 1768. [Google Scholar] [CrossRef] [PubMed]

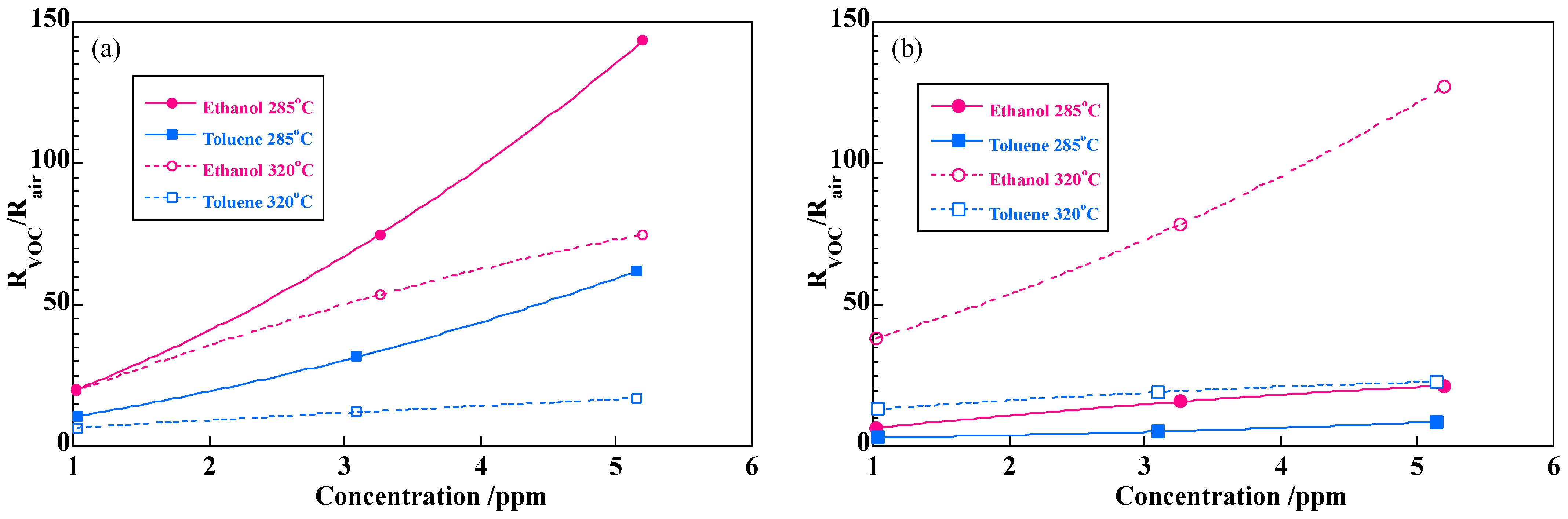

| Target Gas | Temp. (°C) | Sample A | Sample B | ||
|---|---|---|---|---|---|
| a | n | a | n | ||
| Ethanol | 285 | 19.3 | 1.19 | 6.55 | 0.732 |
| 320 | 19.4 | 0.833 | 36.9 | 0.714 | |
| Toluene | 285 | 10.2 | 1.07 | 2.99 | 0.609 |
| 320 | 6.49 | 0.576 | 13.2 | 0.337 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, M.; Noguchi, A.; Itagaki, Y. VOC Detections by p-Type Semiconducting Sensors Using Nano-Sized SmFeO3 Particles. Sensors 2022, 22, 5616. https://doi.org/10.3390/s22155616
Mori M, Noguchi A, Itagaki Y. VOC Detections by p-Type Semiconducting Sensors Using Nano-Sized SmFeO3 Particles. Sensors. 2022; 22(15):5616. https://doi.org/10.3390/s22155616
Chicago/Turabian StyleMori, Masami, Ayumu Noguchi, and Yoshiteru Itagaki. 2022. "VOC Detections by p-Type Semiconducting Sensors Using Nano-Sized SmFeO3 Particles" Sensors 22, no. 15: 5616. https://doi.org/10.3390/s22155616
APA StyleMori, M., Noguchi, A., & Itagaki, Y. (2022). VOC Detections by p-Type Semiconducting Sensors Using Nano-Sized SmFeO3 Particles. Sensors, 22(15), 5616. https://doi.org/10.3390/s22155616





