Using Sensor Graphs for Monitoring the Effect on the Performance of the OTAGO Exercise Program in Older Adults
Abstract
:1. Introduction
2. State of the Art
- ≤10 s: normal, no mobility impairment.
- 11–19 s: minor mobility impairment, not relevant in everyday life.
- 20–29 s: mobility impairment.
- >30 s: severe mobility impairment, need for intervention.
- Stance, semi-tandem stance, full-tandem stance (balance).
- Four-meter walk (gait speed).
- Five-time chair rise (lower-limb strength).
3. Materials and Methods
3.1. Study Design
- Age ≥ 75.
- At least pre-frail by the definition of Fried (Frailty Index ≥ 2) [40].
- Living alone within the city limits of Oldenburg.
- Able to move inside the flat as needed.
- Not able to move inside the flat as needed.
- Keeping pets that move freely inside the flat.
- Living with other people.
- Severe visual impairment.
- Contraindication against the OTAGO exercise program.
- Unable to understand the purpose of the study and the study itself.
3.2. Data Acquisition
3.3. Sensor Graph
3.4. Difference Function
3.5. Preprocessing
- Passed away during the study.
- Change in living situation.
- Incidents compromising mobility.
3.6. Statistical and Computational Methods
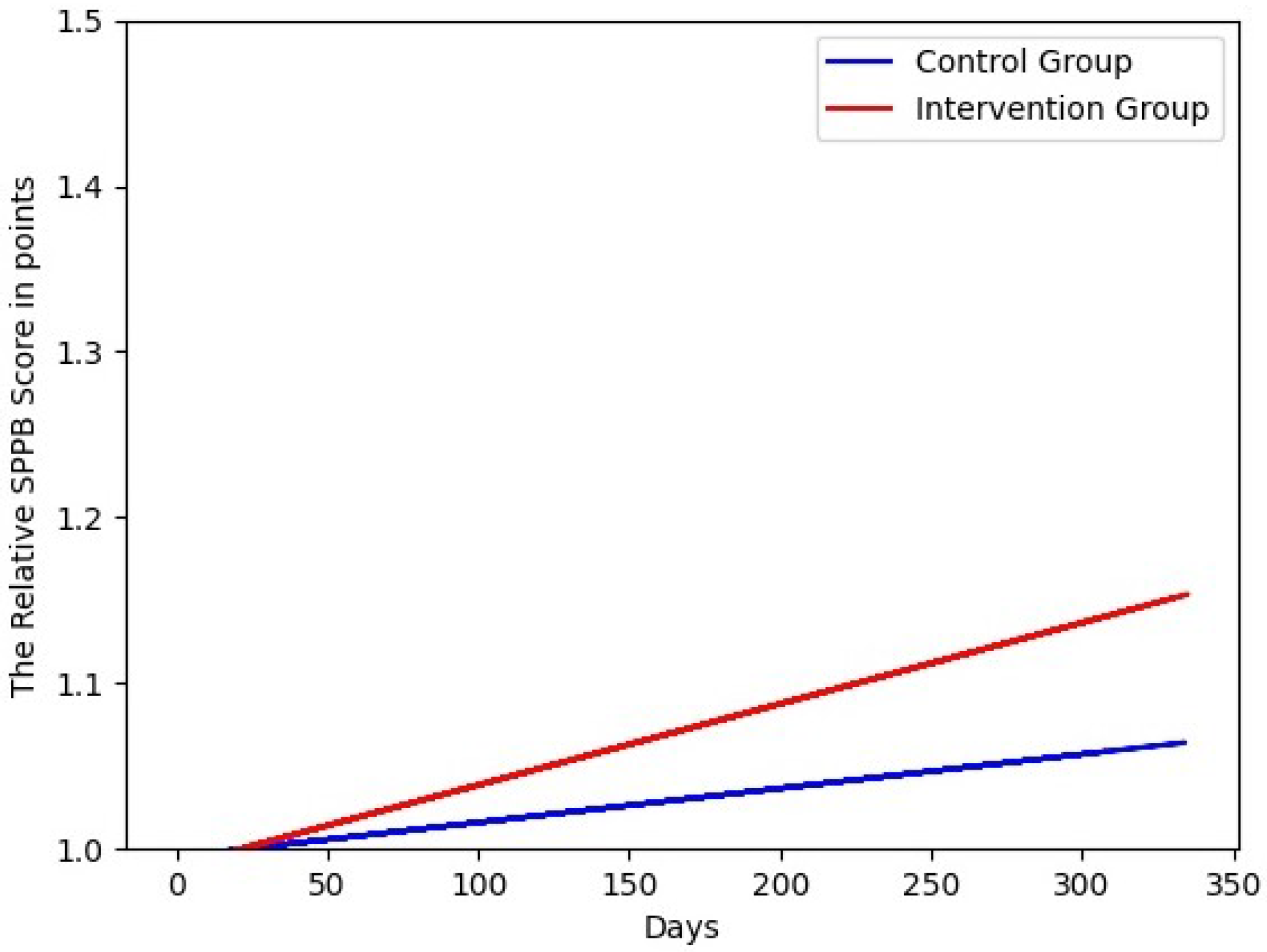
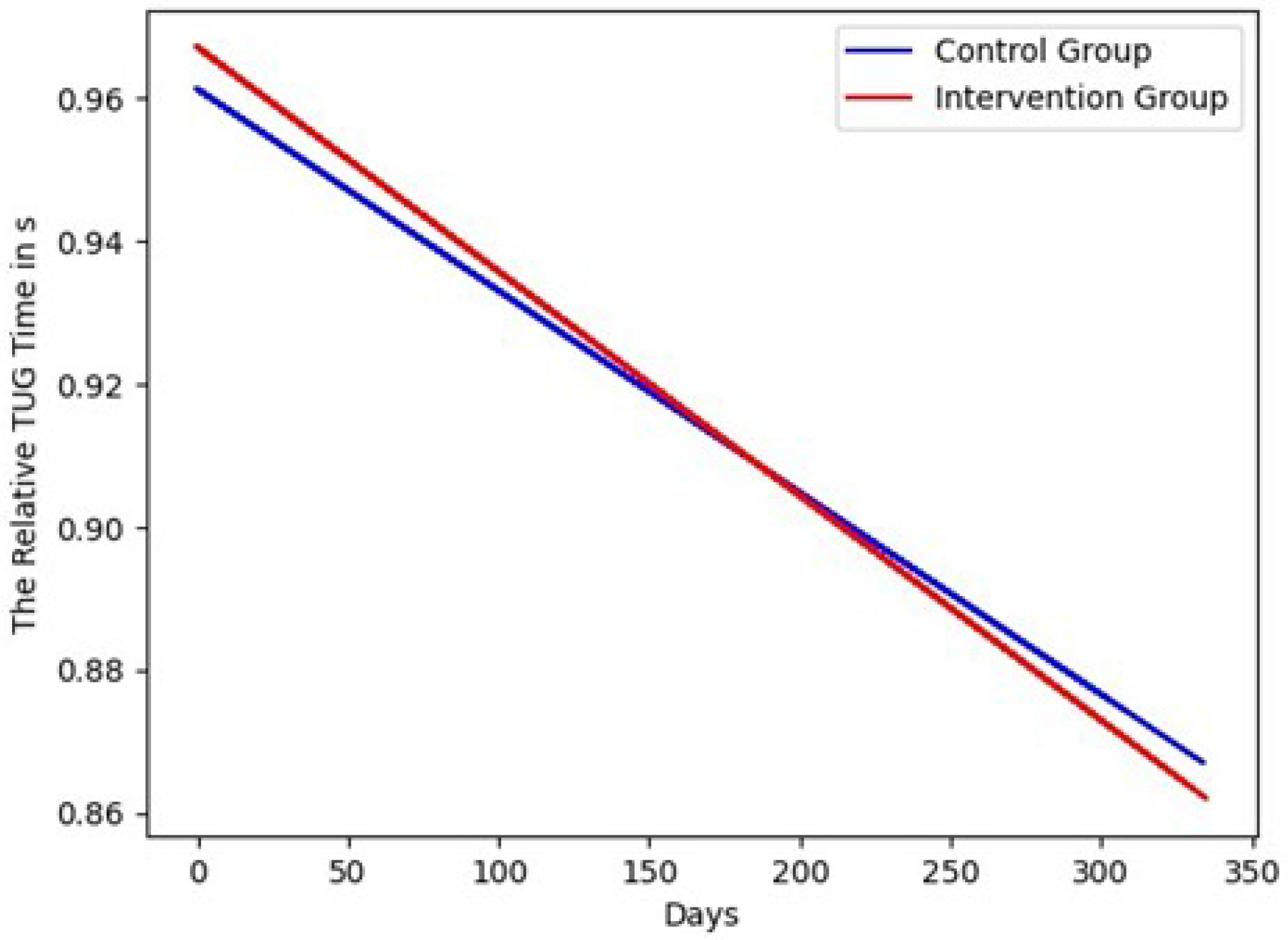
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GPS | Global Positioning System |
| IMU | Inertial measurement unit |
| PIR | Passive infrared |
| SD | Standard deviation |
| SPPB | Short physical performance battery |
| TUG | Timed Up and Go |
Appendix A
| Variable/Parameter | U | z | Middle Rank | Rank Sum |
|---|---|---|---|---|
| Age | 23.50 | −1.24 | 7.70/11.43 | 38.50/171.50 |
| Frailty index | 26.50 | −1.05 | 12.70/9.77 | 63.50/146.50 |
| SPPB | 14.00 | −2.08 | 5.80/12.07 | 29.00/181.00 |
| TUG | 25.00 | −1.09 | 13.00/9.67 | 65.00/145.00 |
| Variable/Parameter | U | z | Middle Rank | Rank Sum |
|---|---|---|---|---|
| Age | 48.00 | −0.15 | 10.70/10.30 | 107.00/103.00 |
| Frailty index | 50.00 | 0.00 | 10.50/10.50 | 105.00/105.00 |
| SPPB | 45.50 | −0.35 | 10.95/10.05 | 109.50/100.50 |
| TUG | 48.00 | −0.15 | 10.70/10.30 | 107.00/103.00 |
References
- Campbell, A.J.; Robertson, M.C. Comprehensive Approach to Fall Prevention on a National Level: New Zealand. Clin. Geriatr. Med. 2010, 26, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Jahanpeyma, P.; Kayhan Koçak, F.Ö.; Yıldırım, Y.; Şahin, S.; Şenuzun Aykar, F. Effects of the Otago exercise program on falls, balance, and physical performance in older nursing home residents with high fall risk: A randomized controlled trial. Eur. Geriatr. Med. 2021, 12, 107–115. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Adair, J.G. The Hawthorne effect: A reconsideration of the methodological artifact. J. Appl. Psychol. 1984, 69, 334–345. [Google Scholar] [CrossRef]
- Giannouli, E.; Bock, O.; Mellone, S.; Zijlstra, W. Mobility in Old Age: Capacity Is Not Performance. BioMed Res. Int. 2016, 2016, 3261567. [Google Scholar] [CrossRef] [Green Version]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Peel, N.M.; Kuys, S.S.; Klein, K. Gait Speed as a Measure in Geriatric Assessment in Clinical Settings: A Systematic Review. J. Gerontol. Ser. A 2012, 68, 39–46. [Google Scholar] [CrossRef]
- Middleton, A.; Fulk, G.D.; Beets, M.W.; Herter, T.M.; Fritz, S.L. Self-Selected Walking Speed Is Predictive of Daily Ambulatory Activity in Older Adults. J. Aging Phys. Act. 2016, 24, 214–222. [Google Scholar] [CrossRef] [Green Version]
- da Câmara, S.M.A.; Alvarado, B.E.; Guralnik, J.M.; Guerra, R.O.; Maciel, Á.C.C. Using the Short Physical Performance Battery to screen for frailty in young-old adults with distinct socioeconomic conditions. Geriatr. Gerontol. Int. 2013, 13, 421–428. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Boolani, A.; Ryan, J.; Vo, T.; Wong, B.; Banerjee, N.K.; Banerjee, S.; Fulk, G.; Smith, M.L.; Martin, R. Do Changes in Mental Energy and Fatigue Impact Functional Assessments Associated with Fall Risks? An Exploratory Study Using Machine Learning. Phys. Occup. Ther. Geriatr. 2020, 38, 283–301. [Google Scholar] [CrossRef]
- Hellmers, S.; Izadpanah, B.; Dasenbrock, L.; Diekmann, R.; Bauer, J.M.; Hein, A.; Fudickar, S. Towards an Automated Unsupervised Mobility Assessment for Older People Based on Inertial TUG Measurements. Sensors 2018, 18, 3310. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, B.; Lau, S.; Elgert, L.; Bauer, J.M.; Hein, A. A Deep Learning Approach for TUG and SPPB Score Prediction of (Pre-) Frail Older Adults on Real-Life IMU Data. Healthcare 2021, 9, 149. [Google Scholar] [CrossRef]
- Fudickar, S.; Kiselev, J.; Frenken, T.; Wegel, S.; Dimitrowska, S.; Steinhagen-Thiessen, E.; Hein, A. Validation of the ambient TUG chair with light barriers and force sensors in a clinical trial. Assist. Technol. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Jung, H.W.; Roh, H.; Cho, Y.; Jeong, J.; Shin, Y.S.; Lim, J.Y.; Guralnik, J.M.; Park, J. Validation of a Multi–Sensor-Based Kiosk for Short Physical Performance Battery. J. Am. Geriatr. Soc. 2019, 67, 2605–2609. [Google Scholar] [CrossRef] [PubMed]
- Vargemidis, D.; Gerling, K.; Spiel, K.; Abeele, V.V.; Geurts, L. Wearable Physical Activity Tracking Systems for Older Adults—A Systematic Review. ACM Trans. Comput. Healthc. 2020, 1, 1–37. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsu, Y.L. A Review of Accelerometry-Based Wearable Motion Detectors for Physical Activity Monitoring. Sensors 2010, 10, 7772–7788. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; O’Shea, E.; Kenny, L.; Barton, J.; Tedesco, S.; Sica, M.; Crowe, C.; Alamäki, A.; Condell, J.; Nordström, A.; et al. Older Adults’ Experiences With Using Wearable Devices: Qualitative Systematic Review and Meta-synthesis. JMIR Mhealth Uhealth 2021, 9, e23832. [Google Scholar] [CrossRef]
- Byun, S.; Lee, H.J.; Han, J.W.; Kim, J.S.; Choi, E.; Kim, K.W. Walking-speed estimation using a single inertial measurement unit for the older adults. PLoS ONE 2019, 14, e0227075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, S.S.; Park, G.Y. Accuracy Verification of Spatio-Temporal and Kinematic Parameters for Gait Using Inertial Measurement Unit System. Sensors 2020, 20, 1343. [Google Scholar] [CrossRef] [Green Version]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Nouredanesh, M.; Godfrey, A.; Howcroft, J.; Lemaire, E.D.; Tung, J. Fall risk assessment in the wild: A critical examination of wearable sensor use in free-living conditions. Gait Posture 2021, 85, 178–190. [Google Scholar] [CrossRef]
- Yu, S.; Chen, H.; Brown, R.; Sherman, S. Motion Sensor-Based Assessment on Fall Risk and Parkinson’s Disease Severity: A Deep Multi-Source Multi-Task Learning (DMML) Approach. In Proceedings of the 2018 IEEE International Conference on Healthcare Informatics (ICHI), New York, NY, USA, 4–7 June 2018; pp. 174–179. [Google Scholar] [CrossRef]
- Nait Aicha, A.; Englebienne, G.; Van Schooten, K.S.; Pijnappels, M.; Kröse, B. Deep Learning to Predict Falls in Older Adults Based on Daily-Life Trunk Accelerometry. Sensors 2018, 18, 1654. [Google Scholar] [CrossRef] [Green Version]
- Luna-Perejón, F.; Domínguez-Morales, M.J.; Civit-Balcells, A. Wearable Fall Detector Using Recurrent Neural Networks. Sensors 2019, 19, 4885. [Google Scholar] [CrossRef] [Green Version]
- Tunca, C.; Salur, G.; Ersoy, C. Deep Learning for Fall Risk Assessment With Inertial Sensors: Utilizing Domain Knowledge in Spatio-Temporal Gait Parameters. IEEE J. Biomed. Health Inf. 2020, 24, 1994–2005. [Google Scholar] [CrossRef]
- Meyer, B.M.; Tulipani, L.J.; Gurchiek, R.D.; Allen, D.A.; Adamowicz, L.; Larie, D.; Solomon, A.J.; Cheney, N.; McGinnis, R.S. Wearables and Deep Learning Classify Fall Risk From Gait in Multiple Sclerosis. IEEE J. Biomed. Health Inf. 2021, 25, 1824–1831. [Google Scholar] [CrossRef]
- Luna-Perejón, F.; Domínguez-Morales, M.; Gutiérrez-Galán, D.; Civit-Balcells, A. Low-Power Embedded System for Gait Classification Using Neural Networks. J. Low Power Electron. Appl. 2020, 10, 14. [Google Scholar] [CrossRef]
- Jung, D.; Nguyen, M.; Park, M.; Kim, M.; Won, C.; Kim, J.; Mun, K. Walking-in-Place Characteristics-Based Geriatric Assessment Using Deep Convolutional Neural Networks. In Proceedings of the 42nd Annual International Conferences of the IEEE Engineering in Medicine and Biology Society, Montreal, QC, Canada, 20–24 July 2020; pp. 3931–3935. [Google Scholar] [CrossRef]
- Kiprijanovska, I.; Gjoreski, H.; Gams, M. Detection of Gait Abnormalities for Fall Risk Assessment Using Wrist-Worn Inertial Sensors and Deep Learning. Sensors 2020, 20, 5373. [Google Scholar] [CrossRef]
- Marschollek, M.; Becker, M.; Bauer, J.M.; Bente, P.; Dasenbrock, L.; Elbers, K.; Hein, A.; Kolb, G.; Künemund, H.; Lammel-Polchau, C.; et al. Multimodal activity monitoring for home rehabilitation of geriatric fracture patients—Feasibility and acceptance of sensor systems in the GAL-NATARS study. Inf. Health Soc. Care 2014, 39, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Pol, M.; van Nes, F.; van Hartingsveldt, M.; Buurman, B.; de Rooij, S.; Kröse, B. Older People’s Perspectives Regarding the Use of Sensor Monitoring in Their Home. Gerontologist 2014, 56, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nait Aicha, A.; Englebienne, G.; Kröse, B. Continuous measuring of the indoor walking speed of older adults living alone. J. Ambient Intell. Humaniz. Comput. 2018, 9, 589–599. [Google Scholar] [CrossRef]
- Chapron, K.; Bouchard, K.; Gaboury, S. Real-time gait speed evaluation at home in a multi residents context. Multimed. Tools Appl. 2021, 80, 12931–12949. [Google Scholar] [CrossRef]
- Frenken, T.; Steen, E.-E.; Brell, M.; Nebel, W.; Hein, A. Motion Pattern Generation and Recognition for Mobility Assessments in Domestic Environments. In Proceedings of the 1st International Living Usability Lab Workshop on AAL Latest Solutions, Trends and Applications, Rome, Italy, 26–29 January 2011; pp. 3–12. [Google Scholar]
- Piau, A.; Mattek, N.; Crissey, R.; Beattie, Z.; Dodge, H.; Kaye, J. When Will My Patient Fall? Sensor-Based In-Home Walking Speed Identifies Future Falls in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.A.; Maxwell, S.A.; Mattek, N.; Hayes, T.L.; Dodge, H.; Pavel, M.; Jimison, H.B.; Wild, K.; Boise, L.; Zitzelberger, T.A. Intelligent Systems For Assessing Aging Changes: Home-based, unobtrusive, and continuous assessment of aging. J. Gerontol. Ser. Psychol. Sci. Soc. Sci. 2011, 66 (Suppl. 1), i180–i190. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Liu, Y.; Kabelac, Z.; Hristov, R.; Katabi, D.; Liu, C. Extracting Gait Velocity and Stride Length from Surrounding Radio Signals. In Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems, Denver, CO, USA, 6–11 May 2017; Association for Computing Machinery: New York, NY, USA, 2017; pp. 2116–2126. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Tinetti, M.E. Performance-Oriented Assessment of Mobility Problems in Elderly Patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.F.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Columbus. V-990 Multifunction GPS Data Logger User Manual. Available online: https://cbgps.com/download/Columbus_V-990_User_Manual_V1.0_ENG.pdf (accessed on 2 May 2020).
- Motion, S.D. Shimmer3 Wireless Sensor Platform. Available online: http://www.shimmersensing.com/images/uploads/docs/Shimmer3_Spec_Sheet_V1.8.pdf (accessed on 2 May 2020).



| n = 20/18 (m = 3/3, f = 17/15) | Age (y) | Frailty Index (Points) | SPPB (Points) | TUG (s) |
|---|---|---|---|---|
| Mean | 84.75/85.44 | 1.90/2.00 | 5.95/6.61 | 17.87/16.12 |
| SD | 5.19/4.92 | 0.72/0.97 | 2.33/2.85 | 5.33/5.85 |
| Range (min–max) | 76.00–92.00/ 77.00–93.00 | 1.00–3.00/ 0.00–4.00 | 3.00–11.00/ 2.00–12.00 | 11.16–31.63/ 8.15–30.06 |
| n = 15/15 (m = 1/1, f = 14/14) | Age (y) | Frailty Index (Points) | SPPB (Points) | TUG (s) |
|---|---|---|---|---|
| Mean | 84.60/85.20 | 1.80/1.73 | 6.53/7.07 | 16.81/14.87 |
| SD | 5.57/5.41 | 0.68/0.80 | 2.36/3.01 | 4.35/5.82 |
| Range (min–max) | 76.00–92.00/ 77.00–93.00 | 1.00–3.00/ 0.00–3.00 | 3.00–11.00/ 2.00–12.00 | 11.16–24.06/ 8.15–30.06 |
| Bedroom | Bathroom | Kitchen | Living Room | Hallway | |
|---|---|---|---|---|---|
| Bedroom | 45.14 | 0 | 0.14 | 34.84 | 1.86 |
| Bathroom | 0 | 114.29 | 0 | 1 | 22.86 |
| Kitchen | 0 | 0 | 144.86 | 38.14 | 0.43 |
| Living room | 35.14 | 1.29 | 38.43 | 294.43 | 50.71 |
| Hallway | 1.71 | 19.57 | 0.14 | 51.86 | 70 |
| Bedroom | Bathroom | Kitchen | Living Room | Hallway | |
|---|---|---|---|---|---|
| Bedroom | 45 | 0 | 0 | 34 | 1 |
| Bathroom | 0 | 98 | 0 | 1 | 25 |
| Kitchen | 0 | 0 | 196 | 39 | 1 |
| Living room | 32 | 1 | 39 | 230 | 68 |
| Hallway | 3 | 25 | 1 | 66 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, B.; Lübbe, C.; Steen, E.-E.; Bauer, J.M.; Hein, A. Using Sensor Graphs for Monitoring the Effect on the Performance of the OTAGO Exercise Program in Older Adults. Sensors 2022, 22, 493. https://doi.org/10.3390/s22020493
Friedrich B, Lübbe C, Steen E-E, Bauer JM, Hein A. Using Sensor Graphs for Monitoring the Effect on the Performance of the OTAGO Exercise Program in Older Adults. Sensors. 2022; 22(2):493. https://doi.org/10.3390/s22020493
Chicago/Turabian StyleFriedrich, Björn, Carolin Lübbe, Enno-Edzard Steen, Jürgen Martin Bauer, and Andreas Hein. 2022. "Using Sensor Graphs for Monitoring the Effect on the Performance of the OTAGO Exercise Program in Older Adults" Sensors 22, no. 2: 493. https://doi.org/10.3390/s22020493






