Waveguide-Enhanced Raman Spectroscopy (WERS): An Emerging Chip-Based Tool for Chemical and Biological Sensing
Abstract
1. Introduction
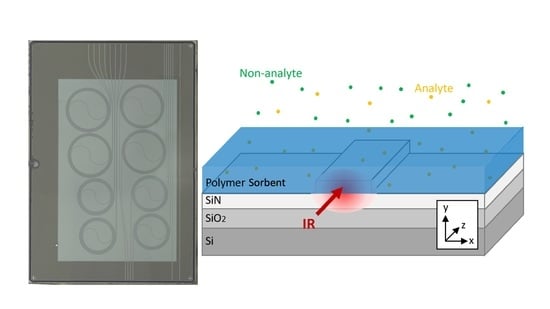
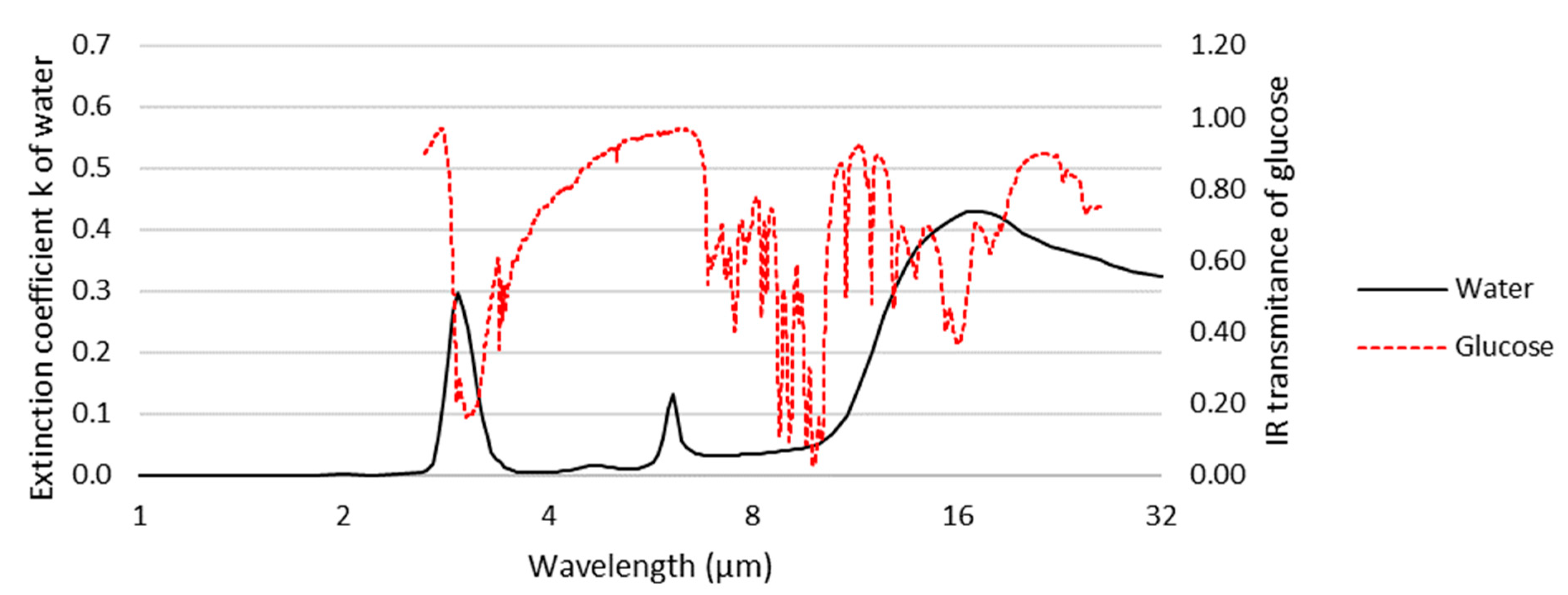
WERS Basics
2. Historical Development of WERS
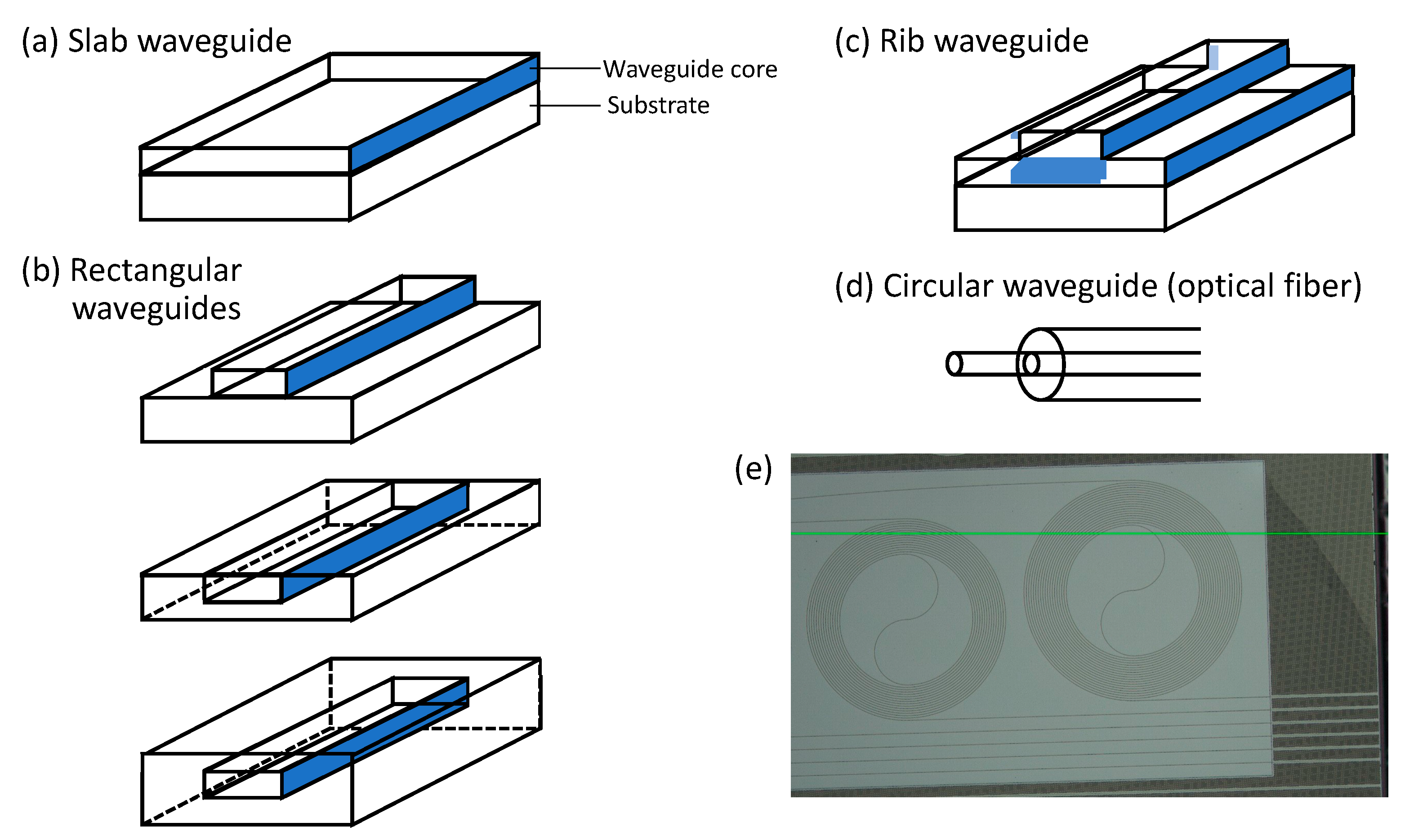
3. WERS Theory for Rectangular/Channel/Strip/Wire Waveguides
4. Experimental Verification of WERS Theory
5. Raman Emission Collected at the End of Waveguide vs. at the Surface of Waveguide
6. Silicon Nitride and Other Materials for WERS Sensing
7. Summary
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| AlN | Aluminum nitride |
| ATR-IR Spectroscopy | Attenuated total reflection infrared spectroscopy |
| BMP | Basic metabolic panel |
| BUN | Blood urea nitrogen |
| CMOS | Complementary metal–oxide–semiconductor |
| CuPc | Copper phthalocyanine |
| DEMP | Diethyl methylphosphonate |
| DMMP | Dimethyl methylphosphonate |
| DMPE | Dimyristoylphosphatidylethanolamine |
| DOS | Density of states |
| HMDS | Hexamethyldisilazane |
| IR | Infrared |
| IRAS or IRRAS | Infrared reflection absorption spectroscopy |
| MIR | Mid-infrared |
| MMA | Methyl methacrylate |
| PICs | Photonic integrated circuits |
| POC | Point-of-care |
| SEIRAS | Surface-enhanced infrared spectroscopy |
| SERS | Surface-enhanced raman scattering |
| SiN | Silicon nitride |
| SOI | Silicon-on-insulator |
| SWAP | Size, weight, and power |
| TEP | Triethyl phosphate |
| TMP | Trimethyl phosphate |
| WERS | Waveguide-enhanced raman spectroscopy |
References
- Rinken, T. State of the Art in Biosensors: Environmental and Medical Applications; Intech Open: London, UK, 2013. [Google Scholar]
- Zhang, W.; Guo, S.; Carvalho, W.S.P.; Jiang, Y.; Serpe, M.J. Portable point-of-care diagnostic devices. Anal. Methods 2016, 8, 7847–7867. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2016, 132, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Prante, M.; Segal, E.; Scheper, T.; Bahnemann, J.; Walter, J. Aptasensors for point-of-care detection of small molecules. Biosensors 2020, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Li, Z.; Li, K.; Yu, X.Q. Small molecular fluorescent probes for the detection of lead, cadmium and mercury ions. Coord. Chem. Rev. 2021, 429, 213691. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luong, J.H. Immunoassays: An overview. In Handbook of Immunoassay Technologies; Vashist, S.K., Luong, J.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–18. [Google Scholar]
- Chittur, K.K. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials 1998, 19, 357–369. [Google Scholar] [CrossRef]
- Sun, H.; Sun, C.; Ding, X.; Lu, H.; Liu, M.; Zhao, G. In situ monitoring of the selective adsorption mechanism of small environmental pollutant molecules on aptasensor interface by attenuated total reflection surface enhanced infrared absorption spectroscopy (ATR–SEIRAS). J. Hazard. Mater. 2021, 403, 123953. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, Q.; Ci, L.; Cao, K.; Mizaikoff, B. Surface-enhanced infrared attenuated total reflection spectroscopy via carbon nanodots for small molecules in aqueous solution. Anal. Bioanal. Chem. 2019, 411, 1863–1871. [Google Scholar] [CrossRef]
- Hoffmann, F.M. Infrared reflection-absorption spectroscopy of adsorbed molecules. Surf. Sci. Rep. 1983, 3, 107–192. [Google Scholar] [CrossRef]
- Boujday, S.; Lamy de la Chapelle, M.; Srajer, J.; Knoll, W. Enhanced vibrational spectroscopies as tools for small molecule biosensing. Sensors 2015, 15, 21239–21264. [Google Scholar] [CrossRef]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- D-Glucose Infrared Spectrum. Available online: https://webbook.nist.gov/cgi/inchi?ID=C2280446&Type=IR-SPEC&Index=1#IR-SPEC (accessed on 22 September 2022).
- Smith, E.; Dent, G. Modern Raman Spectroscopy: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2019; p. 16. [Google Scholar]
- Rohleder, D.R.; Kocherscheidt, G.; Gerber, K.; Kiefer, W.; Köhler, W.; Möcks, J.; Petrich, W.H. Comparison of mid-infrared and Raman spectroscopy in the quantitative analysis of serum. J. Biomed. Opt. 2005, 10, 031108. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J. Present and future of surface-enhanced Raman scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Andrade, G.F.; Brolo, A.G. A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry. Anal. Chim. Acta 2011, 693, 7–25. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-Enhanced raman spectroscopy: Concepts and chemical applications. Angew Chem. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Andrade, G.F.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef]
- Jaworska, A.; Fornasaro, S.; Sergo, V.; Bonifacio, A. Potential of surface enhanced Raman spectroscopy (SERS) in therapeutic drug monitoring (TDM). A critical review. Biosensors 2016, 6, 47. [Google Scholar] [CrossRef]
- Holmstrom, S.A.; Stievater, T.H.; Kozak, D.A.; Pruessner, M.W.; Tyndall, N.; Rabinovich, W.S.; McGill, R.A.; Khurgin, J.B. Trace gas Raman spectroscopy using functionalized waveguides. Optica 2016, 3, 891–896. [Google Scholar] [CrossRef]
- Stievater, T.H.; Khurgin, J.B.; Holmstrom, S.A.; Kozak, D.A.; Pruessner, M.W.; Rabinovich, W.S.; McGill, R.A. Nanophotonic waveguides for chip-scale Raman spectroscopy: Theoretical considerations. In Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XVII, Proceedings of the SPIE Defense + Security, Baltimore, MD, USA, 17–21 April 2016; SPIE: Bellingham, WA, USA, 2016; Volume 9824, pp. 8–13. [Google Scholar]
- Dhakal, A.; Wuytens, P.C.; Peyskens, F.; Jans, K.; Thomas, N.L.; Baets, R. Nanophotonic waveguide enhanced Raman spectroscopy of biological submonolayers. Acs Photonics 2016, 3, 2141–2149. [Google Scholar] [CrossRef]
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon photonic biosensors using label-free detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Hoshino, K. Chapter 5—Optical transducers: Optical molecular sensing and spectroscopy. In Molecular Sensors and Nanodevices: Principles, Designs and Applications in Biomedical Engineering, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 231–309. [Google Scholar]
- Walrafen, G.E.; Stone, J. Intensification of spontaneous Raman spectra by use of liquid core optical fibers. Appl. Spectrosc. 1972, 26, 585–589. [Google Scholar] [CrossRef]
- Coldren, L.A.; Verrinder, P.A.; Klamkin, J. A Review of Photonic Systems-on-Chip Enabled by Widely Tunable Lasers. IEEE J. Quantum Electron. 2022, 58, 1–10. [Google Scholar] [CrossRef]
- Levy, Y.; Imbert, C.; Cipriani, J.; Racine, S.; Dupeyrat, R. Raman scattering of thin films as a waveguide. Opt. Commun. 1974, 11, 66–69. [Google Scholar] [CrossRef]
- Rabolt, J.F.; Santo, R.; Swalen, J.D. Raman spectroscopy of thin polymer films using integrated optical techniques. Appl. Spectrosc. 1979, 33, 549–551. [Google Scholar] [CrossRef]
- Rabolt, J.F.; Santo, R.; Schlotter, N.E.; Swalen, J.D. Integrated optics and Raman scattering: Molecular orientation in thin polymer films and Langmuir-Blodgett monolayers. IBM J. Res. Dev. 1982, 26, 209–216. [Google Scholar] [CrossRef]
- Kanger, J.S.; Otto, C.; Slotboom, M.; Greve, J. Waveguide Raman spectroscopy of thin polymer layers and monolayers of biomolecules using high refractive index waveguides. J. Phys. Chem. 1996, 100, 3288–3292. [Google Scholar] [CrossRef]
- Kanger, J.S.; Otto, C.; Greve, J. Stimulated Raman gain spectroscopy of thin layers using dielectric waveguides. J. Phys. Chem. 1996, 100, 16293–16297. [Google Scholar] [CrossRef]
- O’Connor, P.; Tauc, J. Raman spectrum of optical fiber waveguide—Effect of cladding. Opt. Commun. 1978, 24, 135–138. [Google Scholar] [CrossRef]
- O’Connor, P.; Tauc, J. Light scattering in optical waveguides. Appl. Opt. 1978, 17, 3226–3231. [Google Scholar] [CrossRef]
- Raza, A.; Clemmen, S.; De Goede, M.; Ali, R.; Hua, P.; Garcia-Blanco, S.M.; Honkanen, S.; Wilkinson, J.S.; Baets, R. The performance of high-index-contrast photonics platforms for on-chip raman spectroscopy. In Proceedings of the European Conference on Integrated Optics (ECIO’2018), Valencia, Spain, 30 May–1 June 2018; pp. 35–37. [Google Scholar]
- Mack, C. The multiple lives of Moore’s law. IEEE Spectr. 2015, 52, 31. [Google Scholar] [CrossRef]
- Margalit, N.; Xiang, C.; Bowers, S.M.; Bjorlin, A.; Blum, R.; Bowers, J.E. Perspective on the future of silicon photonics and electronics. Appl. Phys. Lett. 2021, 118, 220501. [Google Scholar] [CrossRef]
- Morton, P.A.; Bowers, J.E.; Khurgin, J.B. ‘Beyond Moore’s Law’ Photonic Integrated Circuits for RF Photonics and Sensing Systems; Morton Photonics Inc.: West Friendship, MD, USA, 2018. [Google Scholar]
- Rahim, A.; Ryckeboer, E.; Subramanian, A.Z.; Clemmen, S.; Kuyken, B.; Dhakal, A.; Raza, A.; Hermans, A.; Muneeb, M.; Dhoore, S.; et al. Expanding the silicon photonics portfolio with silicon nitride photonic integrated circuits. J. Light. Technol. 2017, 35, 639–649. [Google Scholar] [CrossRef]
- Albrecht, A.C. On the theory of Raman intensities. J. Chem. Phys. 1961, 34, 1476–1484. [Google Scholar] [CrossRef]
- Dhakal, A.; Wuytens, P.; Raza, A.; Le Thomas, N.; Baets, R. Silicon nitride background in nanophotonic waveguide enhanced Raman spectroscopy. Materials 2017, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, N.F.; Kozak, D.A.; Pruessner, M.W.; Goetz, P.G.; Rabinovich, W.S.; Stievater, T.H.; Fahrenkopf, N.M.; Antohe, A. A Low-Loss, Broadband, Nitride-Only Photonic Integrated Circuit Platform. In Proceedings of the Quantum 2.0, Boston, MA, USA, 13–16 June 2022; Optica Publishing Group: Washington, DC, USA, 2022; p. QTu4B-5. [Google Scholar]
- Hiller, D.; Zelenina, A.; Gutsch, S.; Dyakov, S.A.; López-Conesa, L.; López-Vidrier, J.; Estradé, S.; Peiró, F.; Garrido, B.; Valenta, J.; et al. Absence of quantum confinement effects in the photoluminescence of Si3N4–embedded Si nanocrystals. J. Appl. Phys. 2014, 115, 204301. [Google Scholar] [CrossRef]
- Optical Properties of Silicon. Available online: https://www.pveducation.org/pvcdrom/materials/optical-properties-of-silicon (accessed on 11 October 2022).
- Jun, Y.C.; Briggs, R.M.; Atwater, H.A.; Brongersma, M.L. Broadband enhancement of light emission in silicon slot waveguides. Opt. Express 2009, 17, 7479–7490. [Google Scholar] [CrossRef]
- Dhakal, A.; Subramanian, A.Z.; Wuytens, P.; Peyskens, F.; Le Thomas, N.; Baets, R. Evanescent excitation and collection of spontaneous Raman spectra using silicon nitride nanophotonic waveguides. Opt. Lett. 2014, 39, 4025–4028. [Google Scholar] [CrossRef]
- Novotny, L.; Hecht, B. Principles of Nano-Optics; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Dhakal, A.; Raza, A.; Peyskens, F.; Subramanian, A.Z.; Clemmen, S.; Le Thomas, N.; Baets, R. Efficiency of evanescent excitation and collection of spontaneous Raman scattering near high index contrast channel waveguides. Opti. Express 2015, 23, 27391–27404. [Google Scholar] [CrossRef]
- Wang, Z.; Zervas, M.N.; Bartlett, P.N.; Wilkinson, J.S. Surface and waveguide collection of Raman emission in waveguide-enhanced Raman spectroscopy. Opt. Lett. 2016, 41, 4146–4149. [Google Scholar] [CrossRef]
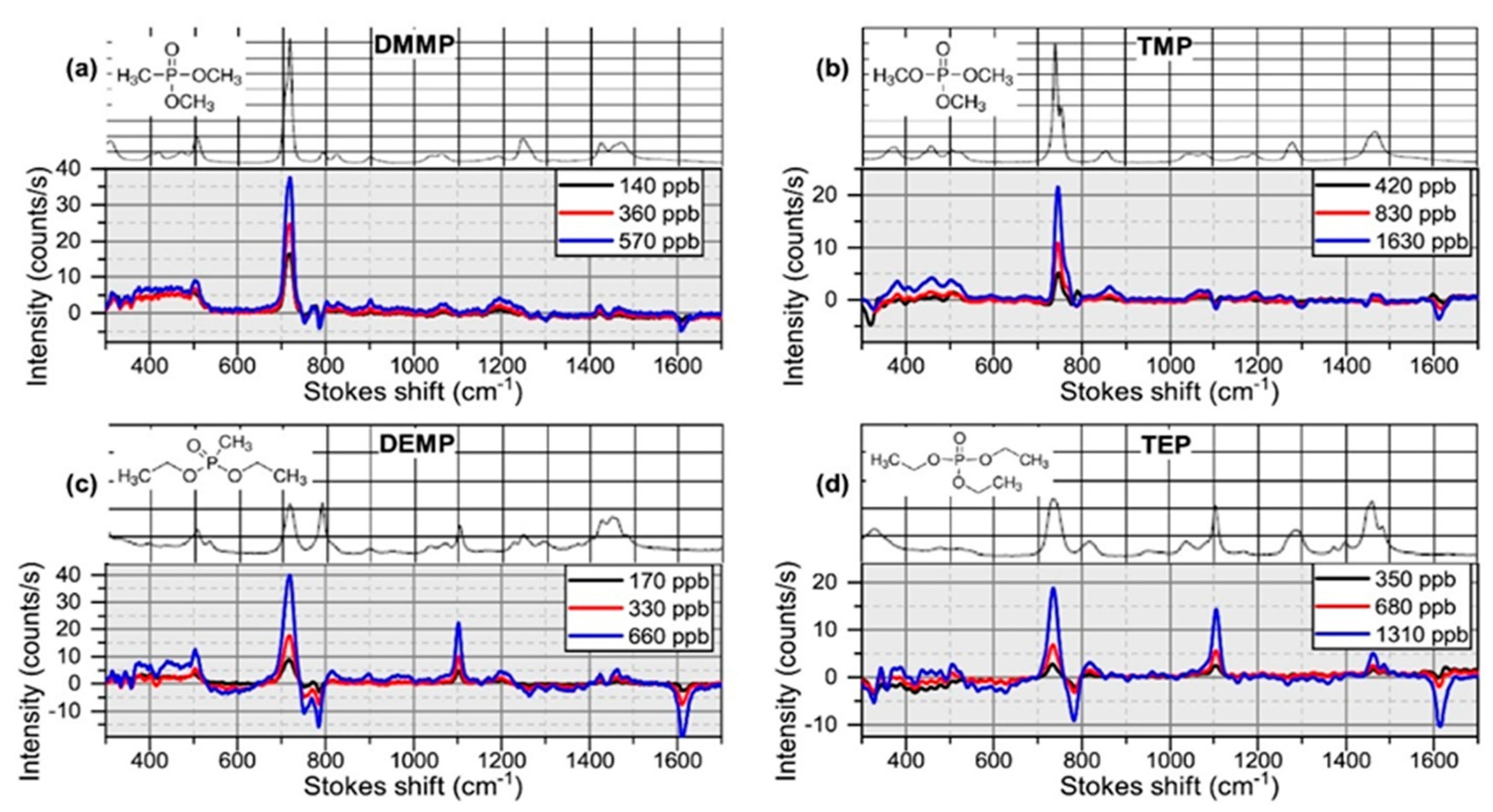
- Tyndall, N.F.; Stievater, T.H.; Kozak, D.A.; Koo, K.; McGill, R.A.; Pruessner, M.W.; Rabinovich, W.S.; Holmstrom, S.A. Waveguide-enhanced Raman spectroscopy of trace chemical warfare agent simulants. Opt. Lett. 2018, 43, 4803–4806. [Google Scholar] [CrossRef]
- Ewing, K.J.; Nau, G.; Bilodeau, T.; Dagenais, D.M.; Bucholtz, F.; Aggarwal, I.D. Monitoring the absorption of organic vapors to a solid phase extraction medium applications to detection of trace volatile organic compounds by integration of solid phase absorbents with fiber optic Raman spectroscopy. Anal. Chim. Acta 1997, 340, 227–232. [Google Scholar] [CrossRef]
- Rein, A.J.; Saperstein, D.; Pines, S.H.; Radlick, C. Blood plasma investigations by resonance Raman spectroscopy: Detection of carotenoid pigment. Experientia 1976, 32, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, N.; Stievater, T.H.; Kozak, D.; Pruessner, M.; Roxworthy, B.; Rabinovich, W.; Roberts, C.; McGill, A.; Miller, B.L.; Luta, E.; et al. Figure-of-merit characterization of hydrogen-bond acidic sorbents for waveguide-enhanced Raman spectroscopy. ACS Sensors 2020, 5, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Kita, D.M.; Michon, J.; Hu, J. A packaged, fiber-coupled waveguide-enhanced Raman spectroscopic sensor. Opt. Express 2020, 28, 14963–14972. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, H.; Baumgartner, B.; Lendl, B.; Stassen, A.; Skirtach, A.; Le Thomas, N.; Baets, R. Ultra-sensitive slot-waveguide-enhanced Raman spectroscopy for aqueous solutions of non-polar compounds using a functionalized silicon nitride photonic integrated circuit. Opt. Lett. 2021, 46, 1153–1156. [Google Scholar] [CrossRef]
- Coucheron, D.A.; Wadduwage, D.N.; Murugan, G.S.; So, P.T.; Ahluwalia, B.S. Chip-based resonance Raman spectroscopy using tantalum pentoxide waveguides. IEEE Photon. Technol. Lett. 2019, 31, 1127–1130. [Google Scholar] [CrossRef]
- Hu, D.B.; Qi, Z.M. Refractive-index-enhanced Raman spectroscopy and absorptiometry of ultrathin film overlaid on an optical waveguide. J. Phys. Chem. C 2013, 117, 16175–16181. [Google Scholar] [CrossRef]
- Raza, A.; Clemmen, S.; Wuytens, P.; De Goede, M.; Tong, A.S.; Le Thomas, N.; Liu, C.; Suntivich, J.; Skirtach, A.G.; Garcia-Blanco, S.M.; et al. High index contrast photonic platforms for on-chip Raman spectroscopy. Opt. Express 2019, 27, 23067–23079. [Google Scholar] [CrossRef]
- Makela, M.; Gordon, P.; Tu, D.; Soliman, C.; Coté, G.L.; Maitland, K.; Lin, P.T. Benzene Derivatives Analysis Using Aluminum Nitride Waveguide Raman Sensors. Anal. Chem. 2020, 92, 8917–8922. [Google Scholar] [CrossRef]
- Turk, N.; Raza, A.; Wuytens, P.; Demol, H.; Van Daele, M.; Detavernier, C.; Skirtach, A.; Gevaert, K.; Baets, R. Comparison of free-space and waveguide-based SERS platforms. Nanomaterials 2019, 9, 1401. [Google Scholar] [CrossRef]
- Turk, N.; Raza, A.; Wuytens, P.; Demol, H.; Van Daele, M.; Detavernier, C.; Skirtach, A.; Gevaert, K.; Baets, R. Waveguide-based surface-enhanced Raman spectroscopy detection of protease activity using non-natural aromatic amino acids. Biomed. Opt. Express 2020, 11, 4800–4816. [Google Scholar] [CrossRef]
- Mu, X.; Wu, S.; Cheng, L.; Fu, H.Y. Edge couplers in silicon photonic integrated circuits: A review. Appl. Sci. 2020, 10, 1538. [Google Scholar] [CrossRef]
- Arbabi, A.; Goddard, L.L. Measurements of the refractive indices and thermo-optic coefficients of Si3N4 and SiOx using microring resonances. Opt. Lett. 2013, 38, 3878–3881. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, N.F.; Stievater, T.H.; Kozak, D.A.; Pruessner, M.W.; Rabinovich, W.S. Passive photonic integration of lattice filters for waveguide-enhanced Raman spectroscopy. Opt. Express 2020, 28, 34927–34934. [Google Scholar] [CrossRef] [PubMed]

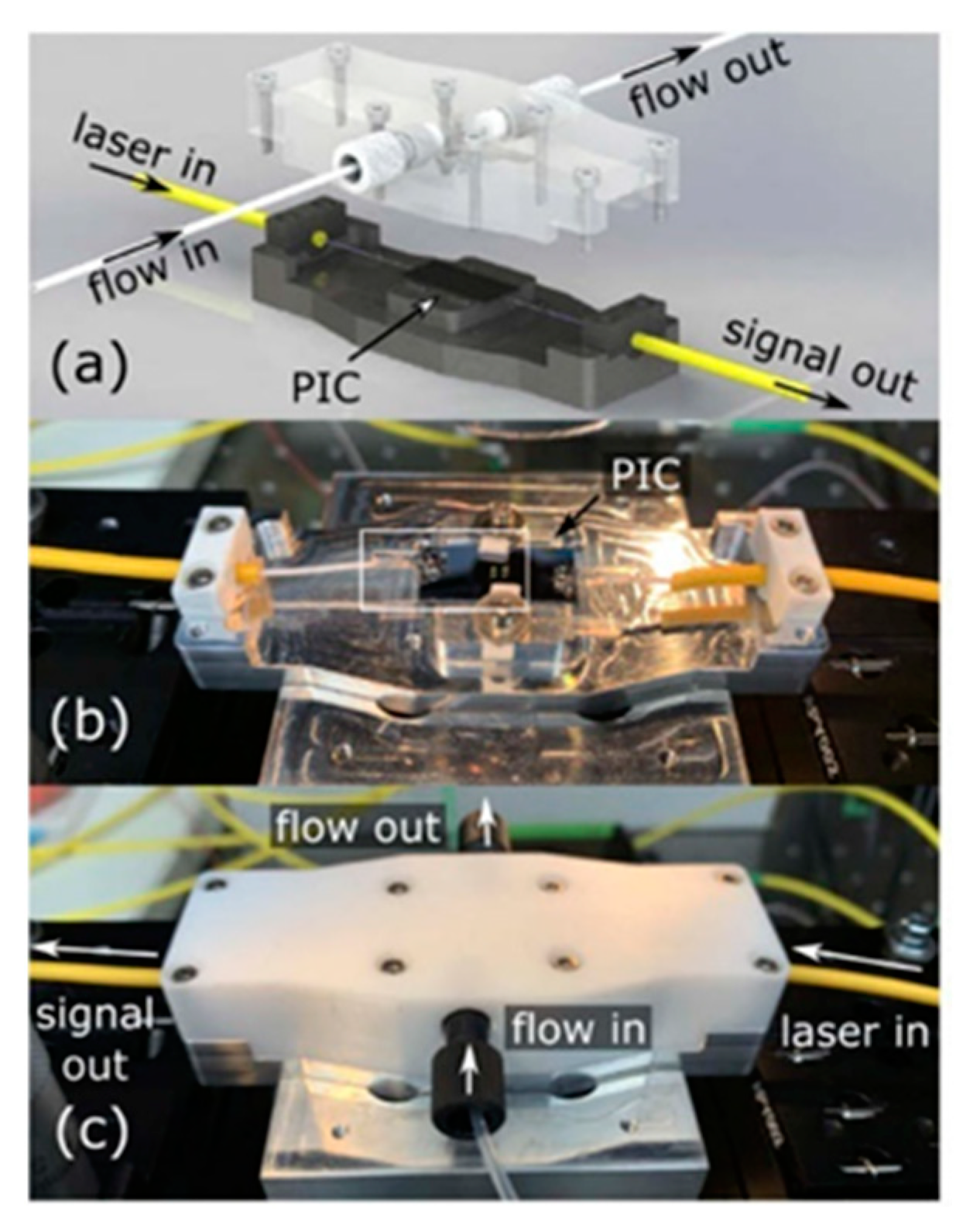
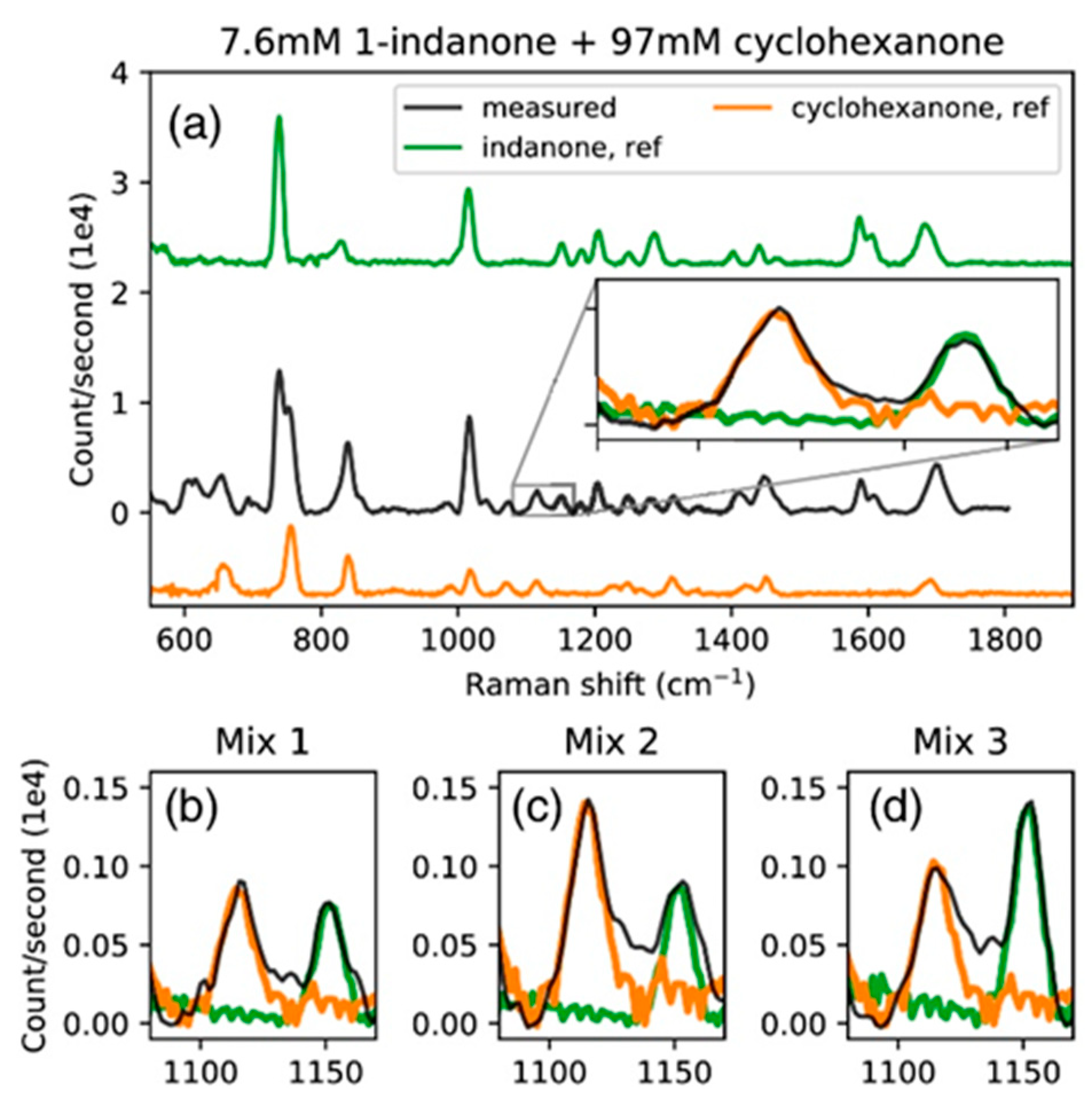
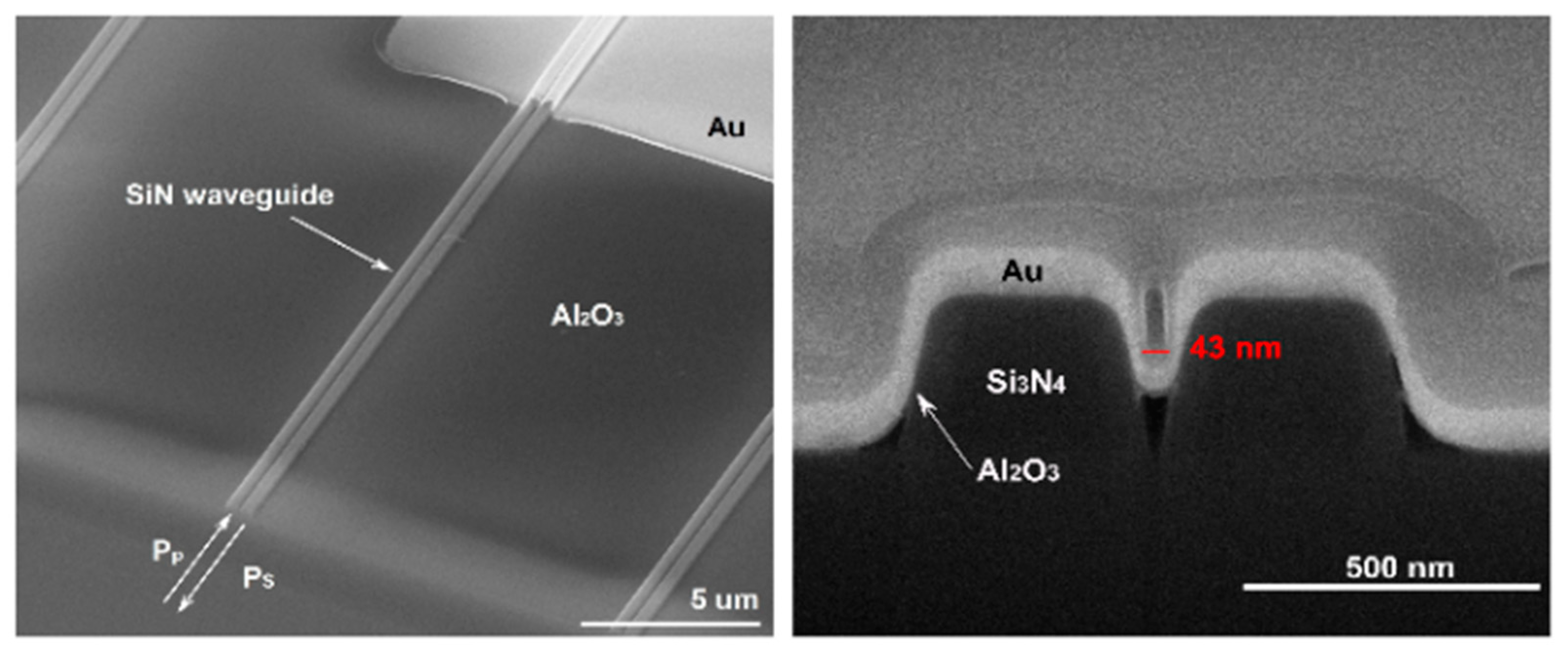
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Miller, B.L. Waveguide-Enhanced Raman Spectroscopy (WERS): An Emerging Chip-Based Tool for Chemical and Biological Sensing. Sensors 2022, 22, 9058. https://doi.org/10.3390/s22239058
Wang P, Miller BL. Waveguide-Enhanced Raman Spectroscopy (WERS): An Emerging Chip-Based Tool for Chemical and Biological Sensing. Sensors. 2022; 22(23):9058. https://doi.org/10.3390/s22239058
Chicago/Turabian StyleWang, Pengyi, and Benjamin L. Miller. 2022. "Waveguide-Enhanced Raman Spectroscopy (WERS): An Emerging Chip-Based Tool for Chemical and Biological Sensing" Sensors 22, no. 23: 9058. https://doi.org/10.3390/s22239058
APA StyleWang, P., & Miller, B. L. (2022). Waveguide-Enhanced Raman Spectroscopy (WERS): An Emerging Chip-Based Tool for Chemical and Biological Sensing. Sensors, 22(23), 9058. https://doi.org/10.3390/s22239058