Electrochemically Deposited Molecularly Imprinted Polymer-Based Sensors
Abstract
:1. Introduction
2. Chemical Formation of Conducting Polymers Based on Redox Processes
3. Formation of Conducting Polymers by Electrochemical Methods
4. MIPs Structures Imprinted by Biomolecules of High Molecular Weight
5. Physicochemical Methods Used in Molecularly Imprinted Polymer Based Analytical Systems
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Progress and Insights in the Application of MXenes as New 2D Nano-Materials Suitable for Biosensors and Biofuel Cell Design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Insights in the Application of Stoichiometric and Non-Stoichiometric Titanium Oxides for the Design of Sensors for the Determination of Gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Fotouhi, L.; Ehsani, A. Recent Progress in the Development of Conducting Polymer-Based Nanocomposites for Electrochemical Biosensors Applications: A Mini-Review. Chem. Rec. 2018, 18, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Pontes, K.; Indrusiak, T.; Soares, B.G. Poly(vinylidene fluoride-co-hexafluorpropylene)/polyaniline conductive blends: Effect of the mixing procedure on the electrical properties and electromagnetic interference shielding effectiveness. J. Appl. Polym. Sci. 2021, 138, 49705. [Google Scholar] [CrossRef]
- Samukaite-Bubniene, U.; Valiūnienė, A.; Bucinskas, V.; Genys, P.; Ratautaite, V.; Ramanaviciene, A.; Aksun, E.; Tereshchenko, A.; Zeybek, B.; Ramanavicius, A. Towards supercapacitors: Cyclic voltammetry and fast Fourier transform electrochemical impedance spectroscopy based evaluation of polypyrrole electrochemically deposited on the pencil graphite electrode. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125750. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, T.; Miao, Y.; Zhao, X. Chloride ion-doped polyaniline/carbon nanotube nanocomposite materials as new cathodes for chloride ion battery. Electrochim. Acta 2018, 270, 30–36. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Liu, Y.; Liu, W.; Zhao, P.; Li, Y.; Dong, Y.; Wang, H.; Yang, J. Urchin-like Ni1/3Co2/3(CO3)0.5OH·0.11H2O anchoring on polypyrrole nanotubes for supercapacitor electrodes. Electrochim. Acta 2019, 295, 989–996. [Google Scholar] [CrossRef]
- Ratautaite, V.; Ramanaviciene, A.; Oztekin, Y.; Voronovic, J.; Balevicius, Z.; Mikoliunaite, L.; Ramanavicius, A. Electrochemical stability and repulsion of polypyrrole film. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 16–21. [Google Scholar] [CrossRef]
- Iroh, J.O.; Su, W. Corrosion performance of polypyrrole coating applied to low carbon steel by an electrochemical process. Electrochim. Acta 2000, 46, 15–24. [Google Scholar] [CrossRef]
- Oztekin, Y.; Ramanaviciene, A.; Yazicigil, Z.; Solak, A.O.; Ramanavicius, A. Direct electron transfer from glucose oxidase immobilized on polyphenanthroline-modified glassy carbon electrode. Biosens. Bioelectron. 2011, 26, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Emir, G.; Dilgin, Y.; Ramanaviciene, A.; Ramanavicius, A. Amperometric nonenzymatic glucose biosensor based on graphite rod electrode modified by Ni-nanoparticle/polypyrrole composite. Microchem. J. 2021, 161, 105751. [Google Scholar] [CrossRef]
- Ratautaite, V.; Plausinaitis, D.; Baleviciute, I.; Mikoliunaite, L.; Ramanaviciene, A.; Ramanavicius, A. Characterization of caffeine-imprinted polypyrrole by a quartz crystal microbalance and electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2015, 212, 63–71. [Google Scholar] [CrossRef]
- Holguín, M.; Rojas Álvarez, O.E.; Arizabaleta, C.A.; Torres, W. Molecular dynamics of the interaction of l-tryptophan with polypyrrole oligomers. Comput. Theor. Chem. 2019, 1147, 29–34. [Google Scholar] [CrossRef]
- Kumar, V.; Mirzaei, A.; Bonyani, M.; Kim, K.-H.; Kim, H.W.; Kim, S.S. Advances in electrospun nanofiber fabrication for polyaniline (PANI)-based chemoresistive sensors for gaseous ammonia. TrAC Trends Anal. Chem. 2020, 129, 115938. [Google Scholar] [CrossRef]
- Tekbaşoğlu, T.Y.; Soganci, T.; Ak, M.; Koca, A.; Şener, M.K. Enhancing biosensor properties of conducting polymers via copolymerization: Synthesis of EDOT-substituted bis(2-pyridylimino)isoindolato-palladium complex and electrochemical sensing of glucose by its copolymerized film. Biosens. Bioelectron. 2017, 87, 81–88. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization Model for Hydrogen Peroxide Initiated Synthesis of Polypyrrole Nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; Andriukonis, E.; Stirke, A.; Mikoliunaite, L.; Balevicius, Z.; Ramanaviciene, A. Synthesis of polypyrrole within the cell wall of yeast by redox-cycling of [Fe(CN)6]3−/[Fe(CN)6]4−. Enzyme Microb. Technol. 2016, 83, 40–47. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Carac, G.; Bahrim, G.; Ramanaviciene, A.; Ramanavicius, A. Modification of Aspergillus niger by conducting polymer, Polypyrrole, and the evaluation of electrochemical properties of modified cells. Bioelectrochemistry 2018, 121, 46–55. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer—Polypyrrole—For the improvement of electric charge transfer through fungal cell wall. Colloids Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Pulsed amperometric detection of DNA with an ssDNA/polypyrrole-modified electrode. Anal. Bioanal. Chem. 2004, 379, 287–293. [Google Scholar] [CrossRef]
- Plikusiene, I.; Balevicius, Z.; Ramanaviciene, A.; Talbot, J.; Mickiene, G.; Balevicius, S.; Stirke, A.; Tereshchenko, A.; Tamosaitis, L.; Zvirblis, G.; et al. Evaluation of affinity sensor response kinetics towards dimeric ligands linked with spacers of different rigidity: Immobilized recombinant granulocyte colony-stimulating factor based synthetic receptor binding with genetically engineered dimeric analyte d. Biosens. Bioelectron. 2020, 156, 112112. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Oztekin, Y.; Ramanaviciene, A. Electrochemical formation of polypyrrole-based layer for immunosensor design. Sens. Actuators B Chem. 2014, 197, 237–243. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Self-encapsulation of oxidases as a basic approach to tune the upper detection limit of amperometric biosensors. Analyst 2008, 133, 1083. [Google Scholar] [CrossRef] [PubMed]
- Lakard, B.; Magnin, D.; Deschaume, O.; Vanlancker, G.; Glinel, K.; Demoustier-Champagne, S.; Nysten, B.; Jonas, A.M.; Bertrand, P.; Yunus, S. Urea potentiometric enzymatic biosensor based on charged biopolymers and electrodeposited polyaniline. Biosens. Bioelectron. 2011, 26, 4139–4145. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanavicius, A.; Voronovic, J.; Ramanaviciene, A. Glucose biosensor based on glucose oxidase and gold nanoparticles of different sizes covered by polypyrrole layer. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 224–230. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Baleviciute, I.; Ratautaite, V.; Ramanaviciene, A.; Balevicius, Z.; Broeders, J.; Croux, D.; McDonald, M.; Vahidpour, F.; Thoelen, R.; de Ceuninck, W.; et al. Evaluation of theophylline imprinted polypyrrole film. Synth. Met. 2015, 209, 206–211. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Sangiorgi, A.; Tarterini, F.; Sanson, A. Molecularly imprinted polypyrrole counter electrode for gel-state dye-sensitized solar cells. Electrochim. Acta 2019, 305, 322–328. [Google Scholar] [CrossRef]
- Syritski, V.; Reut, J.; Öpik, A.; Idla, K. Environmental QCM sensors coated with polypyrrole. Synth. Met. 1999, 102, 1326–1327. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef] [PubMed]
- Keçili, R.; Yılmaz, E.; Ersöz, A.; Say, R. Imprinted materials: From green chemistry to sustainable engineering. In Sustainable Nanoscale Engineering; Szekely, G., Livingston, A.B.T.-S.N.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 317–350. ISBN 978-0-12-814681-1. [Google Scholar]
- Cui, F.; Zhou, Z.; Zhou, H.S. Molecularly Imprinted Polymers and Surface Imprinted Polymers Based Electrochemical Biosensor for Infectious Diseases. Sensors 2020, 20, 996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.-L.; Wang, Y.; Hjertén, S. A novel support with artificially created recognition for the selective removal of proteins and for affinity chromatography. Chromatographia 1996, 42, 259–262. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly Imprinted Polymer Micro- and Nano-Particles: A Review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef] [PubMed]
- Bakirhan, N.K.; Ozcelikay, G.; Ozkan, S.A. Recent progress on the sensitive detection of cardiovascular disease markers by electrochemical-based biosensors. J. Pharm. Biomed. Anal. 2018, 159, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Sankiewicz, A.; Romanowicz, L.; Pyc, M.; Hermanowicz, A.; Gorodkiewicz, E. SPR imaging biosensor for the quantitation of fibronectin concentration in blood samples. J. Pharm. Biomed. Anal. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI–PEDOT Film Deposited on Transparent Electrode. Polymers 2020, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of enzymatic formation of polyaniline nanoparticles. Polymer 2017, 115, 211–216. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Enzymatic Formation of Polyaniline, Polypyrrole, and Polythiophene Nanoparticles with Embedded Glucose Oxidase. Nanomaterials 2019, 9, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krikstolaityte, V.; Kuliesius, J.; Ramanaviciene, A.; Mikoliunaite, L.; Kausaite-Minkstimiene, A.; Oztekin, Y.; Ramanavicius, A. Enzymatic polymerization of polythiophene by immobilized glucose oxidase. Polymer 2014, 55, 1613–1620. [Google Scholar] [CrossRef]
- Genys, P.; Aksun, E.; Tereshchenko, A.; Valiūnienė, A.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical Deposition and Investigation of Poly-9,10-Phenanthrenequinone Layer. Nanomaterials 2019, 9, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kausaite-Minkstimiene, A.; Glumbokaite, L.; Ramanaviciene, A.; Ramanavicius, A. Reagent-less amperometric glucose biosensor based on nanobiocomposite consisting of poly(1,10-phenanthroline-5,6-dione), poly(pyrrole-2-carboxylic acid), gold nanoparticles and glucose oxidase. Microchem. J. 2020, 154, 104665. [Google Scholar] [CrossRef]
- Gicevicius, M.; Bagdziunas, G.; Abduloglu, Y.; Ramanaviciene, A.; Gumusay, O.; Ak, M.; Soganci, T.; Ramanavicius, A. Experimental and Theoretical Investigations of an Electrochromic Azobenzene and 3,4-Ethylenedioxythiophene-based Electrochemically Formed Polymeric Semiconductor. ChemPhysChem 2018, 19, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Olgac, R.; Soganci, T.; Baygu, Y.; Gök, Y.; Ak, M. Zinc(II) phthalocyanine fused in peripheral positions octa-substituted with alkyl linked carbazole: Synthesis, electropolymerization and its electro-optic and biosensor applications. Biosens. Bioelectron. 2017, 98, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Kaseta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of cytotoxicity of polypyrrole nanoparticles synthesized by oxidative polymerization. J. Hazard. Mater. 2013, 250–251, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Vaitkuviene, A.; Ratautaite, V.; Mikoliunaite, L.; Kaseta, V.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Some biocompatibility aspects of conducting polymer polypyrrole evaluated with bone marrow-derived stem cells. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 152–156. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in-vivo study in mice. J. Pharm. Pharmacol. 2010, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation of Polyaniline and Polypyrrole Nanocomposites with Embedded Glucose Oxidase and Gold Nanoparticles. Polymers 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazeiko, V.; Kausaite-Minkstimiene, A.; Ramanaviciene, A.; Balevicius, Z.; Ramanavicius, A. Gold nanoparticle and conducting polymer-polyaniline-based nanocomposites for glucose biosensor design. Sens. Actuators B Chem. 2013, 189, 187–193. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Immobilizing Enzymes: How to Create More Suitable Biocatalysts. Angew. Chem. Int. Ed. 2003, 42, 3336–3337. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [Green Version]
- Miletić, N.; Nastasović, A.; Loos, K. Immobilization of biocatalysts for enzymatic polymerizations: Possibilities, advantages, applications. Bioresour. Technol. 2012, 115, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andriukonis, E.; Ramanaviciene, A.; Ramanavicius, A. Synthesis of Polypyrrole Induced by [Fe(CN)6]3− and Redox Cycling of [Fe(CN)6]4−/[Fe(CN)6]3−. Polymers 2018, 10, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Evaluation of Polyaniline and Polypyrrole Nanocomposites Based on Glucose Oxidase and Gold Nanostructures. Polymers 2020, 12, 3026. [Google Scholar] [CrossRef]
- Ye, L.; Mosbach, K. Molecularly imprinted microspheres as antibody binding mimics. React. Funct. Polym. 2001, 48, 149–157. [Google Scholar] [CrossRef]
- Peeters, M.; Kobben, S.; Jiménez-Monroy, K.L.; Modesto, L.; Kraus, M.; Vandenryt, T.; Gaulke, A.; van Grinsven, B.; Ingebrandt, S.; Junkers, T.; et al. Thermal detection of histamine with a graphene oxide based molecularly imprinted polymer platform prepared by reversible addition–fragmentation chain transfer polymerization. Sens. Actuators B Chem. 2014, 203, 527–535. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Mosbach, K.; Haupt, K. A Simple Method for Spin-Coating Molecularly Imprinted Polymer Films of Controlled Thickness and Porosity. Adv. Mater. 2004, 16, 719–722. [Google Scholar] [CrossRef]
- Lete, C.; Lakard, B.; Hihn, J.-Y.; del Campo, F.J.; Lupu, S. Use of sinusoidal voltages with fixed frequency in the preparation of tyrosinase based electrochemical biosensors for dopamine electroanalysis. Sens. Actuators B Chem. 2017, 240, 801–809. [Google Scholar] [CrossRef]
- Long, Y.-Z.; Li, M.-M.; Gu, C.; Wan, M.; Duvail, J.-L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Patois, T.; Lakard, B.; Martin, N.; Fievet, P. Effect of various parameters on the conductivity of free standing electrosynthesized polypyrrole films. Synth. Met. 2010, 160, 2180–2185. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kumar, P.; Park, D.-S.; Shim, Y.-B. Electrochemical Sensors Based on Organic Conjugated Polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef] [PubMed]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Srilalitha, S.; Jayaveera, K.N.; Madhvendhra, S.S. The Effect of Dopant, Temperature and Band Gap on Conductivity of Conducting Polymers. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 2694–2696. [Google Scholar]
- Lakard, B.; Ploux, L.; Anselme, K.; Lallemand, F.; Lakard, S.; Nardin, M.; Hihn, J.Y. Effect of ultrasounds on the electrochemical synthesis of polypyrrole, application to the adhesion and growth of biological cells. Bioelectrochemistry 2009, 75, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-H.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Hu, Q.; Zeng, Q.; Wang, M.; Wang, L. Variations in Surface Morphologies, Properties, and Electrochemical Responses to Nitro-Analyte by Controlled Electropolymerization of Thiophene Derivatives. ACS Appl. Mater. Interfaces 2018, 10, 11319–11327. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Xu, Y.; Guo, Z.; Jiang, D. Supercapacitive Energy Storage and Electric Power Supply Using an Aza-Fused π-Conjugated Microporous Framework. Angew. Chem. Int. Ed. 2011, 50, 8753–8757. [Google Scholar] [CrossRef]
- Jiang, J.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated Microporous Poly(aryleneethynylene) Networks. Angew. Chem. Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, S.; Wang, Q.; Xiao, Q.; Yue, Y.; Ren, S. Fluorene-Based Conjugated Microporous Polymers: Preparation and Chemical Sensing Application. Macromol. Rapid Commun. 2017, 38, 1700445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.; Füser, M.; Bolte, M.; Terfort, A. Self-assembled monolayers of aromatic pyrrole derivatives: Electropolymerization and electrocopolymerization with pyrrole. Electrochim. Acta 2017, 246, 853–863. [Google Scholar] [CrossRef]
- Wu, Y.; Li, G.; Tian, Y.; Feng, J.; Xiao, J.; Liu, J.; Liu, X.; He, Q. Electropolymerization of molecularly imprinted polypyrrole film on multiwalled carbon nanotube surface for highly selective and stable determination of carcinogenic amaranth. J. Electroanal. Chem. 2021, 895, 115494. [Google Scholar] [CrossRef]
- Peng, Y.; Su, H. Recent Innovations of Molecularly Imprinted Electrochemical Sensors Based on Electropolymerization Technique. Curr. Anal. Chem. 2015, 11, 307–317. [Google Scholar] [CrossRef]
- Moreira Gonçalves, L. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2021, 25, 100640. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent Advances in Electrosynthesized Molecularly Imprinted Polymer Sensing Platforms for Bioanalyte Detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaabal, M.; Bakirhan, N.K.; Doulache, M.; Kaddour, S.; Saidat, B.; Ozkan, S.A. A New Approach on Sensitive Assay of Adefovir in Pharmaceutical and Biological Fluid Samples Using Polypyrrole Modified Glassy Carbon Electrode. Sens. Actuators B Chem. 2020, 323, 128657. [Google Scholar] [CrossRef]
- Karadas, N.; Ozkan, S.A. Electrochemical preparation of sodium dodecylsulfate doped over-oxidized polypyrrole/multi-walled carbon nanotube composite on glassy carbon electrode and its application on sensitive and selective determination of anticancer drug: Pemetrexed. Talanta 2014, 119, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Devkota, L.; Nguyen, L.T.; Vu, T.T.; Piro, B. Electrochemical determination of tetracycline using AuNP-coated molecularly imprinted overoxidized polypyrrole sensing interface. Electrochim. Acta 2018, 270, 535–542. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, F.; Kan, X. Voltammetric dopamine sensor based on three-dimensional electrosynthesized molecularly imprinted polymers and polypyrrole nanowires. Microchim. Acta 2017, 184, 2515–2522. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Zhang, L.; Zuo, P.; Ye, B.; Yao, J.; Chen, W. Supportless electrochemical sensor based on molecularly imprinted polymer modified nanoporous microrod for determination of dopamine at trace level. Biosens. Bioelectron. 2016, 78, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Janssens, S.D.; Haenen, K.; Nesládek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Molecularly Imprinted Polypyrrole Based Impedimentric Sensor for Theophylline Determination. Electrochim. Acta 2014, 130, 361–367. [Google Scholar] [CrossRef]
- Betlem, K.; Mahmood, I.; Seixas, R.D.; Sadiki, I.; Raimbault, R.L.D.; Foster, C.W.; Crapnell, R.D.; Tedesco, S.; Banks, C.E.; Gruber, J.; et al. Evaluating the temperature dependence of heat-transfer based detection: A case study with caffeine and Molecularly Imprinted Polymers as synthetic receptors. Chem. Eng. J. 2019, 359, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Ratautaite, V.; Nesladek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Evaluation of Histamine Imprinted Polypyrrole Deposited on Boron Doped Nanocrystalline Diamond. Electroanalysis 2014, 26, 2458–2464. [Google Scholar] [CrossRef]
- Zhang, W.; Zong, L.; Geng, G.; Li, Y.; Zhang, Y. Enhancing determination of quercetin in honey samples through electrochemical sensors based on highly porous polypyrrole coupled with nanohybrid modified GCE. Sens. Actuators B Chem. 2018, 257, 1099–1109. [Google Scholar] [CrossRef]
- Ye, C.; Chen, X.; Xu, J.; Xi, H.; Wu, T.; Deng, D.; Zhang, J.; Huang, G. Highly sensitive detection to gallic acid by polypyrrole-based MIES supported by MOFs-Co2+@Fe3O4. J. Electroanal. Chem. 2020, 859, 113839. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, W.; Yang, Z. A novel photoelectrochemical sensor for bilirubin based on porous transparent TiO2 and molecularly imprinted polypyrrole. Electrochim. Acta 2016, 187, 451–456. [Google Scholar] [CrossRef]
- Nguy, T.P.; van Phi, T.; Tram, D.T.N.; Eersels, K.; Wagner, P.; Lien, T.T.N. Development of an impedimetric sensor for the label-free detection of the amino acid sarcosine with molecularly imprinted polymer receptors. Sens. Actuators B Chem. 2017, 246, 461–470. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Chen, Z. Ultrasensitive electrochemical microcystin-LR immunosensor using gold nanoparticle functional polypyrrole microsphere catalyzed silver deposition for signal amplification. Sens. Actuators B Chem. 2017, 246, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Tadi, K.K.; Motghare, R.V.; Ganesh, V. Electrochemical Detection of Sulfanilamide Using Pencil Graphite Electrode Based on Molecular Imprinting Technology. Electroanalysis 2014, 26, 2328–2336. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Karimian, N. Fabrication of a highly selective and sensitive voltammetric ganciclovir sensor based on electropolymerized molecularly imprinted polymer and gold nanoparticles on multiwall carbon nanotubes/glassy carbon electrode. Sens. Actuators B Chem. 2015, 215, 471–479. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Sinkevicius, L.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Evaluation of electrochemical quartz crystal microbalance based sensor modified by uric acid-imprinted polypyrrole. Talanta 2020, 220, 121414. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. A novel detection approach for serotonin by graphene quantum dots/two-dimensional (2D) hexagonal boron nitride nanosheets with molecularly imprinted polymer. Appl. Surf. Sci. 2018, 458, 648–655. [Google Scholar] [CrossRef]
- Syritski, V.; Reut, J.; Menaker, A.; Gyurcsányi, R.E.; Öpik, A. Electrosynthesized molecularly imprinted polypyrrole films for enantioselective recognition of l-aspartic acid. Electrochim. Acta 2008, 53, 2729–2736. [Google Scholar] [CrossRef]
- Gu, J.; Dai, H.; Kong, Y.; Tao, Y.; Chu, H.; Tong, Z. Chiral electrochemical recognition of cysteine enantiomers with molecularly imprinted overoxidized polypyrrole-Au nanoparticles. Synth. Met. 2016, 222, 137–143. [Google Scholar] [CrossRef]
- Işık, D.; Şahin, S.; Caglayan, M.O.; Üstündağ, Z. Electrochemical impedimetric detection of kanamycin using molecular imprinting for food safety. Microchem. J. 2021, 160, 105713. [Google Scholar] [CrossRef]
- Bolat, G.; Yaman, Y.T.; Abaci, S. Molecularly imprinted electrochemical impedance sensor for sensitive dibutyl phthalate (DBP) determination. Sens. Actuators B Chem. 2019, 299, 127000. [Google Scholar] [CrossRef]
- Zaidi, S.A. Utilization of an environmentally-friendly monomer for an efficient and sustainable adrenaline imprinted electrochemical sensor using graphene. Electrochim. Acta 2018, 274, 370–377. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, G.; Zhu, A.; Zhao, Z.; Ren, C.; Nie, L.; Kan, X. A multiporous electrochemical sensor for epinephrine recognition and detection based on molecularly imprinted polypyrrole. RSC Adv. 2012, 2, 7803. [Google Scholar] [CrossRef]
- Radi, A.-E.; El-Naggar, A.-E.; Nassef, H.M. Determination of coccidiostat clopidol on an electropolymerized-molecularly imprinted polypyrrole polymer modified screen printed carbon electrode. Anal. Methods 2014, 6, 7967–7972. [Google Scholar] [CrossRef]
- Xing, X.; Liu, S.; Yu, J.; Lian, W.; Huang, J. Electrochemical sensor based on molecularly imprinted film at polypyrrole-sulfonated graphene/hyaluronic acid-multiwalled carbon nanotubes modified electrode for determination of tryptamine. Biosens. Bioelectron. 2012, 31, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, Y.; Sun, G.; Wang, S.; Deng, J.; Wei, H. Molecularly imprinted polymers on graphene oxide surface for EIS sensing of testosterone. Biosens. Bioelectron. 2017, 92, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Nezhadali, A.; Feizy, J.; Beheshti, H.R. A Molecularly Imprinted Polymer for the Selective Extraction and Determination of Fenvalerate from Food Samples Using High-Performance Liquid Chromatography. Food Anal. Methods 2015, 8, 1225–1237. [Google Scholar] [CrossRef]
- Agrisuelas, J.; Gabrielli, C.; García-Jareño, J.J.; Perrot, H.; Sanchis-Gual, R.; Sel, O.; Vicente, F. Evaluation of the electrochemical anion recognition of NO3−-imprinted poly(Azure A) in NO3−/Cl− mixed solutions by ac-electrogravimetry. Electrochim. Acta 2016, 194, 292–303. [Google Scholar] [CrossRef]
- Zang, Y.; Nie, J.; He, B.; Yin, W.; Zheng, J.; Hou, C.; Huo, D.; Yang, M.; Liu, F.; Sun, Q.; et al. Fabrication of S-MoSe2/NSG/Au/MIPs imprinted composites for electrochemical detection of dopamine based on synergistic effect. Microchem. J. 2020, 156, 104845. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, Q.; Zang, L.; Zhao, M.; Chen, X.; Shen, D. Enhancement anti-interference ability of photoelectrochemical sensor via differential molecularly imprinting technique demonstrated by dopamine determination. Anal. Chim. Acta 2020, 1125, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Zhou, H.; Li, C.; Zhu, A.; Xing, Z.; Zhao, Z. Imprinted electrochemical sensor for dopamine recognition and determination based on a carbon nanotube/polypyrrole film. Electrochim. Acta 2012, 63, 69–75. [Google Scholar] [CrossRef]
- Peeters, M.; Troost, F.J.; van Grinsven, B.; Horemans, F.; Alenus, J.; Murib, M.S.; Keszthelyi, D.; Ethirajan, A.; Thoelen, R.; Cleij, T.J.; et al. MIP-based biomimetic sensor for the electronic detection of serotonin in human blood plasma. Sens. Actuators B Chem. 2012, 171–172, 602–610. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Wu, G.; Zhu, L.; Lu, X. Development and Application of the Serotonin Voltametric Sensors Based on Molecularly Imprinting Technology. J. Electrochem. Soc. 2015, 162, B201–B206. [Google Scholar] [CrossRef]
- Akhoundian, M.; Rüter, A.; Shinde, S. Ultratrace Detection of Histamine Using a Molecularly-Imprinted Polymer-Based Voltammetric Sensor. Sensors 2017, 17, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, S.; Yeung, C.-C.; Li, T.; Lau, S.C.; Sun, Q.-J.; Li, L.-Y.; Li, J.H.; Lam, M.H.W.; Roy, V.A.L. Portable molecularly imprinted polymer-based platform for detection of histamine in aqueous solutions. J. Hazard. Mater. 2021, 410, 124609. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhong, T.; Long, Q.; Huang, C.; Chen, L.; Lu, D.; Li, X.; Zhang, Z.; Shen, G.; Hou, X. A gold nanoparticles-based molecularly imprinted electrochemical sensor for histamine specific-recognition and determination. Microchem. J. 2021, 171, 106844. [Google Scholar] [CrossRef]
- Kim, J.M.; Yang, J.C.; Park, J.Y. Quartz crystal microbalance (QCM) gravimetric sensing of theophylline via molecularly imprinted microporous polypyrrole copolymers. Sens. Actuators B Chem. 2015, 206, 50–55. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Lu, Z.; Liu, T.; Zhao, L.; Ding, F.; Zou, P.; Wang, X.; Zhao, Q.; Rao, H. Molecularly imprinted polydopamine modified with nickel nanoparticles wrapped with carbon: Fabrication, characterization and electrochemical detection of uric acid. Microchim. Acta 2019, 186, 414. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Deng, P.; Wu, Y.; Ding, Z.; Li, G.; Liu, J.; He, Q. A Simple and Efficient Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Tryptophan. Biomolecules 2019, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Ayankojo, A.G.; Reut, J.; Ciocan, V.; Öpik, A.; Syritski, V. Molecularly imprinted polymer-based sensor for electrochemical detection of erythromycin. Talanta 2020, 209, 120502. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B.; Erkmen, C.; Uslu, B. Molecularly Imprinted Electrochemical Sensors: Analytical and Pharmaceutical Applications Based on Ortho-Phenylenediamine Polymerization. Curr. Pharm. Anal. 2020, 16, 350–366. [Google Scholar] [CrossRef]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef] [PubMed]
- Ozcelikay, G.; Karadas-Bakirhan, N.; Taskin-Tok, T.; Ozkan, S.A. A selective and molecular imaging approach for anticancer drug: Pemetrexed by nanoparticle accelerated molecularly imprinting polymer. Electrochim. Acta 2020, 354, 136665. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Kurbanoglu, S.; Zhang, X.; Kosak Soz, C.; Wollenberger, U.; Ozkan, S.A.; Yarman, A.; Scheller, F.W. Electrochemical MIP Sensor for Butyrylcholinesterase. Polymers 2019, 11, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Li, Y.; Liu, T.; Wang, G.; Sun, M.; Jiang, Y.; He, H.; Wang, Y.; Zou, P.; Wang, X.; et al. A dual-template imprinted polymer electrochemical sensor based on AuNPs and nitrogen-doped graphene oxide quantum dots coated on NiS2/biomass carbon for simultaneous determination of dopamine and chlorpromazine. Chem. Eng. J. 2020, 389, 124417. [Google Scholar] [CrossRef]
- Kumar Prusty, A.; Bhand, S. Molecularly Imprinted Polyresorcinol Based Capacitive Sensor for Sulphanilamide Detection. Electroanalysis 2019, 31, 1797–1808. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Li, Y. Molecularly imprinted polymer-decorated signal on-off ratiometric electrochemical sensor for selective and robust dopamine detection. Biosens. Bioelectron. 2019, 135, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Piri, S.; Piri, F.; Yaftian, M.R.; Zamani, A. Imprinted Azorubine electrochemical sensor based upon composition of MnO2 and 1-naphthylamine on graphite nanopowder. J. Iran. Chem. Soc. 2018, 15, 2713–2720. [Google Scholar] [CrossRef]
- Yarman, A.; Kurbanoglu, S.; Jetzschmann, K.J.; Ozkan, S.A.; Wollenberger, U.; Scheller, F.W. Electrochemical MIP-Sensors for Drugs. Curr. Med. Chem. 2018, 25, 4007–4019. [Google Scholar] [CrossRef] [PubMed]
- Gokoglan, T.C.; Soylemez, S.; Kesik, M.; Unay, H.; Sayin, S.; Yildiz, H.B.; Cirpan, A.; Toppare, L. A novel architecture based on a conducting polymer and calixarene derivative: Its synthesis and biosensor construction. RSC Adv. 2015, 5, 35940–35947. [Google Scholar] [CrossRef]
- Ahuja, T.; Mir, I.; Kumar, D. Biomolecular immobilization on conducting polymers for biosensing applications. Biomaterials 2007, 28, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Neo, W.T.; Ye, Q.; Chua, S.-J.; Xu, J. Conjugated polymer-based electrochromics: Materials, device fabrication and application prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Soylemez, S.; Hacioglu, S.O.; Kesik, M.; Unay, H.; Cirpan, A.; Toppare, L. A Novel and Effective Surface Design: Conducting Polymer/β-Cyclodextrin Host–Guest System for Cholesterol Biosensor. ACS Appl. Mater. Interfaces 2014, 6, 18290–18300. [Google Scholar] [CrossRef]
- Wang, B.; Tang, J.; Wang, F. Electrochemical polymerization of aniline. Synth. Met. 1987, 18, 323–328. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makaraviciute, A.; Ramanaviciene, A. Site-directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron. 2013, 50, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Baleviciute, I.; Balevicius, Z.; Makaraviciute, A.; Ramanaviciene, A.; Ramanavicius, A. Study of antibody/antigen binding kinetics by total internal reflection ellipsometry. Biosens. Bioelectron. 2013, 39, 170–176. [Google Scholar] [CrossRef]
- Rattanarat, P.; Suea-Ngam, A.; Ruecha, N.; Siangproh, W.; Henry, C.S.; Srisa-Art, M.; Chailapakul, O. Graphene-polyaniline modified electrochemical droplet-based microfluidic sensor for high-throughput determination of 4-aminophenol. Anal. Chim. Acta 2016, 925, 51–60. [Google Scholar] [CrossRef]
- Gurudatt, N.G.; Chung, S.; Kim, J.-M.; Kim, M.-H.; Jung, D.-K.; Han, J.-Y.; Shim, Y.-B. Separation detection of different circulating tumor cells in the blood using an electrochemical microfluidic channel modified with a lipid-bonded conducting polymer. Biosens. Bioelectron. 2019, 146, 111746. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Tsai, W.-B.; Garrison, M.D.; Ferrari, S.; Ratner, B.D. Template-imprinted nanostructured surfaces for protein recognition. Nature 1999, 398, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Jeans, C.W.; Brain, K.R.; Allender, C.J.; Hlady, V.; Britt, D.W. From 3D to 2D: A review of the molecular imprinting of proteins. Biotechnol. Prog. 2006, 22, 1474–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, S.; Whitcombe, M.J.; Piletsky, S.A. Size matters: Challenges in imprinting macromolecules. Prog. Polym. Sci. 2014, 39, 145–163. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Molecularly imprinted polypyrrole-based synthetic receptor for direct detection of bovine leukemia virus glycoproteins. Biosens. Bioelectron. 2004, 20, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Hishiya, T. Molecular imprinting of proteins emerging as a tool for protein recognition. Org. Biomol. Chem. 2008, 6, 2459. [Google Scholar] [CrossRef]
- Erdőssy, J.; Horváth, V.; Yarman, A.; Scheller, F.W.; Gyurcsányi, R.E. Electrosynthesized molecularly imprinted polymers for protein recognition. Trends Anal. Chem. 2016, 79, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Kryscio, D.R.; Fleming, M.Q.; Peppas, N.A. Protein Conformational Studies for Macromolecularly Imprinted Polymers. Macromol. Biosci. 2012, 12, 1137–1144. [Google Scholar] [CrossRef]
- Menaker, A.; Syritski, V.; Reut, J.; Öpik, A.; Horváth, V.; Gyurcsányi, R.E. Electrosynthesized Surface-Imprinted Conducting Polymer Microrods for Selective Protein Recognition. Adv. Mater. 2009, 21, 2271–2275. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template-“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosini, S.; Beyazit, S.; Haupt, K.; Tse Sum Bui, B. Solid-phase synthesis of molecularly imprinted nanoparticles for protein recognition. Chem. Commun. 2013, 49, 6746. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Y.; Guo, M.; Lin, B.; Zhang, L. A sensitive determination of albumin in urine by molecularly imprinted electrochemical biosensor based on dual-signal strategy. Sens. Actuators B Chem. 2019, 288, 564–570. [Google Scholar] [CrossRef]
- Tretjakov, A.; Syritski, V.; Reut, J.; Boroznjak, R.; Volobujeva, O.; Öpik, A. Surface molecularly imprinted polydopamine films for recognition of immunoglobulin G. Microchim. Acta 2013, 180, 1433–1442. [Google Scholar] [CrossRef]
- Tamboli, V.K.; Bhalla, N.; Jolly, P.; Bowen, C.R.; Taylor, J.T.; Bowen, J.L.; Allender, C.J.; Estrela, P. Hybrid Synthetic Receptors on MOSFET Devices for Detection of Prostate Specific Antigen in Human Plasma. Anal. Chem. 2016, 88, 11486–11490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q. A Molecularly Imprinted Electrochemical Sensor Based on Polypyrrole/Carbon Nanotubes Composite for the Detection of S-ovalbumin in Egg White. Int. J. Electrochem. Sci. 2017, 12, 3965–3981. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Sigolaeva, L.V.; Kuzikov, A.V.; Archakov, A.I. Electrosynthesis and binding properties of molecularly imprinted poly-o-phenylenediamine for selective recognition and direct electrochemical detection of myoglobin. Biosens. Bioelectron. 2016, 86, 330–336. [Google Scholar] [CrossRef]
- Stojanovic, Z.; Erdőssy, J.; Keltai, K.; Scheller, F.W.; Gyurcsányi, R.E. Electrosynthesized molecularly imprinted polyscopoletin nanofilms for human serum albumin detection. Anal. Chim. Acta 2017, 977, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarman, A.; Dechtrirat, D.; Bosserdt, M.; Jetzschmann, K.J.; Gajovic-Eichelmann, N.; Scheller, F.W. Cytochrome c-Derived Hybrid Systems Based on Moleculary Imprinted Polymers. Electroanalysis 2015, 27, 573–586. [Google Scholar] [CrossRef]
- Qian, L.-W.; Hu, X.-L.; Guan, P.; Gao, B.; Wang, D.; Wang, C.-L.; Li, J.; Du, C.-B.; Song, W.-Q. Thermal preparation of lysozyme-imprinted microspheres by using ionic liquid as a stabilizer. Anal. Bioanal. Chem. 2014, 406, 7221–7231. [Google Scholar] [CrossRef] [PubMed]
- Dolak, I.; Canpolat, G.; Ersöz, A.; Say, R. Metal chelate based site recognition of ceruloplasmin using molecularly imprinted polymer/cryogel system. Sep. Sci. Technol. 2020, 55, 199–208. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef] [PubMed]
- Kalecki, J.; Cieplak, M.; Dąbrowski, M.; Lisowski, W.; Kuhn, A.; Sharma, P.S. Hexagonally Packed Macroporous Molecularly Imprinted Polymers for Chemosensing of Follicle-Stimulating Hormone Protein. ACS Sens. 2020, 5, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Zhang, C.; Deng, Y.; Yang, G.; Li, S.; Tang, C.; He, N. A novel α -fetoprotein-MIP immunosensor based on AuNPs/PTh modified glass carbon electrode. Chin. Chem. Lett. 2018, 30, 160–162. [Google Scholar] [CrossRef]
- Karami, P.; Bagheri, H.; Johari-Ahar, M.; Khoshsafar, H.; Arduini, F.; Afkhami, A. Dual-modality impedimetric immunosensor for early detection of prostate-specific antigen and myoglobin markers based on antibody-molecularly imprinted polymer. Talanta 2019, 202, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tan, W.; Xu, H. Protein molecularly imprinted polyacrylamide membrane: For hemoglobin sensing. Analyst 2010, 135, 2523. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Turner, A.P.F.; Tiwari, A. Electrochemical evaluation of troponin T imprinted polymer receptor. Biosens. Bioelectron. 2014, 59, 160–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, S.; Dehghani, M.; Nasirizadeh, N.; Azimzadeh, M. An azithromycin electrochemical sensor based on an aniline MIP film electropolymerized on a gold nano urchins/graphene oxide modified glassy carbon electrode. J. Electroanal. Chem. 2018, 829, 27–34. [Google Scholar] [CrossRef]
- Nikitina, V.N.; Zaryanov, N.V.; Kochetkov, I.R.; Karyakina, E.E.; Yatsimirsky, A.K.; Karyakin, A.A. Molecular imprinting of boronate functionalized polyaniline for enzyme-free selective detection of saccharides and hydroxy acids. Sens. Actuators B Chem. 2017, 246, 428–433. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Z.; Si, S. Potentiometric urea biosensor based on immobilization of urease onto molecularly imprinted TiO2 film. J. Electroanal. Chem. 2009, 635, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Caserta, G.; Yarman, A.; Supala, E.; Waffo, A.F.T.; Wollenberger, U.; Gyurcsányi, R.E.; Zebger, I.; Scheller, F.W. “Out of Pocket” Protein Binding—A Dilemma of Epitope Imprinted Polymers Revealed for Human Hemoglobin. Chemosensors 2021, 9, 128. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies—Synthesis and Applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slinchenko, O.; Rachkov, A.; Miyachi, H.; Ogiso, M.; Minoura, N. Imprinted polymer layer for recognizing double-stranded DNA. Biosens. Bioelectron. 2004, 20, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Babamiri, B.; Salimi, A.; Hallaj, R. A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens. Bioelectron. 2018, 117, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Muti, M.; Soysal, M.; Nacak, F.M.; Gençdağ, K.; Karagözler, A.E. A Novel DNA Probe Based on Molecularly Imprinted Polymer Modified Electrode for the Electrochemical Monitoring of DNA. Electroanalysis 2015, 27, 1368–1377. [Google Scholar] [CrossRef]
- Zamora-Gálvez, A.; Morales-Narváez, E.; Mayorga-Martinez, C.C.; Merkoçi, A. Nanomaterials connected to antibodies and molecularly imprinted polymers as bio/receptors for bio/sensor applications. Appl. Mater. Today 2017, 9, 387–401. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, Z.; Li, J.; Ma, X.; Chen, L.; Yang, X. Molecular imprinting technology for microorganism analysis. TrAC Trends Anal. Chem. 2018, 106, 190–201. [Google Scholar] [CrossRef]
- Iskierko, Z.; Sharma, P.S.; Bartold, K.; Pietrzyk-Le, A.; Noworyta, K.; Kutner, W. Molecularly imprinted polymers for separating and sensing of macromolecular compounds and microorganisms. Biotechnol. Adv. 2016, 34, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, X.; Zhang, L.; Gao, J.; Ma, Q. Electrochemiluminescence Detection of Escherichia coli O157:H7 Based on a Novel Polydopamine Surface Imprinted Polymer Biosensor. ACS Appl. Mater. Interfaces 2017, 9, 5430–5436. [Google Scholar] [CrossRef]
- Ait Lahcen, A.; Arduini, F.; Lista, F.; Amine, A. Label-free electrochemical sensor based on spore-imprinted polymer for Bacillus cereus spore detection. Sens. Actuators B Chem. 2018, 276, 114–120. [Google Scholar] [CrossRef]
- Hayden, O.; Lieberzeit, P.A.; Blaas, D.; Dickert, F.L. Artificial Antibodies for Bioanalyte Detection—Sensing Viruses and Proteins. Adv. Funct. Mater. 2006, 16, 1269–1278. [Google Scholar] [CrossRef]
- Takátsy, A.; Végvári, Á.; Hjertén, S.; Kilár, F. Universal method for synthesis of artificial gel antibodies by the imprinting approach combined with a unique electrophoresis technique for detection of minute structural differences of proteins, viruses and cells (bacteria). Ib. Gel antibodies against pr. Electrophoresis 2007, 28, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Hayden, O.; Dickert, F.L. Selective Microorganism Detection with Cell Surface Imprinted Polymers. Adv. Mater. 2001, 13, 1480–1483. [Google Scholar] [CrossRef]
- Zaidi, S.A. Bacterial Imprinting Methods and Their Applications: An Overview. Crit. Rev. Anal. Chem. 2020, 51, 609–618. [Google Scholar] [CrossRef]
- Golabi, M.; Kuralay, F.; Jager, E.W.H.; Beni, V.; Turner, A.P.F. Electrochemical bacterial detection using poly(3-aminophenylboronic acid)-based imprinted polymer. Biosens. Bioelectron. 2017, 93, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef]
- Gupta, G.; Singh, P.K.; Boopathi, M.; Kamboj, D.V.; Singh, B.; Vijayaraghavan, R. Molecularly imprinted polymer for the recognition of biological warfare agent staphylococcal enterotoxin B based on Surface Plasmon Resonance. Thin Solid Films 2010, 519, 1115–1121. [Google Scholar] [CrossRef]
- Bartold, K.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Oligonucleotide Analogs and Mimics for Sensing Macromolecular Biocompounds. Trends Biotechnol. 2019, 37, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Bochenkov, V.E.; Runager, K.; Aslan, H.; Dong, M.; Enghild, J.J.; de Freitas, V.; Ferreira Sales, M.G.; Sutherland, D.S. Molecular Imprinting of Complex Matrices at Localized Surface Plasmon Resonance Biosensors for Screening of Global Interactions of Polyphenols and Proteins. ACS Sens. 2016, 1, 258–264. [Google Scholar] [CrossRef]
- Lin, Z.-T.; DeMarr, V.; Bao, J.; Wu, T. Molecularly Imprinted Polymer-Based Biosensors: For the Early, Rapid Detection of Pathogens, Biomarkers, and Toxins in Clinical, Environmental, or Food Samples. IEEE Nanotechnol. Mag. 2018, 12, 6–13. [Google Scholar] [CrossRef]
- Rachkov, A.E.; Cheong, S.-H.; El’skaya, A.V.; Yano, K.; Karube, I. Molecularly imprinted polymers as artificial steroid receptors. Polym. Adv. Technol. 1998, 9, 511–519. [Google Scholar] [CrossRef]
- Des Azevedo, S.; Lakshmi, D.; Chianella, I.; Whitcombe, M.J.; Karim, K.; Ivanova-Mitseva, P.K.; Subrahmanyam, S.; Piletsky, S.A. Molecularly Imprinted Polymer-Hybrid Electrochemical Sensor for the Detection of β-Estradiol. Ind. Eng. Chem. Res. 2013, 52, 13917–13923. [Google Scholar] [CrossRef]
- Spychalska, K.; Zając, D.; Baluta, S.; Halicka, K.; Cabaj, J. Functional Polymers Structures for (Bio)Sensing Application—A Review. Polymers 2020, 12, 1154. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Tharpa, K.; Dima, Ş.-O. Molecularly Imprinted Membranes: Past, Present, and Future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing—Synthesis, characterisation and application. Sens. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of High Polymer Solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Lu, H.; Du, S. A phenomenological thermodynamic model for the chemo-responsive shape memory effect in polymers based on Flory-Huggins solution theory. Polym. Chem. 2014, 5, 1155–1162. [Google Scholar] [CrossRef]
- Antipchik, M.; Dzhuzha, A.; Sirotov, V.; Tennikova, T.; Korzhikova-Vlakh, E. Molecularly imprinted macroporous polymer monolithic layers for L-phenylalanine recognition in complex biological fluids. J. Appl. Polym. Sci. 2021, 138, 50070. [Google Scholar] [CrossRef]
- Hildebrand, J.H. The term ‘regular solution’. Nature 1951, 168, 868. [Google Scholar] [CrossRef]
- Menge, H.; Hotopf, S.; Pönitzsch, S.; Richter, S.; Arndt, K.F.; Schneider, H.; Heuert, U. Investigation on the swelling behaviour in poly(dimethylsiloxane) rubber networks using nmr and compression measurements. Polymer 1999, 40, 5303–5313. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Leng, J.; Du, S. Qualitative separation of the physical swelling effect on the recovery behavior of shape memory polymer. Eur. Polym. J. 2010, 46, 1908–1914. [Google Scholar] [CrossRef]
- Zanuy, D.; Fabregat, G.; Ferreira, C.A.; Alemán, C. A molecular dynamics study on glucose molecular recognition by a non-enzymatic selective sensor based on a conducting polymer. Phys. Chem. Chem. Phys. 2019, 21, 8099–8107. [Google Scholar] [CrossRef] [PubMed]
- Boroznjak, R.; Reut, J.; Tretjakov, A.; Lomaka, A.; Öpik, A.; Syritski, V. A computational approach to study functional monomer-protein molecular interactions to optimize protein molecular imprinting. J. Mol. Recognit. 2017, 30, e2635. [Google Scholar] [CrossRef] [PubMed]
- Lach, P.; Sharma, P.S.; Golebiewska, K.; Cieplak, M.; D’Souza, F.; Kutner, W. Molecularly Imprinted Polymer Chemosensor for Selective Determination of an N -Nitroso- l -proline Food Toxin. Chem. A Eur. J. 2017, 23, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Garcia-Cruz, A.; Cieplak, M.; Noworyta, K.R.; Kutner, W. ‘Gate effect’ in molecularly imprinted polymers: The current state of understanding. Curr. Opin. Electrochem. 2019, 16, 50–56. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.-T.; Liu, Y.; Qi, X.-M.; Jin, H.-G.; Yang, C.; Liu, J.; Li, G.-L.; He, Q.-G. Recent advances in black phosphorus-based electrochemical sensors: A review. Anal. Chim. Acta 2021, 1170, 338480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, G.; Wu, D.; Xiong, C.; Zheng, L.; Ding, Y.; Lu, H.; Zhang, G.; Qiu, L. Highly selective and sensitive sensor based on an organic electrochemical transistor for the detection of ascorbic acid. Biosens. Bioelectron. 2018, 100, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Turemis, M.; Zappi, D.; Giardi, M.T.; Basile, G.; Ramanaviciene, A.; Kapralovs, A.; Ramanavicius, A.; Viter, R. ZnO/polyaniline composite based photoluminescence sensor for the determination of acetic acid vapor. Talanta 2020, 211, 120658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.P.; Rahman, M.A.; Srivastava, S.; Kang, J.-S.; McGillivray, D.; Abd-Ellah, M.; Heinig, N.F.; Leung, K.T. Highly Conducting Hybrid Silver-Nanowire-Embedded Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) for High-Efficiency Planar Silicon/Organic Heterojunction Solar Cells. ACS Nano 2018, 12, 9495–9503. [Google Scholar] [CrossRef] [PubMed]
- Benachio, I.; Lobato, A.; Gonçalves, L.M. Employing molecularly imprinted polymers in the development of electroanalytical methodologies for antibiotic determination. J. Mol. Recognit. 2021, 34, e2878. [Google Scholar] [CrossRef] [PubMed]
- Yarman, A.; Scheller, F.W. How Reliable Is the Electrochemical Readout of MIP Sensors? Sensors 2020, 20, 2677. [Google Scholar] [CrossRef] [PubMed]
- Yarman, A. Development of a molecularly imprinted polymer-based electrochemical sensor for tyrosinase. Turk. J. Chem. 2018, 42, 346–354. [Google Scholar] [CrossRef]
- Burow, M.; Minoura, N. Molecular Imprinting: Synthesis of Polymer Particles with Antibody-like Binding Characteristics for Glucose Oxidase. Biochem. Biophys. Res. Commun. 1996, 227, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Jetzschmann, K.J.; Jágerszki, G.; Dechtrirat, D.; Yarman, A.; Gajovic-Eichelmann, N.; Gilsing, H.-D.; Schulz, B.; Gyurcsányi, R.E.; Scheller, F.W. Vectorially Imprinted Hybrid Nanofilm for Acetylcholinesterase Recognition. Adv. Funct. Mater. 2015, 25, 5178–5183. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-Y.; Chen, Y.-C.; Sheu, D.-C.; Chou, T.C. Molecularly imprinted polymers for the recognition of sodium dodecyl sulfate denatured creatine kinase. J. Taiwan Inst. Chem. Eng. 2012, 43, 188–194. [Google Scholar] [CrossRef]
- Jetzschmann, K.J.; Yarman, A.; Rustam, L.; Kielb, P.; Urlacher, V.B.; Fischer, A.; Weidinger, I.M.; Wollenberger, U.; Scheller, F.W. Molecular LEGO by domain-imprinting of cytochrome P450 BM3. Colloids Surf. B Biointerfaces 2018, 164, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yarman, A.; Jetzschmann, K.J.; Jeoung, J.-H.; Schad, D.; Dobbek, H.; Wollenberger, U.; Scheller, F.W. Molecularly Imprinted Electropolymer for a Hexameric Heme Protein with Direct Electron Transfer and Peroxide Electrocatalysis. Sensors 2016, 16, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarman, A. Electrosynthesized Molecularly Imprinted Polymer for Laccase Using the Inactivated Enzyme as the Target. Bull. Korean Chem. Soc. 2018, 39, 483–488. [Google Scholar] [CrossRef]
- Bossi, A.; Piletsky, S.A.; Piletska, E.V.; Righetti, P.G.; Turner, A.P.F. Surface-Grafted Molecularly Imprinted Polymers for Protein Recognition. Anal. Chem. 2001, 73, 5281–5286. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Bai, C.; Teng, Y.; Zhang, J.; Peng, J.; Fang, Z.; Xu, W. Study of horseradish peroxidase and hydrogen peroxide bi-analyte sensor with boronate affinity-based molecularly imprinted film. Can. J. Chem. 2019, 97, 833–839. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, R.; Guo, H.; Wei, Y.; Yang, W. A facile horseradish peroxidase electrochemical biosensor with surface molecular imprinting based on polyaniline nanotubes. J. Electroanal. Chem. 2018, 817, 184–194. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, H.; Zhang, C.; Qu, L.; Yu, L. A novel molecularly imprinted sensor based on PtCu bimetallic nanoparticle deposited on PSS functionalized graphene with peroxidase-like activity for selective determination of puerarin. Talanta 2020, 210, 120621. [Google Scholar] [CrossRef] [PubMed]
- Cutivet, A.; Schembri, C.; Kovensky, J.; Haupt, K. Molecularly Imprinted Microgels as Enzyme Inhibitors. J. Am. Chem. Soc. 2009, 131, 14699–14702. [Google Scholar] [CrossRef] [PubMed]
- Emir Diltemiz, S.; Keçili, R.; Ersöz, A.; Say, R. Molecular Imprinting Technology in Quartz Crystal Microbalance (QCM) Sensors. Sensors 2017, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- Merkoçi, A.; Alegret, S. New materials for electrochemical sensing IV. Molecular imprinted polymers. TrAC Trends Anal. Chem. 2002, 21, 717–725. [Google Scholar] [CrossRef]
- Eslami, M.R.; Alizadeh, N. Nanostructured conducting molecularly imprinted polypyrrole based quartz crystal microbalance sensor for naproxen determination and its electrochemical impedance study. RSC Adv. 2016, 6, 9387–9395. [Google Scholar] [CrossRef]
- Pietrzyk, A.; Suriyanarayanan, S.; Kutner, W.; Chitta, R.; D’Souza, F. Selective Histamine Piezoelectric Chemosensor Using a Recognition Film of the Molecularly Imprinted Polymer of Bis(bithiophene) Derivatives. Anal. Chem. 2009, 81, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Noworyta, K.; Kutner, W. Imprinted polymer-based enantioselective acoustic sensor using a quartz crystal microbalance. Anal. Commun. 1999, 36, 391. [Google Scholar] [CrossRef]
- Eslami, M.R.; Alizadeh, N. A dual usage smart sorbent/recognition element based on nanostructured conducting molecularly imprinted polypyrrole for simultaneous potential-induced nanoextraction/determination of ibuprofen in biomedical samples by quartz crystal microbalance sensor. Sens. Actuators B Chem. 2015, 220, 880–887. [Google Scholar] [CrossRef]
- Dabrowski, M.; Lach, P.; Cieplak, M.; Kutner, W. Nanostructured molecularly imprinted polymers for protein chemosensing. Biosens. Bioelectron. 2018, 102, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Boysen, R.I.; Schwarz, L.J.; Nicolau, D.V.; Hearn, M.T.W. Molecularly imprinted polymer membranes and thin films for the separation and sensing of biomacromolecules. J. Sep. Sci. 2017, 40, 314–335. [Google Scholar] [CrossRef]
- Saylan, Y.; Yilmaz, F.; Özgür, E.; Derazshamshir, A.; Yavuz, H.; Denizli, A. Molecular Imprinting of Macromolecules for Sensor Applications. Sensors 2017, 17, 898. [Google Scholar] [CrossRef] [PubMed]
- Karaseva, N.A.; Pluhar, B.; Beliaeva, E.A.; Ermolaeva, T.N.; Mizaikoff, B. Synthesis and application of molecularly imprinted polymers for trypsin piezoelectric sensors. Sens. Actuators B Chem. 2019, 280, 272–279. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, D.; Guo, T. Construction of a novel macroporous imprinted biosensor based on quartz crystal microbalance for ribonuclease Adetection. Biosens. Bioelectron. 2013, 42, 80–86. [Google Scholar] [CrossRef]
- Chunta, S.; Suedee, R.; Boonsriwong, W.; Lieberzeit, P.A. Biomimetic sensors targeting oxidized-low-density lipoprotein with molecularly imprinted polymers. Anal. Chim. Acta 2020, 1116, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bartold, K.; Pietrzyk-Le, A.; Huynh, T.-P.; Iskierko, Z.; Sosnowska, M.; Noworyta, K.; Lisowski, W.; Sannicolò, F.; Cauteruccio, S.; Licandro, E.; et al. Programmed Transfer of Sequence Information into a Molecularly Imprinted Polymer for Hexakis(2,2′-bithien-5-yl) DNA Analogue Formation toward Single-Nucleotide-Polymorphism Detection. ACS Appl. Mater. Interfaces 2017, 9, 3948–3958. [Google Scholar] [CrossRef] [Green Version]
- Mosch, H.L.K.S.; Akintola, O.; Plass, W.; Höppener, S.; Schubert, U.S.; Ignaszak, A. Specific Surface versus Electrochemically Active Area of the Carbon/Polypyrrole Capacitor: Correlation of Ion Dynamics Studied by an Electrochemical Quartz Crystal Microbalance with BET Surface. Langmuir 2016, 32, 4440–4449. [Google Scholar] [CrossRef]
- Hu, Y.; Xing, H.; Li, G.; Wu, M. Magnetic Imprinted Polymer-Based Quartz Crystal Microbalance Sensor for Sensitive Label-Free Detection of Methylene Blue in Groundwater. Sensors 2020, 20, 5506. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Nishitani, S.; Kajisa, T. Molecularly imprinted polymer-based bioelectrical interfaces with intrinsic molecular charges. RSC Adv. 2020, 10, 16999–17013. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, Q.; Wang, Y.; Liu, X.; Wei, T. Surface plasmon resonance sensor for theophylline using a water-compatible molecularly imprinted film. Anal. Methods 2016, 8, 2349–2356. [Google Scholar] [CrossRef]
- Bhunia, S.; Dey, N.; Pradhan, A.; Bhattacharya, S. A conjugated microporous polymer based visual sensing platform for aminoglycoside antibiotics in water. Chem. Commun. 2018, 54, 7495–7498. [Google Scholar] [CrossRef]
- Tan, J.; Chen, W.-J.; Guo, J. Conjugated microporous polymers with distinctive π -electronic properties exhibiting enhanced optical applications. Chin. Chem. Lett. 2016, 27, 1405–1411. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Wang, Z. Creation of Carbazole-Based Fluorescent Porous Polymers for Recognition and Detection of Various Pesticides in Water. ACS Sens. 2020, 5, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Mo, W.; Tang, H.; Cheng, Z.; Chen, Y.; Zhang, S.; Ma, H.; Li, B.; Li, X. Dendrimer-Based, High-Luminescence Conjugated Microporous Polymer Films for Highly Sensitive and Selective Volatile Organic Compound Sensor Arrays. Adv. Funct. Mater. 2020, 30, 1910275. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kurilcik, N.; Jursenas, S.; Finkelsteinas, A.; Ramanaviciene, A. Conducting polymer based fluorescence quenching as a new approach to increase the selectivity of immunosensors. Biosens. Bioelectron. 2007, 23, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Baleg, A.A.; Baker, P.; Iwuoha, E.; Amine, A. Synthesis and electrochemical characterization of nanostructured magnetic molecularly imprinted polymers for 17-β-Estradiol determination. Sens. Actuators B Chem. 2017, 241, 698–705. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Y.; Bai, J.; Ning, B.; Sun, S.; Hong, X.; Liu, Y.; Liu, Y.; Gao, Z. A novel electrochemical sensor based on electropolymerized molecularly imprinted polymer and gold nanomaterials amplification for estradiol detection. Sens. Actuators B Chem. 2014, 200, 69–75. [Google Scholar] [CrossRef]
- Duan, D.; Si, X.; Ding, Y.; Li, L.; Ma, G.; Zhang, L.; Jian, B. A novel molecularly imprinted electrochemical sensor based on double sensitization by MOF/CNTs and Prussian blue for detection of 17β-estradiol. Bioelectrochemistry 2019, 129, 211–217. [Google Scholar] [CrossRef]
- Lee, M.-H.; Chen, Y.-C.; Ho, M.-H.; Lin, H.-Y. Optical recognition of salivary proteins by use of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles. Anal. Bioanal. Chem. 2010, 397, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Cui, H.; Chao, J.; Huang, K.; Wang, X.; Zhou, Y.; Jing, T. Colorimetric determination of tetrabromobisphenol A based on enzyme-mimicking activity and molecular recognition of metal-organic framework-based molecularly imprinted polymers. Microchim. Acta 2020, 187, 142. [Google Scholar] [CrossRef] [PubMed]
- Bosserdt, M.; Erdőssy, J.; Lautner, G.; Witt, J.; Köhler, K.; Gajovic-Eichelmann, N.; Yarman, A.; Wittstock, G.; Scheller, F.W.; Gyurcsányi, R.E. Microelectrospotting as a new method for electrosynthesis of surface-imprinted polymer microarrays for protein recognition. Biosens. Bioelectron. 2015, 73, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Meng, Z.; Xue, M.; Shea, K.J. Molecular imprinted photonic crystal for sensing of biomolecules. Mol. Impr. 2016, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cieplak, M.; Węgłowski, R.; Iskierko, Z.; Węgłowska, D.; Sharma, P.S.; Noworyta, K.R.; D’Souza, F.; Kutner, W. Protein Determination with Molecularly Imprinted Polymer Recognition Combined with Birefringence Liquid Crystal Detection. Sensors 2020, 20, 4692. [Google Scholar] [CrossRef] [PubMed]
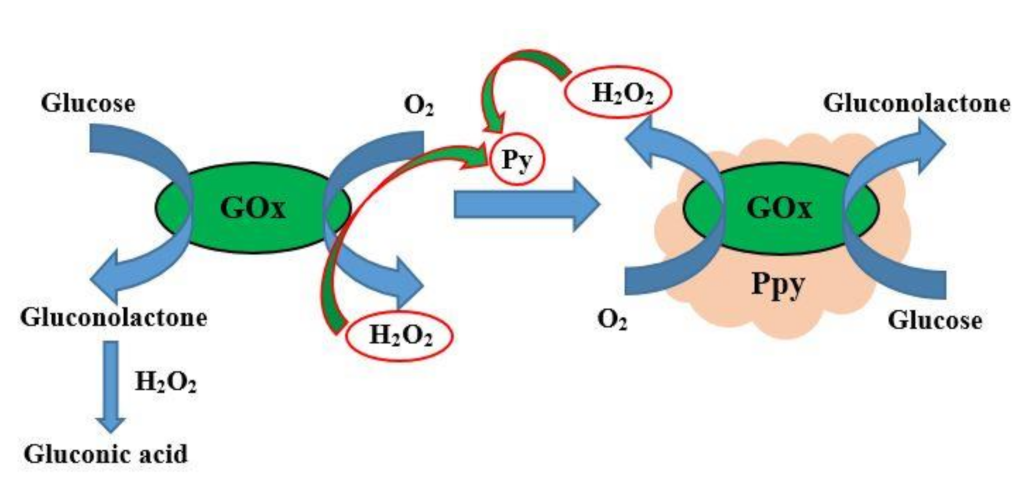
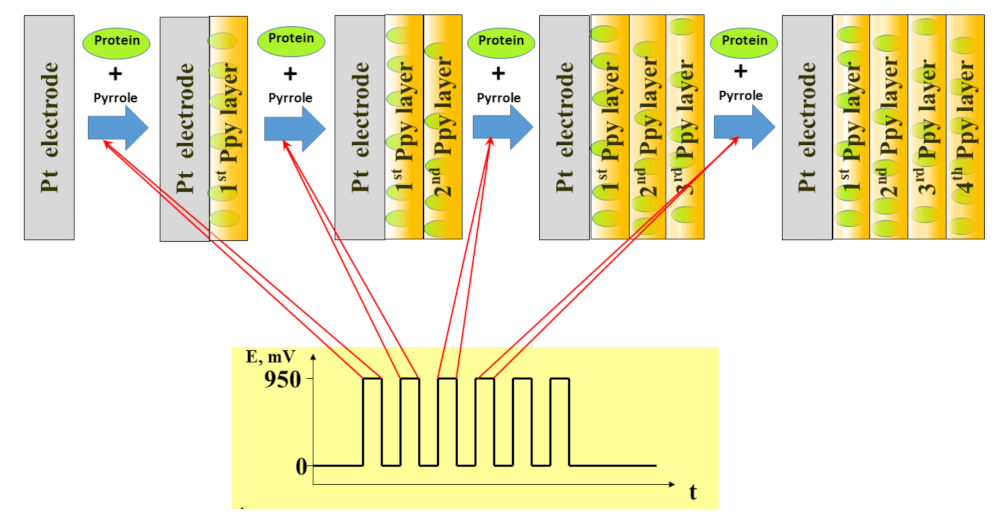

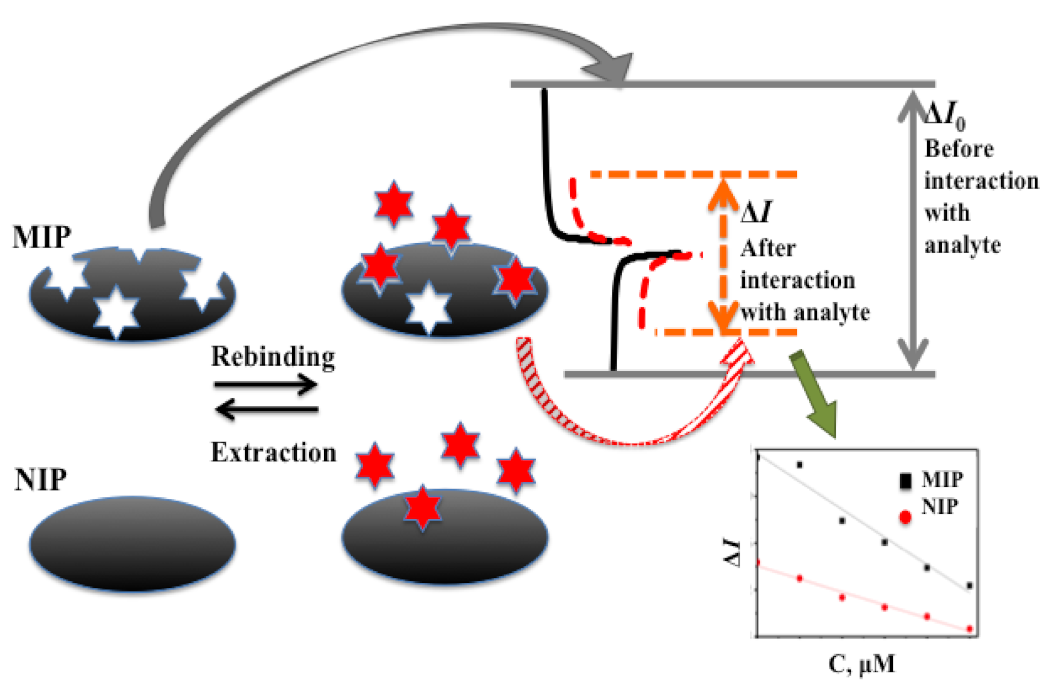
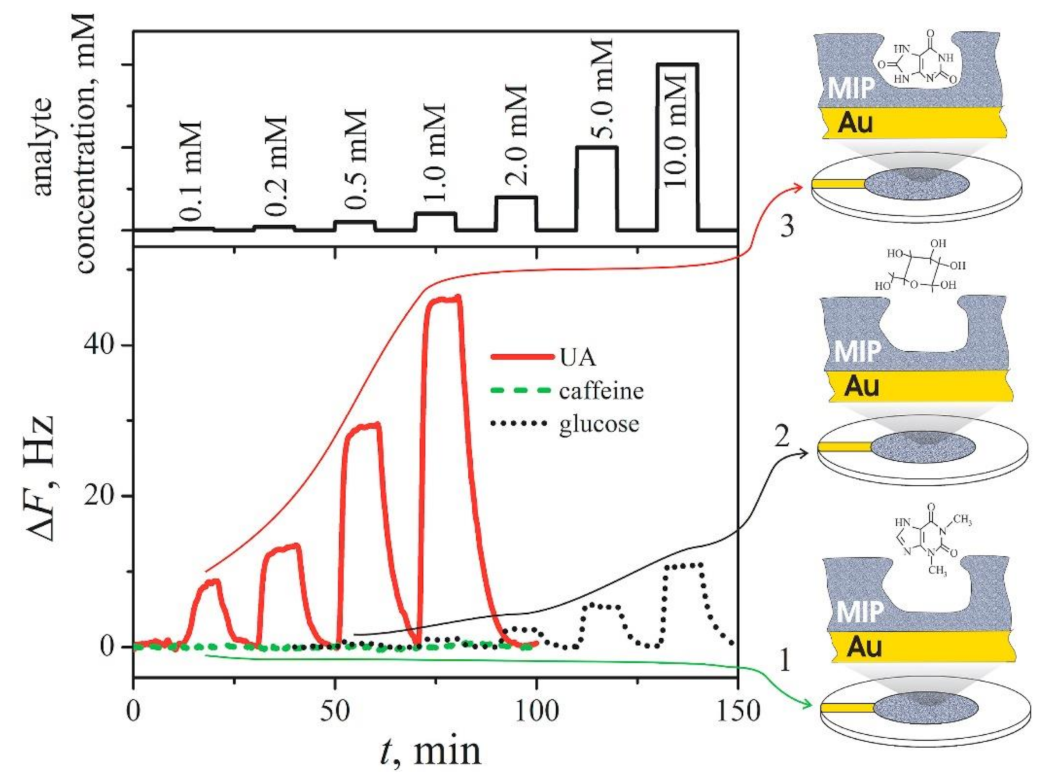
| Analyte | Polymer and Modifiers of the Polymer | Method of Analysis | Analytical Parameters: Linear Range (LR), Limit of Detection (LOD) | Ref. |
|---|---|---|---|---|
| Neurotransmitters: | ||||
| Dopamine | o-phenylenediamine (OPD)-based MIP with polypyrrole nanowires on glassy carbon electrode | Differential pulse voltammetry (DPV) | LR from 50 nM to 0.1 mM; LOD 33 nM | [84] |
| o-aminophenol (other monomers were bromophenol blue, picolinic acid, lactamic acid, and pyrrole) on nanoporous Au–Ag alloy microrod (NPAMR) as working electrode | Cyclic voltammetry (CV) | LR from 0.2 pM to 20 nM; LOD 76.3 fM | [85] | |
| Polypyrrole on S-MoSe2/NSG/Au nanocomposite on GCE | DPV | LR from 0.05 μM to 1000 μM; LOD 0.02 μM | [109] | |
| Polypyrrole on graphene quantum dots (GQDs)/TiO2 nanotubes (NTs) on Ti foil | Photoelectrochemical | LR from 0.05 μM to 12.5 μM; LOD 0.018 μM | [110] | |
| Polypyrrole on carboxyl-functionalized multi-walled carbon nanotubes (MWNTs-COOH) onto a glassy carbon electrode (GCE) | DPV | LR from 0.625 μM to 100 μM; LOD 60 nM | [111] | |
| Serotonin | Graphene quantum dots on two dimensional hexagonal boron nitride on GCE | CV | LT from 1 pM to 0.1 nM; LOD 0.2 pM | [97] |
| Acrylate-based MIP | EIS | LOD 3.2 nM | [112] | |
| 5-hydroxy tryptophan (5-HTP) and acrylamide (AM) with carbon nanotubes on GCE | DPV and CV | LR from 5.4 nM to 1.8 μM; LOD 0.18 nM | [113] | |
| Histamine | Polypyrrole on boron doped nanocrystalline diamond electrode | EIS | [88] | |
| Metacrylic acid-based MIP on carbon paste electrode | CV | LR from 0.1 nM to 7 nM and from 7 nM to 40 μM; LOD 74 nM | [114] | |
| Metacrylic acid-based MIP on interdigitated electrode | EIS | LR from 100 ppm to 500 ppm In seafood samples | [115] | |
| p-aminobenzene sulfonic acid (p-ABSA) based MIP on GCE with gold nanoparticles | DPV | LR from 1 μM to 40 μM and 40 μM to 107 μM; LOD 0.6 μM, In beer and wine | [116] | |
| Epinephrine (adrenaline) | Polypyrrole-based MIP with silica nanoparticles and multiwalled carbon nanotubes on GCE | DPV | LR from 0.3 μM to 1 mM; LOD 30 nM | [104] |
| Nicotinamide-based MIP with reduced graphene oxide on GCE | CV | LR from 0.015 μM to 40 μM; LOD 1.97 nM | [102] | |
| Purine derivatives: | ||||
| Theophylline | Polypyrrole based MIP on boron-doped oxygen terminated nanocrystalline diamond as working electrode | EIS | [31] | |
| Polypyrrole based MIP on boron-doped oxygen terminated nanocrystalline diamond as working electrode | EIS | [86] | ||
| Poly(pyrrole-co-pyrrole-3-carboxylic acid) based MIP with polystyrene colloidal particles on QCM sensor | QCM | [117] | ||
| Caffeine | Polypyrrole based MIP on the gold coated QCM sensor | QCM | [15] | |
| Acrylate based MIP on SPE (Screen Printed Electrode). | Heat-Transfer Method (HTM) | 1 nM | [87] | |
| Uric acid | Polypyrrole based MIP on Gold- coated quartz crystal resonator | EQCM | [96] | |
| Polydopamine based MIP with carbon-enwrapped nickel nanoparticles (Ni@BC) on GCE | DPV | LR from 0.01 μM to 30 μM, LOD 8 nM | [118] | |
| Amino acids | ||||
| L-aspartic acid | Overoxidized polypyrrole based MIP on Gold- coated quartz crystal resonator | EQCM | - | [98] |
| Cysteine | Overoxidized polypyrrole based MIP on GCE with Au nanoparticles (AuNPs) | CV | [99] | |
| Tryptophan | Chitosan based MIP on an acetylene black paste electrode | CV | LR from 0.01 μM to 4 μM, LOD 8.0 nM | [119] |
| Sarcosine | Poly-aminothiophenol (p-ATP) based MIP on screen-printed gold electrode | EIS | LR from 0.011 μM to 17.9 μM, LOD 8.5 nM | [92] |
| Other analytes: | ||||
| Quercetin | Polypyrrole based MIP on GCE with MIL-101 (Cr) and MoS2 | DPV | LR from 0.1 μM to 10.5 μM and from 10.5 μM to 700 μM, LOD 0.06 μM | [89] |
| Gallic acid | Polypyrrole based MIP on gold electrode with Fe3O4@ZIF-67 | DPV | LR from 6 pM to 600 pM, LOD 0.297 pM | [90] |
| Bilirubin | Polypyrrole based MIP on ITO electrode coated with TiO2 | Photoelectrochemical | LR from 0.03 μM to 28 μM, LOD 1 nM | [91] |
| Testosterone | O-phenylenediamine (o-PD) based on GCE with graphene oxide (GO) | EIS | LR from 1 fM to 1 μm, LOD 0.4 fM | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramanavičius, S.; Morkvėnaitė-Vilkončienė, I.; Samukaitė-Bubnienė, U.; Ratautaitė, V.; Plikusienė, I.; Viter, R.; Ramanavičius, A. Electrochemically Deposited Molecularly Imprinted Polymer-Based Sensors. Sensors 2022, 22, 1282. https://doi.org/10.3390/s22031282
Ramanavičius S, Morkvėnaitė-Vilkončienė I, Samukaitė-Bubnienė U, Ratautaitė V, Plikusienė I, Viter R, Ramanavičius A. Electrochemically Deposited Molecularly Imprinted Polymer-Based Sensors. Sensors. 2022; 22(3):1282. https://doi.org/10.3390/s22031282
Chicago/Turabian StyleRamanavičius, Simonas, Inga Morkvėnaitė-Vilkončienė, Urtė Samukaitė-Bubnienė, Vilma Ratautaitė, Ieva Plikusienė, Roman Viter, and Arūnas Ramanavičius. 2022. "Electrochemically Deposited Molecularly Imprinted Polymer-Based Sensors" Sensors 22, no. 3: 1282. https://doi.org/10.3390/s22031282
APA StyleRamanavičius, S., Morkvėnaitė-Vilkončienė, I., Samukaitė-Bubnienė, U., Ratautaitė, V., Plikusienė, I., Viter, R., & Ramanavičius, A. (2022). Electrochemically Deposited Molecularly Imprinted Polymer-Based Sensors. Sensors, 22(3), 1282. https://doi.org/10.3390/s22031282











