1. Introduction
Nowadays, many elderly people have motor dysfunction and joint problems because of age factors, stroke, and spinal cord injuries. Due to this, they face many problems when walking, which strongly influences their lives [
1]. According to WHO data, mental illnesses and neurological disorders are major sources of morbidity, death, and disability. Mental, neurological, and behavioral diseases have a major impact on the world’s population, impacting more than 450 million individuals. According to the Global Burden of Disease Report, neurological and mental disorders account for four out of the six primary causes of years lived with disability, accounting for 33 percent of the years lived with disability and 13 percent of disability-adjusted life years (DALYs) [
2]. People with walking disabilities need to improve their walking patterns or capability by using rehabilitation and assistive devices [
3]. The brain-computer interface (BCI) is the best way to accommodate the neuro-rehabilitation process, by providing a communication pathway between the brain and the peripheral devices [
4]. The field of perceptible neuroscience scrutinizes itself with calibrating information processing models of the brain with the operational and structural (e.g., hemodynamic, metabolic, and electrical) features of the brain [
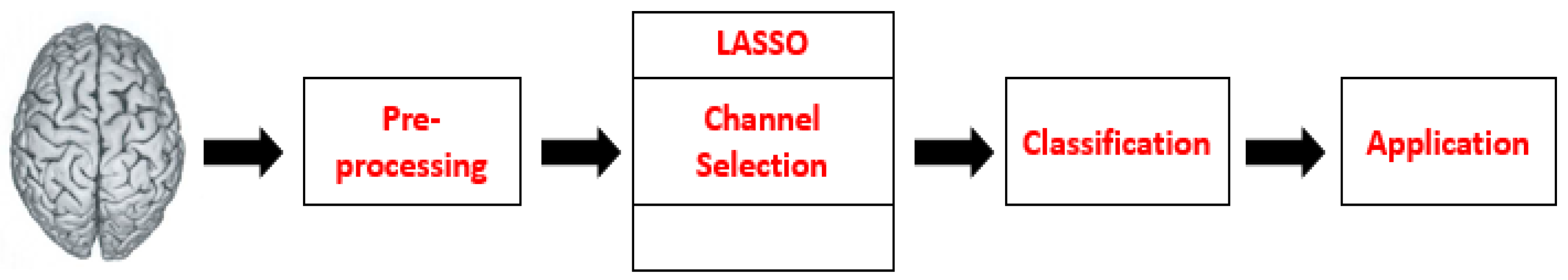
5]. In the last few years, the development of BCI has played an important role in the analysis of brain dysfunction disorders and musculoskeletal gait. A typical BCI system consists of the following five major stages: signal acquisition, pre-processing, feature extraction, classification, and control commands to peripheral devices. In the signal acquisition stage, brain signals are acquired using brain signal acquisition modalities. The acquired signals contain noises such as physiological, instrumentation, and motion artifacts. These errors can be removed with the help of appropriate filters in the pre-processing stage. After obtaining filtered and processed data, some useful features can be extracted. These extracted features contain intrinsic information related to brain activity. In the classification stage, the most suitable classifier is employed for the extracted features, to predict the response to a particular class. Furthermore, the brain activity discriminated by the classifier is used as a command to control the external devices. The general flow diagram of functional near-infrared spectroscopy (fNIRS)-BCI is shown in
Figure 1. BCI systems provide the end-user with full control over the channels used to communicate with the brain and external devices, regardless of the level of dependence on the output channel [
1].
For improving mild cognitive impairment (MCI), BCI based on functional near-infrared spectroscopy (fNIRS) had a positive result [
3]. It has been widely used in the rehabilitation process [
6]. fNIRS is utilized to concentrate on the brain areas of interest in eleven sicknesses, including stroke, MCI, traumatic brain injury, and harm recognition [
7]. There are several modalities used to acquire brain signals for rehabilitation, such as magnetic field measurement using magnetoencephalography (MEG) [
8,
9], electroencephalography (EEG), radioactive tracer-based positron emission tomography (PET) [
10,
11], functional magnetic resonance imaging (fMRI) [
12,
13], gamma emission-based single-photon emission computed tomography (SPECT) [
14,
15], and fNIRS. fNIRS is widely used due to its advantages of mobility and ease of use compared to other neuroimaging modalities when researching the brain basis of cognitive inputs during gait [
16,
17]. The fNIRS modality has been most commonly used over the recent decades, because of its portability and high spatial resolution. fNIRS is operated in wavelengths between 650 and 1000 nm; in this range, the blood oxygenation concentration (
HbO) and the blood deoxygenation concentration (HbR) are more clear [
7]. Several classifiers and techniques are applied to fNIRS signals [
6,
18], to improve the accuracy and efficiency of BCI systems, to help disabled and elderly people in their daily life [
7,
19].
For the classification of different brain activity, fNIRS-based BCI mostly extracted features such as mean, peak, variance, skewness, kurtosis values, etc., from the obtained data [
20]. In the literature, studies have been performed using single, multiple or a combination of features to classify two- or multiple-class fNIRS-BCI problems [
21]. Support vector machine (SVM) and linear discrimination analysis (LDA) are mainly used to classify walking and resting states, but the classification accuracy is low and needs to be improved [
22].
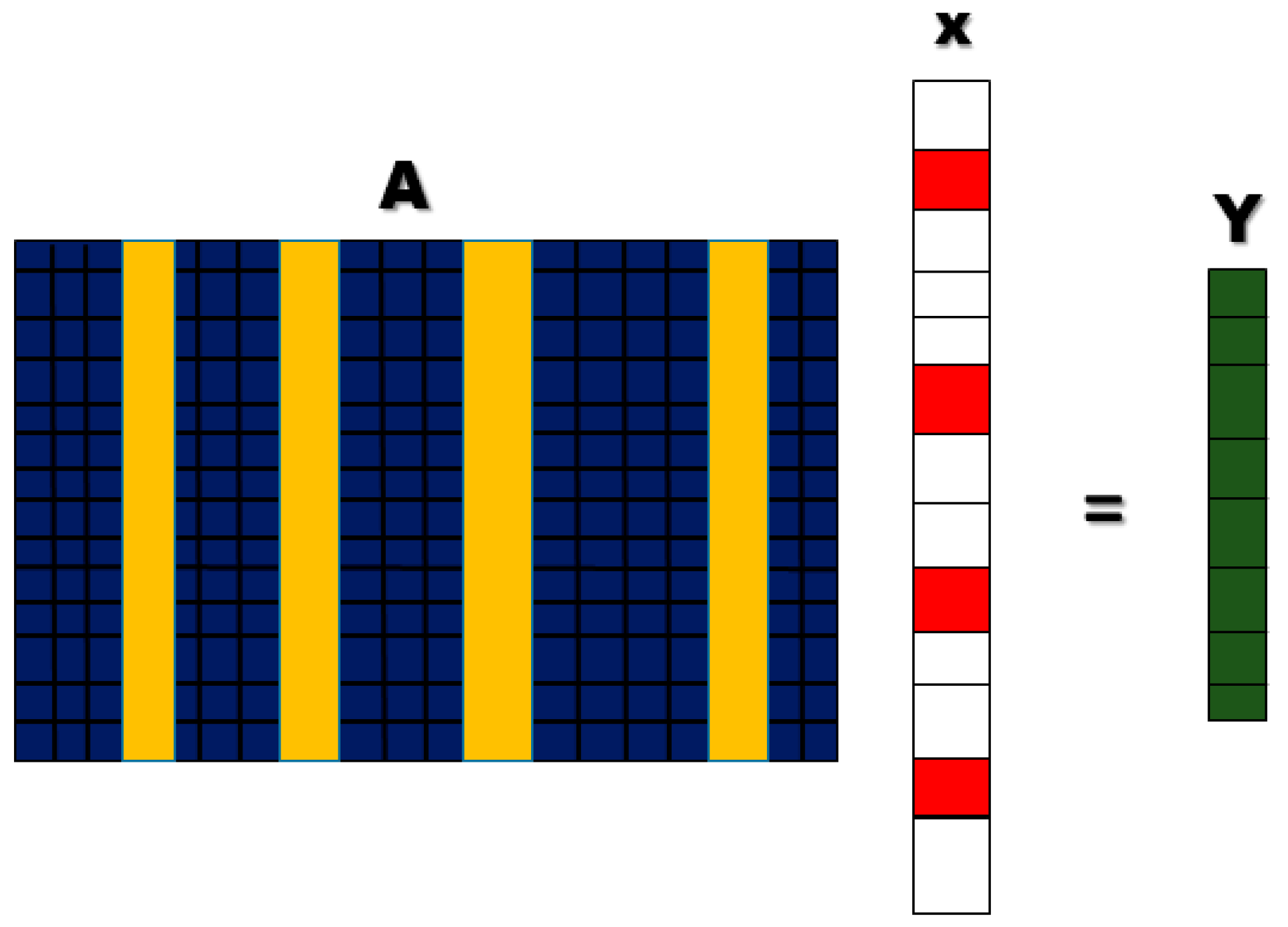
To improve the classification accuracy, it is important to introduce some new methods and technologies in the field of fNIRS-BCI. In this study, a new classification method is discussed, which is sparse representation-based classification (SRC). SRC has been used in the compressed sensing (CS) theory; the core concept of CS is that we can represent a huge amount of data with a few data points [
23]. Weighted SRC was applied to EEG-BCI to classify motor imagery, and achieved good classification accuracy results [
24]. Sparse representation-based classification was used to translate the motor imagery of a single index finger classification, with an accuracy of 81.32%; the results were used to construct a BCI-enhanced finger rehabilitation system [
25]. Optimization features, such as spatial-frequency-temporal, were calculated from the public dataset of EEG, and were used as predictors for SRC. The classification accuracy achieved was higher than on the original basis [
26]. Shin et al. classified motor images using SRC and compared the results with SVM. They discovered that SRC had better results than SVM and LDA, in terms of classification accuracy, testing duration, and noise robustness [
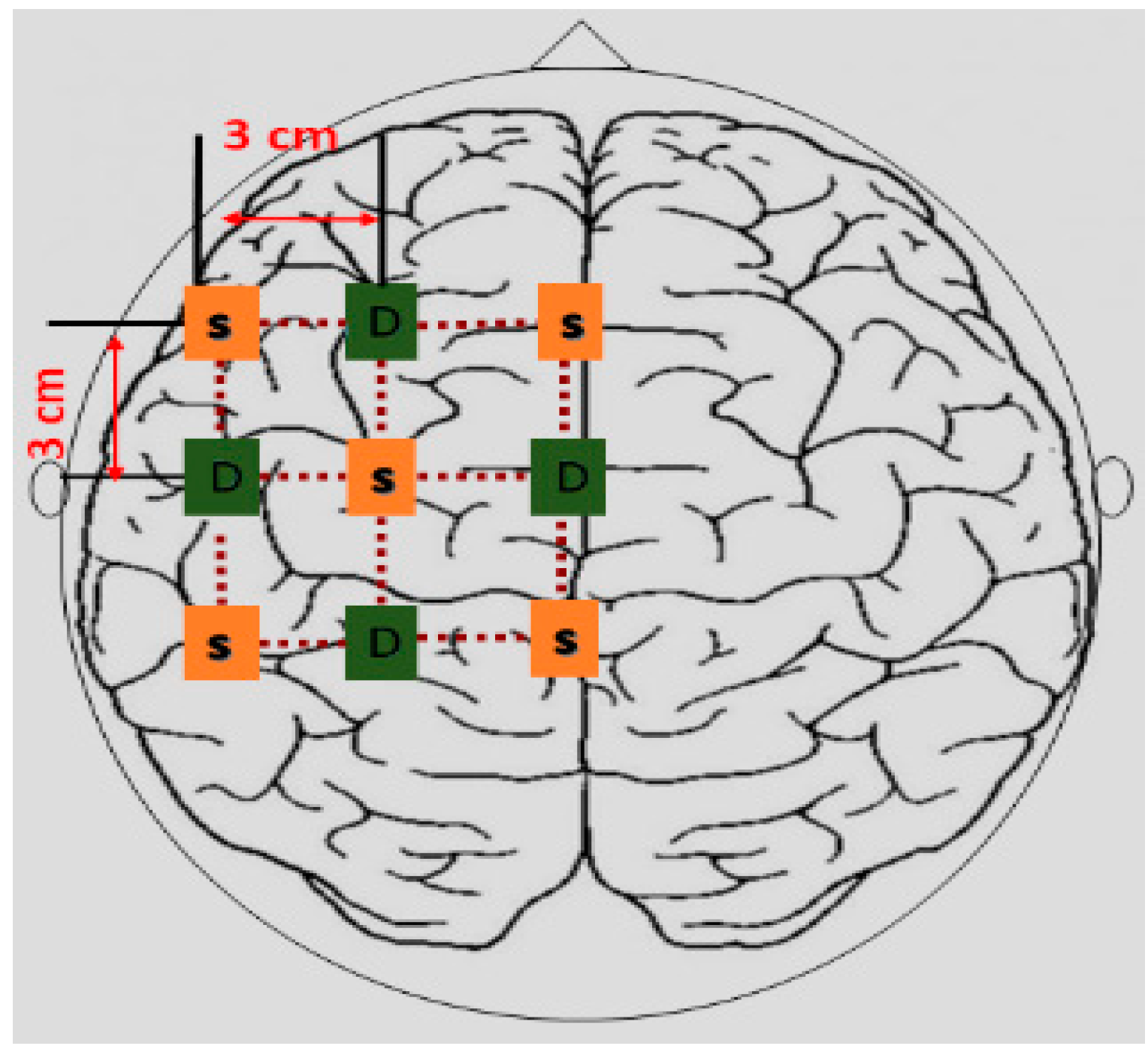
27]. This study includes the use of LASSO homotopy-based SRC for channel selection for the fNIRS-BCI system, to identify walking and resting states,
Figure 2.
4. Discussion
In the literature, recent studies have focused on enhancing the classification accuracies of fNIRS-BCI systems using the optimal classification technique [
22], general linear model [
38], vector-based phase analysis [
38,
39,
40,
41], optimal feature selection [
31,
38], optimal feature combination [
42],
t-value method [
43,
44], cross-correlation [
45], and dominant channel selection [
46]. An accurate and reliable fNIRS-BCI performance may lead to producing applications in neuro-robotics, rehabilitation, clinical BCI, for monitoring and analysis, and neuro-ergonomics.
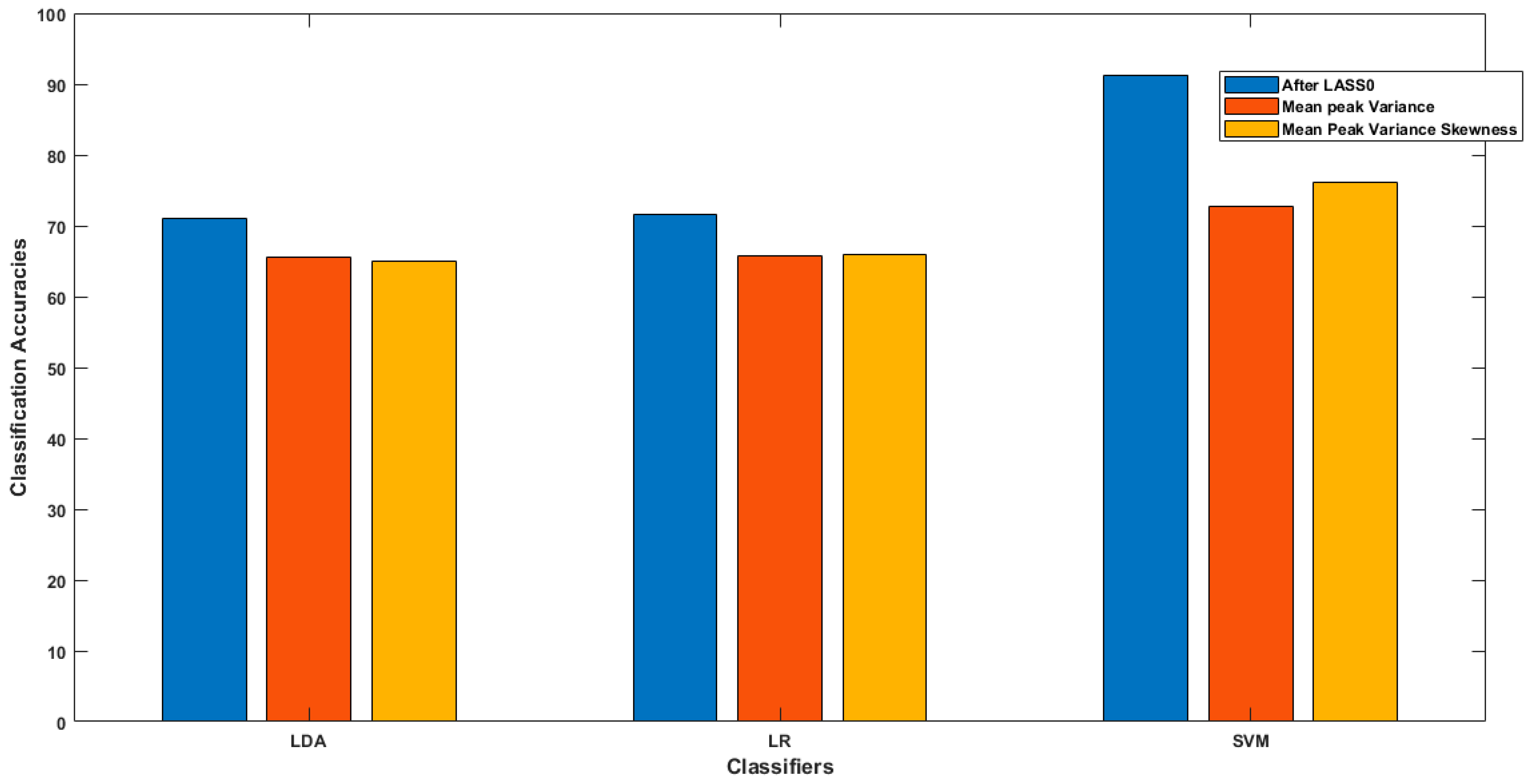
In the present study, a new method for selecting channels on the basis of the strong influence of individual input variables on the output response was introduced to increase fNIRS-BCI performance, especially in terms of classification accuracy. In the literature, there were many optimization techniques used to enhance the classification accuracy of the fNIRS-BCI system, to make it more robust and reliable. A comparative analysis between classifications of fNIRS-BCI, based on two methods, was conducted. The classification accuracies based on the proposed method were compared with the accuracies based on the conventional method of excessively used feature extractions, without channel selection, using all the channel data. In the first method, we observed that by using two different combinations of spatial features, we achieved average classification accuracies, for LDA, LR, and SVM, of 65 ± 1.34%, 65 ± 1.6%, and 72 ± 4.9%, respectively. After the implementation of the other method, LASSO homotopy-based sparse representation for channel selection, the classification accuracies of LDA, LR, and SVM improved to 71.01, 71.6, and 91.32%, respectively. This study shows that selecting the channels with intrinsic brain information as features for classification improves the classification accuracy of fNIRS-BCI. LASSO homotopy-based SRC enhances both the prediction accuracy and model interpretability. It lowers the variability of the system estimations, by precisely decreasing some of the coefficients, and making models that are easy to understand, produce, and interpret [
47]. For the channel selection method used for EEG-BCI, the classification accuracy was 93.08%, by selecting only eight channels out of 64 when classifying motor imagery tasks [
48]. A similar study was performed to select cortical activation-based channel selection using the
z-score method for fNIRS-BCI problems, achieving a classification accuracy of 88% [
21]. LASSO homotopy-based SRC autonomously selects the most significant channels for the fNIRS-BCI system, thus greatly improving the overall classification accuracy.
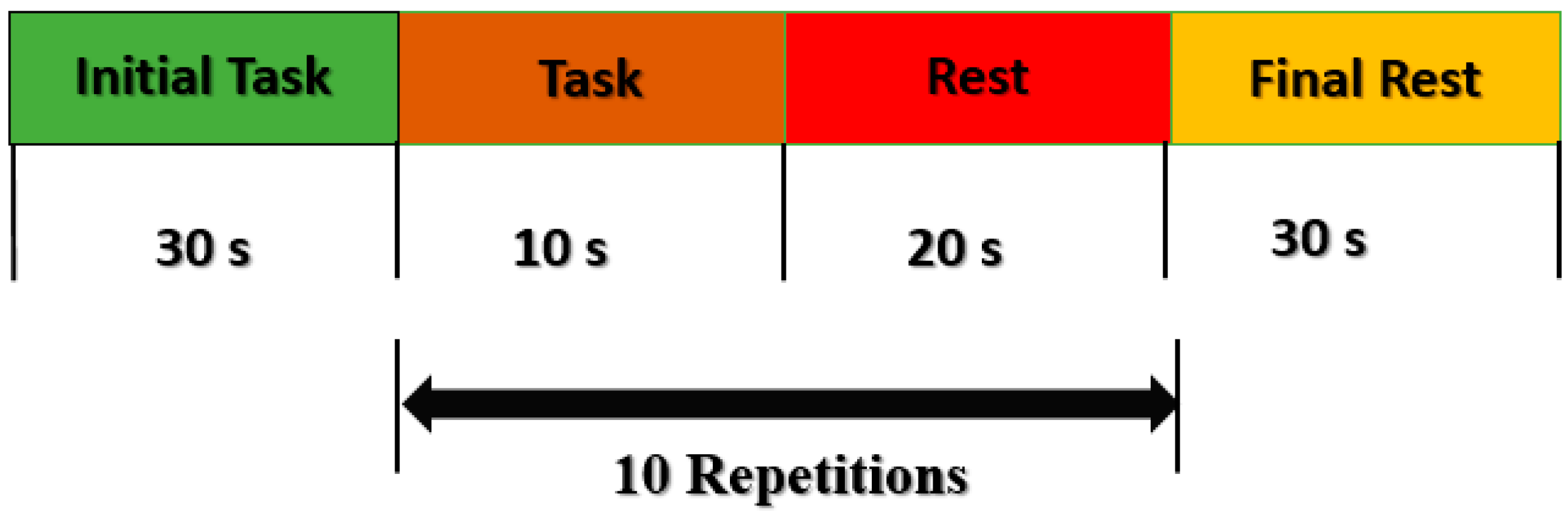
This study has a few limitations, including the fact that it only applies to a single activity at a time, because specific tasks are linked to certain brain regions, and subject-based channels were selected due to the different brain sizes. LASSO homotopy-based SRC selects channels with the minimum residual sum of error. Furthermore, the offline study is performed and analyzed, while the online study may be conducted for other cognitive activities. Moreover, several machine learning algorithms are applied in this study to analyze performance. Further deep learning algorithms may be implemented with LASSO homotopy-based SRC for analysis, and may perform better.