Functional Enhancement and Characterization of an Electrophysiological Mapping Electrode Probe with Carbonic, Directional Macrocontacts
Abstract
:1. Introduction
- Increasing the safety and electrical efficiency of charge injection during current stimulation using the advantageous electrochemical properties of conductive carbon;
- Providing directionally-selective capabilities for the sensing and stimulation functions by patterning these macrocontacts in a circumferentially segmented geometry (offering 2–3 distinct distal-end orientations), aiming again at improving the assessment accuracy of the anatomical target and in line with the recent trends in chronic probes [22].
- This work does not address enhancing the performance of the bipolar electrode, but its functional capabilities. In addition, the use of a specific commercial electrode for this study was made in order to include its architectural characteristics and constraints as in a realistic ‘testbed’ for this type of development.
2. Materials and Methods
2.1. Substrates
2.2. Functional Materials
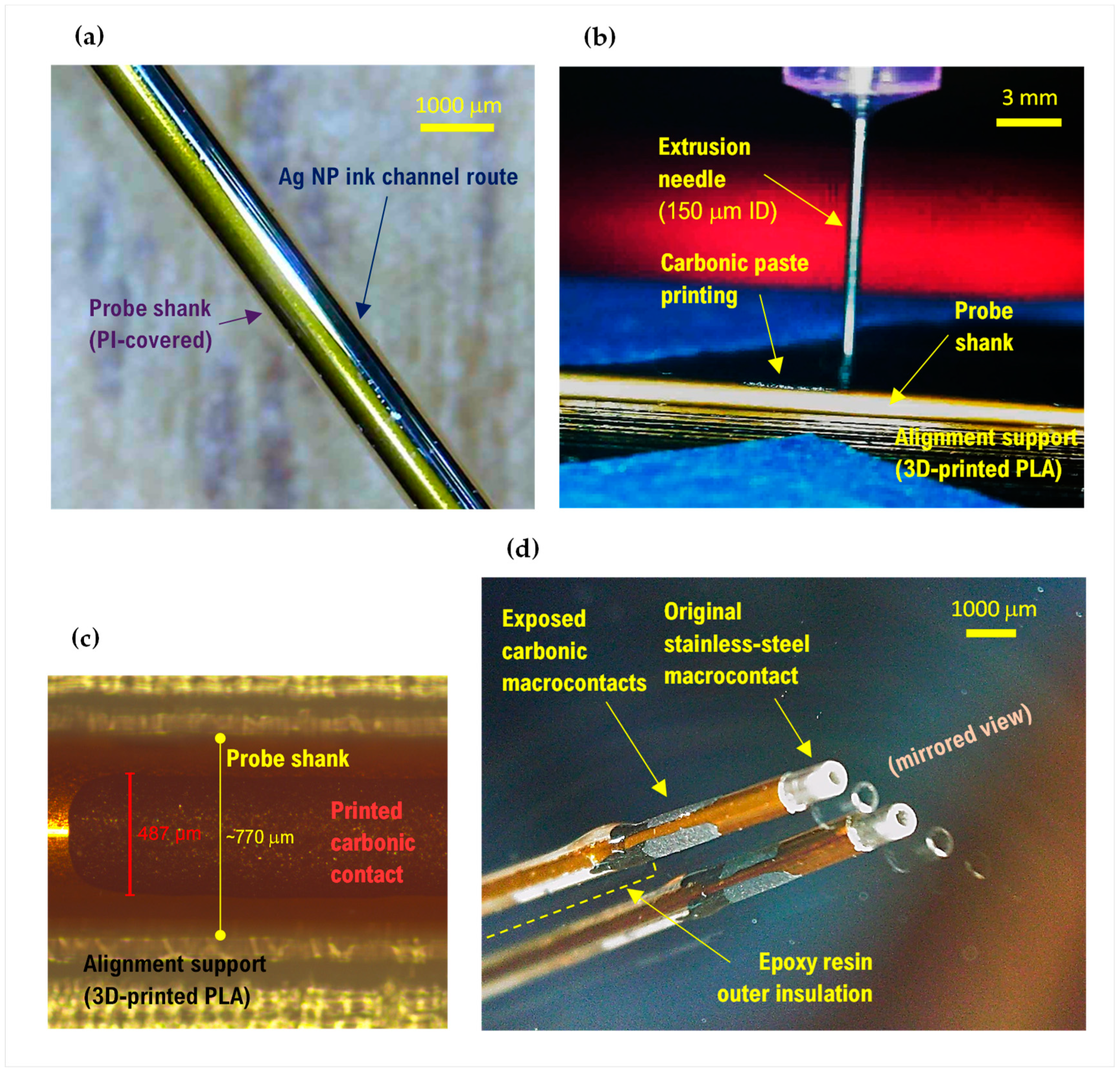
2.3. Probe Manufacturing
2.3.1. Process Development on Planar Geometry Substrate
2.3.2. Process Transfer to the 3D Substrate
2.4. Characterization Methods
2.4.1. Morphology and Structure
2.4.2. Electrochemical Characterizations
2.5. In Vivo Recording and Stimulation
3. Results
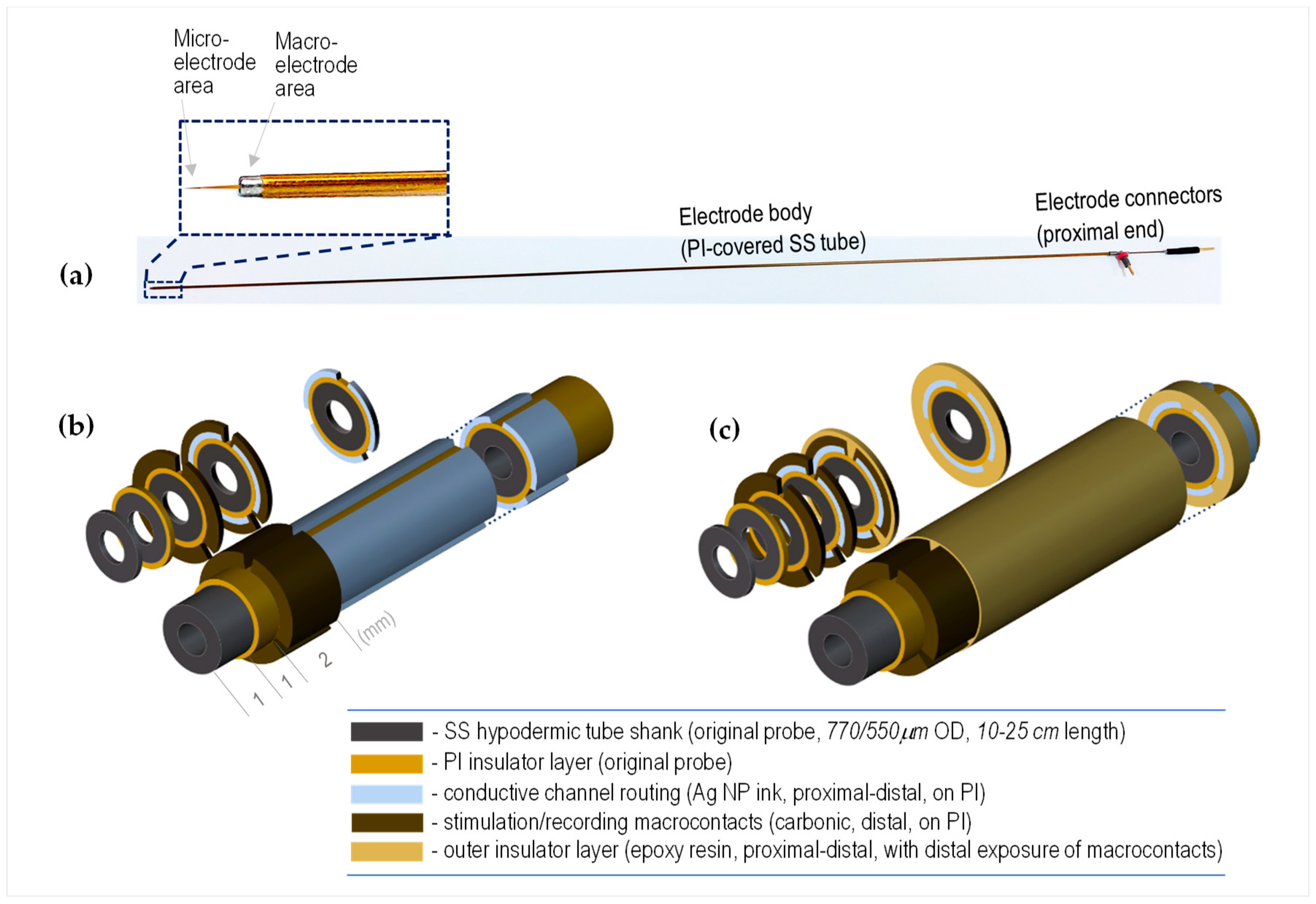
3.1. Constructive Concept
- The non-planar shape of the substrate, characterized by a small radius of curvature (~280–490 μm), as well as the high aspect ratio (up to ~600, for the 23 cm long electrodes);
- The need to make the depositions over an electrically insulating layer, a fact that limits the temperature regime of the subsequent processes;
- The need to realize the patterns as separated (segmented) parallel lines along several generators of the cylindrical shaft;
- In addition to the need to rotate the shaft for each such channel, each material needs a post-curing treatment to become functional.
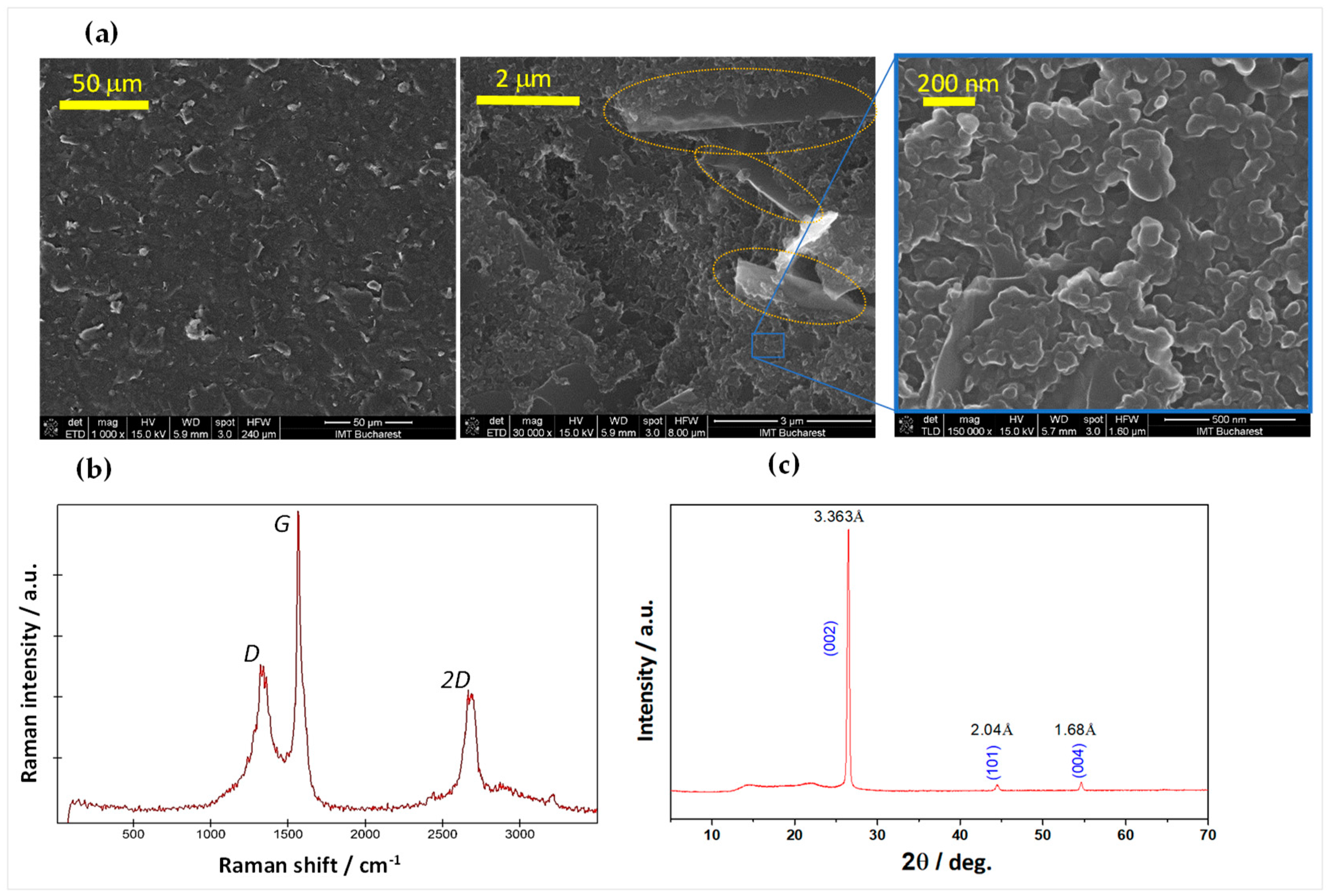
3.2. Characterization of the Carbonic Material
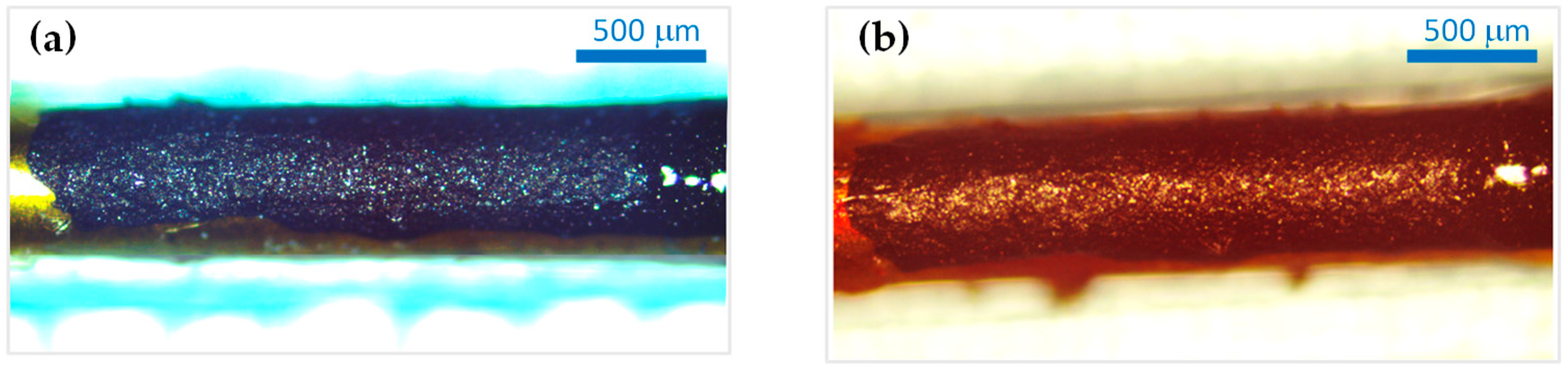
3.2.1. Morpho-Structural Analysis
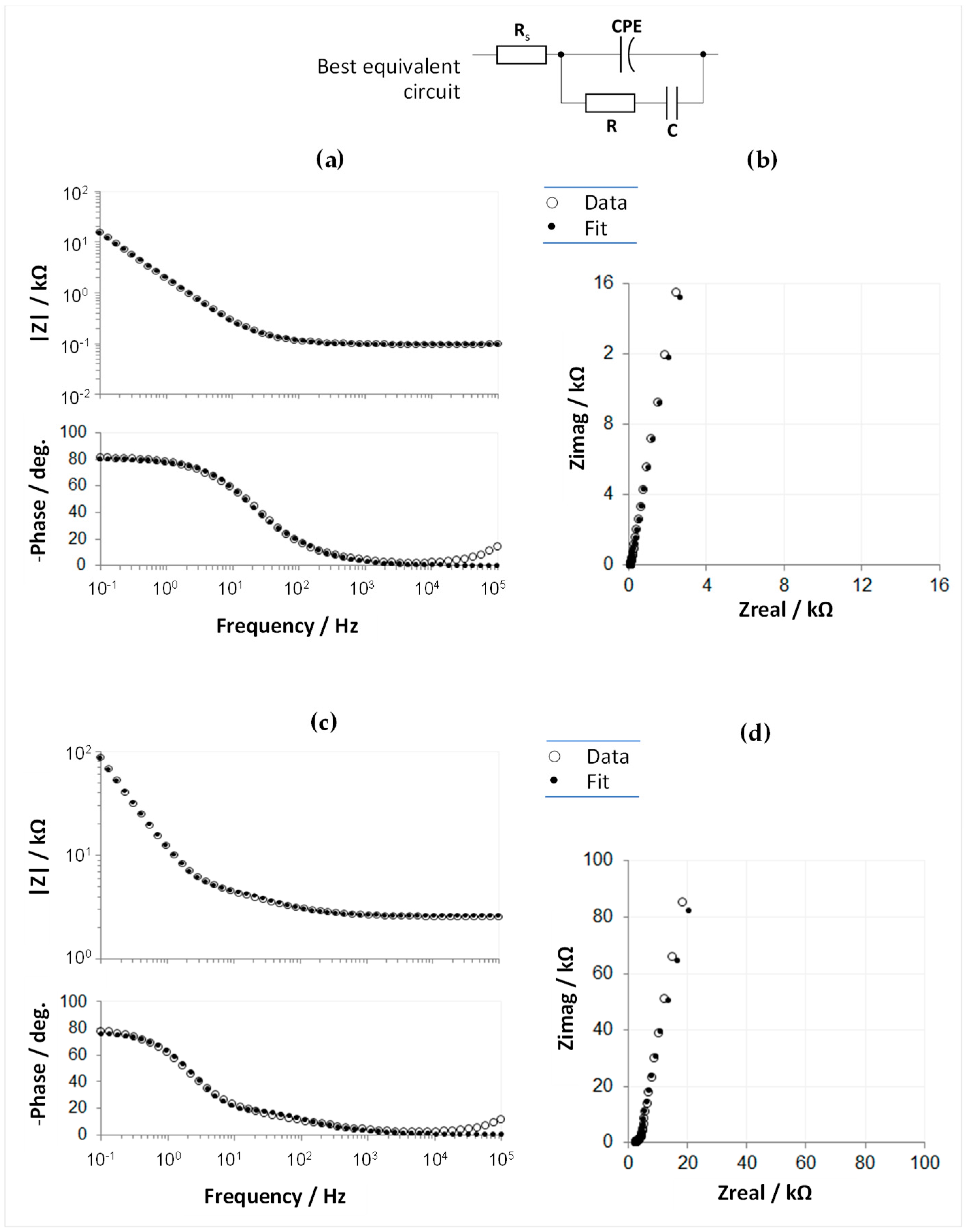
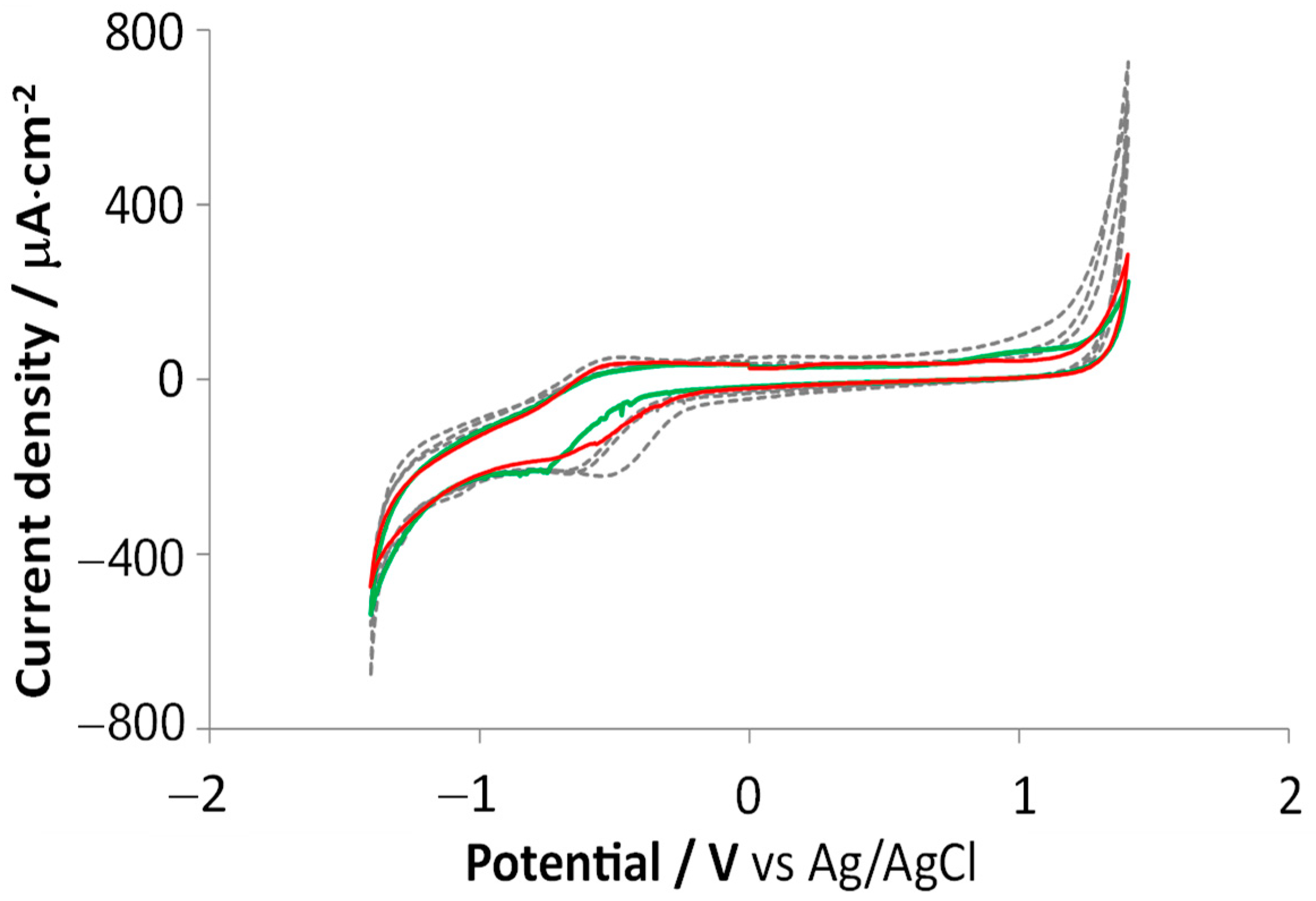
3.2.2. Cyclic Voltammetry and Electrochemical Impedance Spectroscopy
3.3. Planar Substrate Probes
3.4. Cylindrical Substrate Probes
3.4.1. Electrochemical Assessments
3.4.2. Charge Injection Capacity
3.5. In Vivo Experiments
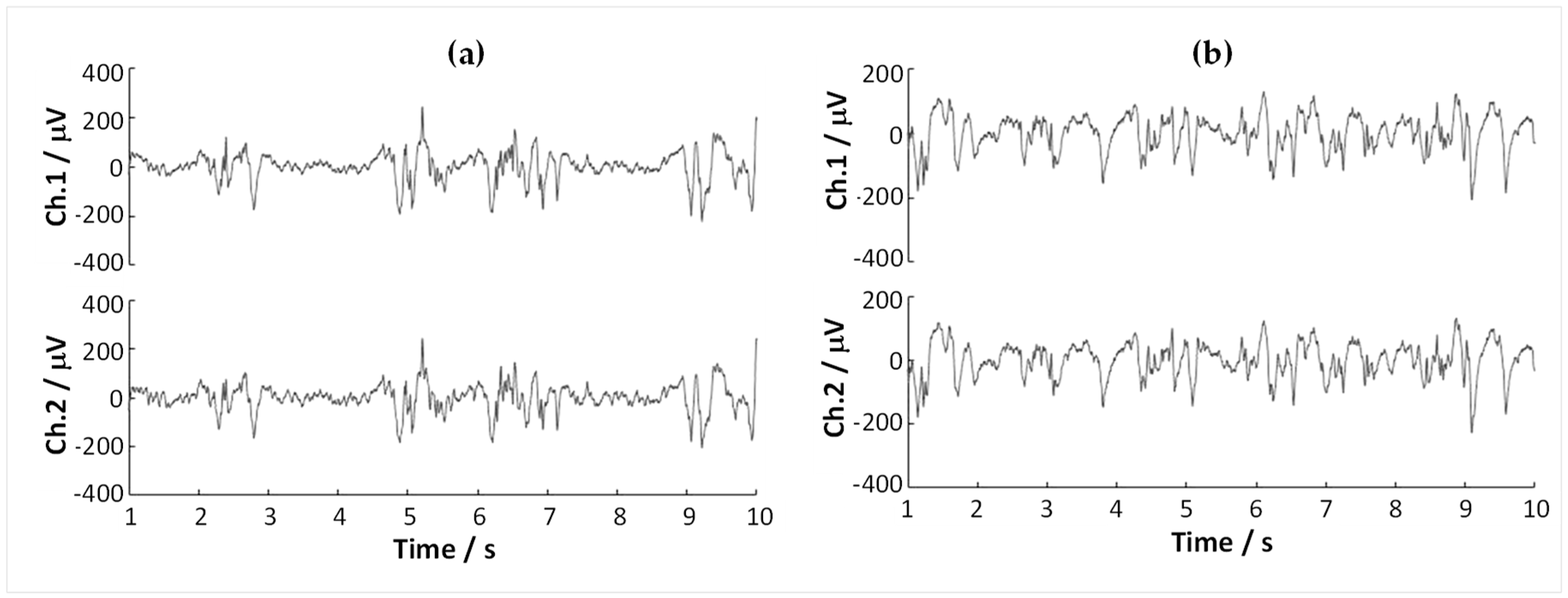
3.5.1. Macro-Recording Tests
3.5.2. Macrostimulation Tests
3.5.3. Overpotential Analysis
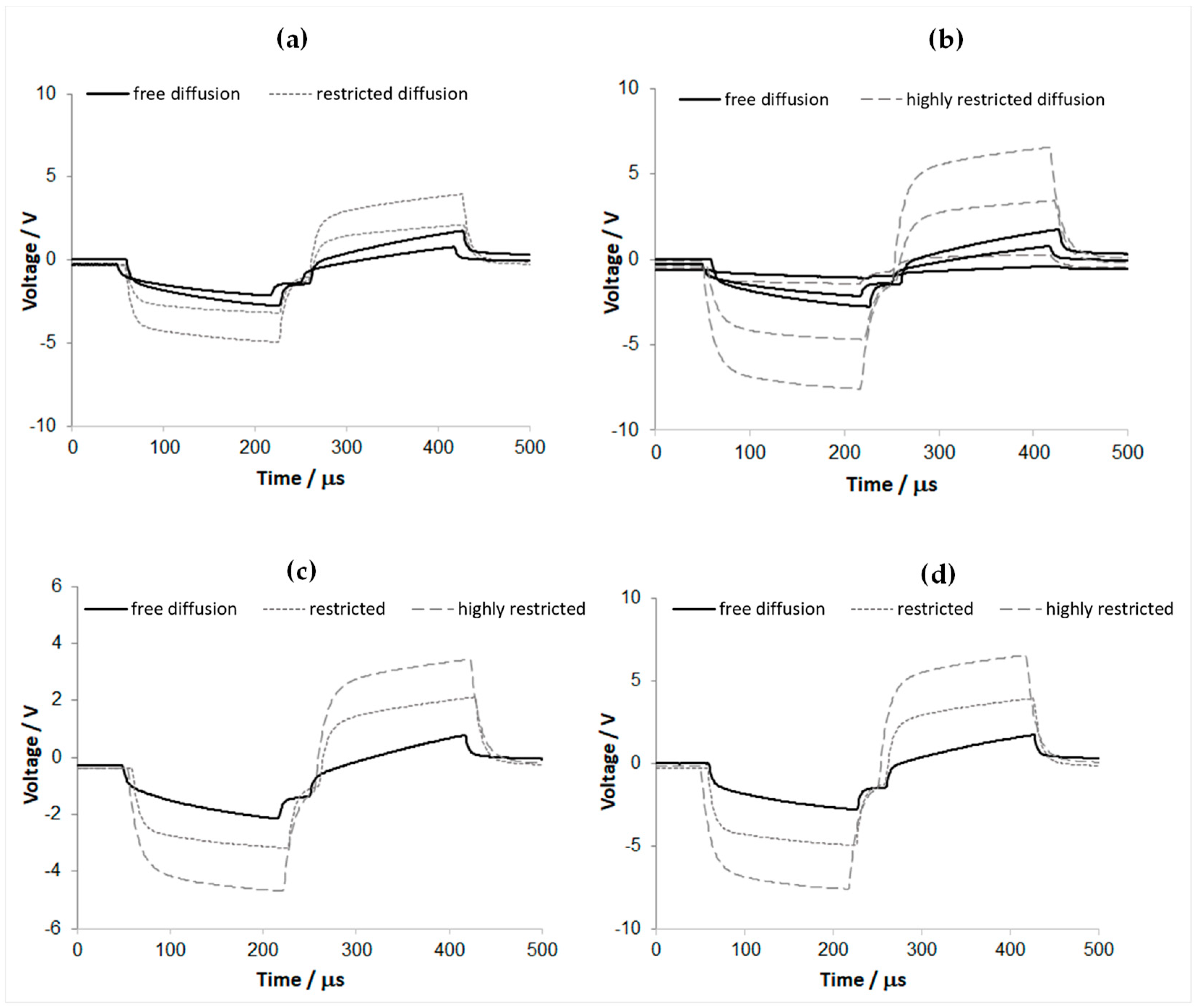
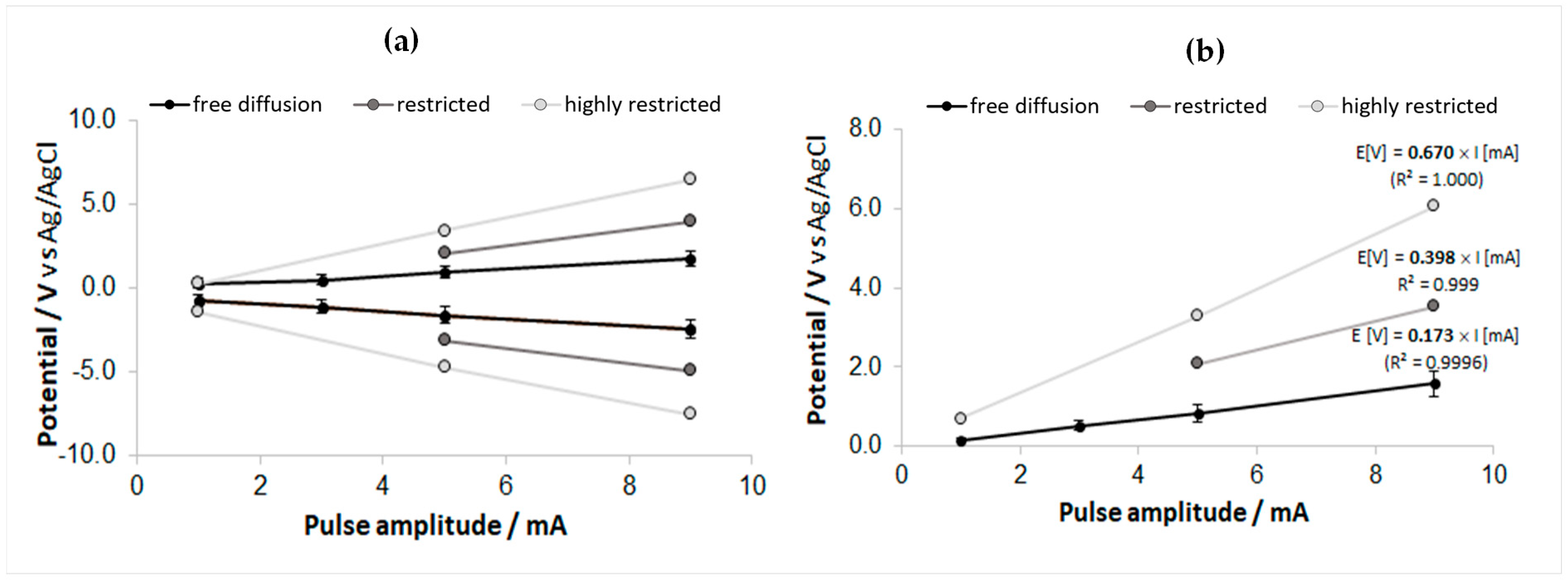
3.6. Emulative Experiment for the In Vivo Overpotential
4. Discussion—Limitations of the Study
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep Brain Stimulation: Current Challenges and Future Directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N. Probing and Regulating Dysfunctional Circuits Using Deep Brain Stimulation. Neuron 2013, 77, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of Deep Brain Stimulation: Current Status and Future Directions. Nat. Rev. Neurol. 2021, 17, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, W.D.; Lang, A.E.; Dostrovsky, J.O.; Lozano, A.M. Pallidal Neuronal Activity: Implications for Models of Dystonia. Ann. Neurol. 2003, 53, 480–488. [Google Scholar] [CrossRef]
- Montgomery, E.B. Intraoperative Neurophysiological Monitoring for Deep Brain Stimulation: Principles, Practice, and Cases; Oxford University Press: New York, NY, USA, 2014; ISBN 9780199389384. [Google Scholar]
- FHC. STarFix Stereotactic Microelectrodes and Insertion Tubes. Available online: https://www.fh-co.com/product/starfix-stereotactic-microelectrodes-insertion-tubes/ (accessed on 17 July 2023).
- NeuroProbe—Sonus. Available online: https://www.alphaomega-eng.com/NeuroProbe-Sonus (accessed on 17 July 2023).
- Accessories That Set New Standards in Brain Surgery. Available online: https://www.en.inomed.com/products/accessories/fns/ (accessed on 17 July 2023).
- Plexon Electrodes, Probes and Arrays. Available online: https://plexon.com/products/plexon-electrodes-probes-and-arrays/ (accessed on 17 July 2023).
- Wellman, S.M.; Eles, J.R.; Ludwig, K.A.; Seymour, J.P.; Michelson, N.J.; McFadden, W.E.; Vazquez, A.L.; Kozai, T.D.Y. A Materials Roadmap to Functional Neural Interface Design. Adv. Funct. Mater. 2018, 28, 1701269. [Google Scholar] [CrossRef]
- Hejazi, M.A.; Tong, W.; Stacey, A.; Sun, S.H.; Yunzab, M.; Almasi, A.; Jung, Y.J.; Meffin, H.; Fox, K.; Edalati, K.; et al. High Fidelity Bidirectional Neural Interfacing with Carbon Fiber Microelectrodes Coated with Boron-Doped Carbon Nanowalls: An Acute Study. Adv. Funct. Mater. 2020, 30, 2006101. [Google Scholar] [CrossRef]
- Vomero, M.; Castagnola, E.; Ciarpella, F.; Maggiolini, E.; Goshi, N.; Zucchini, E.; Carli, S.; Fadiga, L.; Kassegne, S.; Ricci, D. Highly Stable Glassy Carbon Interfaces for Long-Term Neural Stimulation and Low-Noise Recording of Brain Activity. Sci. Rep. 2017, 7, 40332. [Google Scholar] [CrossRef]
- Nimbalkar, S.; Castagnola, E.; Balasubramani, A.; Scarpellini, A.; Samejima, S.; Khorasani, A.; Boissenin, A.; Thongpang, S.; Moritz, C.; Kassegne, S. Ultra-Capacitive Carbon Neural Probe Allows Simultaneous Long-Term Electrical Stimulations and High-Resolution Neurotransmitter Detection. Sci. Rep. 2018, 8, 6958. [Google Scholar] [CrossRef]
- Cisnal, A.; Ihmig, F.R.; Fraile, J.-C.; Pérez-Turiel, J.; Muñoz-Martinez, V. Application of a Novel Measurement Setup for Characterization of Graphene Microelectrodes and a Comparative Study of Variables Influencing Charge Injection Limits of Implantable Microelectrodes. Sensors 2019, 19, 2725. [Google Scholar] [CrossRef]
- Boehler, C.; Vieira, D.M.; Egert, U.; Asplund, M. NanoPt—A Nanostructured Electrode Coating for Neural Recording and Microstimulation. ACS Appl. Mater. Interfaces 2020, 12, 14855–14865. [Google Scholar] [CrossRef]
- Sikder, K.U.; Shivdasani, M.N.; Fallon, J.B.; Seligman, P.; Ganesan, K.; Villalobos, J.; Prawer, S.; Garrett, D.J. Electrically Conducting Diamond Films Grown on Platinum Foil for Neural Stimulation. J. Neural Eng. 2019, 16, 066002. [Google Scholar] [CrossRef]
- Hadjinicolaou, A.E.; Leung, R.T.; Garrett, D.J.; Ganesan, K.; Fox, K.; Nayagam, D.A.X.; Shivdasani, M.N.; Meffin, H.; Ibbotson, M.R.; Prawer, S.; et al. Electrical Stimulation of Retinal Ganglion Cells with Diamond and the Development of an All Diamond Retinal Prosthesis. Biomaterials 2012, 33, 5812–5820. [Google Scholar] [CrossRef]
- Ansaldo, A.; Castagnola, E.; Maggiolini, E.; Fadiga, L.; Ricci, D. Superior Electrochemical Performance of Carbon Nanotubes Directly Grown on Sharp Microelectrodes. ACS Nano 2011, 5, 2206–2214. [Google Scholar] [CrossRef]
- Bareket-Keren, L.; Hanein, Y. Carbon Nanotube-Based Multi Electrode Arrays for Neuronal Interfacing: Progress and Prospects. Front. Neural Circuits 2013, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.T.; Vetter, R.J.; Hetke, J.F.; Teplitzky, B.A.; Kipke, D.R.; Pellinen, D.S.; Anderson, D.J.; Baker, K.B.; Vitek, J.L.; Johnson, M.D. A Novel Lead Design for Modulation and Sensing of Deep Brain Structures. IEEE Trans. Biomed. Eng. 2016, 63, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Hariz, M. Deep Brain Stimulation: New Techniques. Park. Relat. Disord. 2014, 20, S192–S196. [Google Scholar] [CrossRef]
- Alonso, F.; Latorre, M.; Göransson, N.; Zsigmond, P.; Wårdell, K. Investigation into Deep Brain Stimulation Lead Designs: A Patient-Specific Simulation Study. Brain Sci. 2016, 6, 39. [Google Scholar] [CrossRef]
- Contarino, M.F.; Bour, L.J.; Verhagen, R.; Lourens, M.A.J.; De Bie, R.M.A.; Van Den Munckhof, P.; Schuurman, P.R. Directional Steering: A Novel Approach to Deep Brain Stimulation. Neurology 2014, 83, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F.; Garrett, D.J.; Green, R.A. Electrochemical Principles of Safe Charge Injection. In Neurobionics: The Biomedical Engineering of Neural Prostheses; Shepherd, R.K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 55–88. ISBN 9781118814871. [Google Scholar]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G.R. Electrical Stimulation of Excitable Tissue: Design of Efficacious and Safe Protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Meijs, S.; Alcaide, M.; Sørensen, C.; McDonald, M.; Sørensen, S.; Rechendorff, K.; Gerhardt, A.; Nesladek, M.; Rijkhoff, N.J.M.; Pennisi, C.P. Biofouling Resistance of Boron-Doped Diamond Neural Stimulation Electrodes Is Superior to Titanium Nitride Electrodes in Vivo. J. Neural Eng. 2016, 13, 056011. [Google Scholar] [CrossRef]
- Devi, M.; Vomero, M.; Fuhrer, E.; Castagnola, E.; Gueli, C.; Nimbalkar, S.; Hirabayashi, M.; Kassegne, S.; Stieglitz, T.; Sharma, S. Carbon-Based Neural Electrodes: Promises and Challenges. J. Neural Eng. 2021, 18, 041007. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Bak, B.H. Multichannel Brain Probe. U.S. Patent 9731114B2, 15 August 2017. [Google Scholar]
- AlphaProbe (since December 2020). Available online: https://www.alphaomega-eng.com/AlphaProbe (accessed on 17 July 2023).
- Ad-Tech Medical. Epilepsy. Available online: https://adtechmedical.com/epilepsy (accessed on 17 July 2023).
- Sinclair, N.C.; McDermott, H.J.; Bulluss, K.J.; Fallon, J.B.; Perera, T.; Xu, S.S.; Brown, P.; Thevathasan, W. Subthalamic Nucleus Deep Brain Stimulation Evokes Resonant Neural Activity. Ann. Neurol. 2018, 83, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Tekriwal, A.; Afshar, N.M.; Santiago-Moreno, J.; Kuijper, F.M.; Kern, D.S.; Halpern, C.H.; Felsen, G.; Thompson, J.A. Neural Circuit and Clinical Insights from Intraoperative Recordings during Deep Brain Stimulation Surgery. Brain Sci. 2019, 9, 173. [Google Scholar] [CrossRef]
- Telkes, I.; Sabourin, S.; Durphy, J.; Adam, O.; Sukul, V.; Raviv, N.; Staudt, M.D.; Pilitsis, J.G. Functional Use of Directional Local Field Potentials in the Subthalamic Nucleus Deep Brain Stimulation. Front. Hum. Neurosci. 2020, 14, 145. [Google Scholar] [CrossRef]
- Vlahov, A. XRD Graphitization Degrees: A Review of the Published Data and New Calculations, Correlations, and Applications. Geol. Balc. 2021, 50, 11–35. [Google Scholar] [CrossRef]
- Yeager, E.; Gupta, S.; Molla, J.A. The Electrochemical Properties of Graphite and Carbon. In Proceedings of the Workshop on the Electrochemistry of Carbon; Electrochemical Society Inc.: Pennington, NJ, USA, 1983; pp. 123–157. [Google Scholar]
- Norlin, A.; Pan, J.; Leygraf, C. Investigation of Electrochemical Behavior of Stimulation/Sensing Materials for Pacemaker Electrode Applications, I. Pt, Ti, and TiN Coated Electrodes. J. Electrochem. Soc. 2005, 152, J7. [Google Scholar] [CrossRef]
- Rossi, L.; Foffani, G.; Marceglia, S.; Bracchi, F.; Barbieri, S.; Priori, A. An Electronic Device for Artefact Suppression in Human Local Field Potential Recordings during Deep Brain Stimulation. J. Neural Eng. 2007, 4, 96–106. [Google Scholar] [CrossRef]
- Rose, T.L.; Robblee, L.S. Electrical Stimulation with Pt Electrodes. VIII. Electrochemically Safe Charge Injection Limits with 0.2 Ms Pulses (Neuronal Application). IEEE Trans. Biomed. Eng. 1990, 37, 1118–1120. [Google Scholar] [CrossRef]
- Boehler, C.; Carli, S.; Fadiga, L.; Stieglitz, T.; Asplund, M. Tutorial: Guidelines for Standardized Performance Tests for Electrodes Intended for Neural Interfaces and Bioelectronics. Nat. Protoc. 2020, 15, 3557–3578. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.V. A Model of Safe Levels for Electrical Stimulation. IEEE Trans. Biomed. Eng. 1992, 39, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Kuncel, A.M.; Grill, W.M. Selection of Stimulus Parameters for Deep Brain Stimulation. Clin. Neurophysiol. 2004, 115, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Greatbatch, W.; Chardack, W.M. Myocardial and Endocardiac Electrodes for Chronic Implantation. Ann. N. Y. Acad. Sci. 1968, 148, 234–251. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, D.; Amzica, F. Hypersensitivity of the Anesthesia-Induced Comatose Brain. J. Neurosci. 2007, 27, 10597–10607. [Google Scholar] [CrossRef]
- Cogan, S.F. In Vivo and In Vitro Differences in the Charge-Injection and Electrochemical Properties of Iridium Oxide Electrodes. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 882–885. [Google Scholar]
- Hu, Z.; Troyk, P.R.; Brawn, T.P.; Margoliash, D.; Cogan, S.F. In Vitro and In Vivo Charge Capacity of AIROF Microelectrodes. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 886–889. [Google Scholar]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Plante, T.D. In Vitro Comparison of the Charge-Injection Limits of Activated Iridium Oxide (AIROF) and Platinum-Iridium Microelectrodes. IEEE Trans. Biomed. Eng. 2005, 52, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Newbold, C.; Stathopoulos, D.; Carter, P.; Cowan, R.; Wallace, G.G. Comparison of the In Vitro and In Vivo Electrochemical Performance of Bionic Electrodes. Micromachines 2022, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Carvallo, A.; Modolo, J.; Benquet, P.; Lagarde, S.; Bartolomei, F.; Wendling, F. Biophysical Modeling for Brain Tissue Conductivity Estimation Using SEEG Electrodes. IEEE Trans. Biomed. Eng. 2019, 66, 1695–1704. [Google Scholar] [CrossRef]
- Emin, D.; Akhtari, M.; Ellingson, B.M.; Mathern, G.W. Ionic Charge Transport between Blockages: Sodium Cation Conduction in Freshly Excised Bulk Brain Tissue. AIP Adv. 2015, 5, 087133. [Google Scholar] [CrossRef]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Gasbarro, C.M.; Plante, T.D. The Influence of Electrolyte Composition on the in Vitro Charge-Injection Limits of Activated Iridium Oxide (AIROF) Stimulation Electrodes. J. Neural Eng. 2007, 4, 79–86. [Google Scholar] [CrossRef]
- Saito, Y. Ion Transport in Solid Medium—Evaluation of Ionic Mobility for Design of Ion Transport Pathways in Separator and Gel Electrolyte. Membranes 2021, 11, 277. [Google Scholar] [CrossRef]
- Gao, H.; Wu, Q.; Hu, Y.; Zheng, J.P.; Amine, K.; Chen, Z. Revealing the Rate-Limiting Li-Ion Diffusion Pathway in Ultrathick Electrodes for Li-Ion Batteries. J. Phys. Chem. Lett. 2018, 9, 5100–5104. [Google Scholar] [CrossRef] [PubMed]
- Zouhri, K.; Lee, S.-Y. Exergy Study on the Effect of Material Parameters and Operating Conditions on the Anode Diffusion Polarization of the SOFC. Int. J. Energy Environ. Eng. 2016, 7, 211–224. [Google Scholar] [CrossRef]
- Tesfai, A.; Irvine, J.T.S. 4.10—Solid Oxide Fuel Cells: Theory and Materials. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Oxford, UK, 2012; pp. 261–276. ISBN 9780080878737. [Google Scholar]
- Newbold, C.; Richardson, R.; Huang, C.Q.; Milojevic, D.; Cowan, R.; Shepherd, R. An in Vitro Model for Investigating Impedance Changes with Cell Growth and Electrical Stimulation: Implications for Cochlear Implants. J. Neural Eng. 2004, 1, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Li, N.; Dembek, T.A.; Kappel, A.; Boulay, C.; Ewert, S.; Tietze, A.; Husch, A.; Perera, T.; Neumann, W.-J.; et al. Lead-DBS v2: Towards a Comprehensive Pipeline for Deep Brain Stimulation Imaging. NeuroImage 2019, 184, 293–316. [Google Scholar] [CrossRef]
- Baniasadi, M.; Proverbio, D.; Gonçalves, J.; Hertel, F.; Husch, A. FastField: An Open-Source Toolbox for Efficient Approximation of Deep Brain Stimulation Electric Fields. NeuroImage 2020, 223, 117330. [Google Scholar] [CrossRef]
- McCann, H.; Pisano, G.; Beltrachini, L. Variation in Reported Human Head Tissue Electrical Conductivity Values. Brain Topogr. 2019, 32, 825–858. [Google Scholar] [CrossRef]
- Keller, D.; Erö, C.; Markram, H. Cell Densities in the Mouse Brain: A Systematic Review. Front. Neuroanat. 2018, 12, 83. [Google Scholar] [CrossRef] [PubMed]



| Probe Channel | Rs (Ω·cm2) | CPE-Q0 (S·sn·cm−2) | CPE-n | R (Ω·cm2) | C (F·cm−2) | Fitting χ2 | |Z|EEI-1kHz (Ω) | |Z|EEI-10Hz (Ω) |
|---|---|---|---|---|---|---|---|---|
| P1—plane P2—plane P3—plane | 1.0 | 1.1 × 10−4 | 0.71 | 0.6 | 3.7 × 10−5 | 2.4 × 10−3 | 275 | 25006 |
| 0.9 | 1.0 × 10−4 | 0.69 | 0.8 | 2.8 × 10−5 | 3.3 × 10−3 | 383 | 31466 | |
| 1.4 | 2.3 × 10−4 | 0.77 | 1.3 | 2.8 × 10−5 | 2.6 × 10−3 | 190 | 14596 | |
| Ca—cylinder Cb—cylinder | 0.3 | 1.0 × 10−4 | 0.54 | 2.9 | 0.9 × 10−5 | 5.0 × 10−3 | 1273 | 67663 |
| 0.8 | 0.9 × 10−4 | 0.65 | 1.3 | 1.4 × 10−5 | 3.6 × 10−3 | 740 | 46081 |
| Carbon, In Vitro-PBS n = 6 | Carbon, In Vivo Hippocamp., n = 6 | SS, In Vivo Hippocamp., n = 1 | SS, In Vivo Thalamus, n = 1 | |
|---|---|---|---|---|
| Average Std. dev. | 705 | 3353 | 2670 | 3950 |
| 125 | 513 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, R.C.; Serban, C.-A.; Barborica, A.; Zagrean, A.-M.; Buiu, O.; Dumbravescu, N.; Paslaru, A.-C.; Obreja, C.; Pachiu, C.; Stoian, M.; et al. Functional Enhancement and Characterization of an Electrophysiological Mapping Electrode Probe with Carbonic, Directional Macrocontacts. Sensors 2023, 23, 7497. https://doi.org/10.3390/s23177497
Popa RC, Serban C-A, Barborica A, Zagrean A-M, Buiu O, Dumbravescu N, Paslaru A-C, Obreja C, Pachiu C, Stoian M, et al. Functional Enhancement and Characterization of an Electrophysiological Mapping Electrode Probe with Carbonic, Directional Macrocontacts. Sensors. 2023; 23(17):7497. https://doi.org/10.3390/s23177497
Chicago/Turabian StylePopa, Radu C., Cosmin-Andrei Serban, Andrei Barborica, Ana-Maria Zagrean, Octavian Buiu, Niculae Dumbravescu, Alexandru-Catalin Paslaru, Cosmin Obreja, Cristina Pachiu, Marius Stoian, and et al. 2023. "Functional Enhancement and Characterization of an Electrophysiological Mapping Electrode Probe with Carbonic, Directional Macrocontacts" Sensors 23, no. 17: 7497. https://doi.org/10.3390/s23177497
APA StylePopa, R. C., Serban, C.-A., Barborica, A., Zagrean, A.-M., Buiu, O., Dumbravescu, N., Paslaru, A.-C., Obreja, C., Pachiu, C., Stoian, M., Marculescu, C., Radoi, A., Vulpe, S., & Ion, M. (2023). Functional Enhancement and Characterization of an Electrophysiological Mapping Electrode Probe with Carbonic, Directional Macrocontacts. Sensors, 23(17), 7497. https://doi.org/10.3390/s23177497












