Comparative Evaluations on Real-Time Monitoring of Temperature Sensors during Endoscopic Laser Application
Abstract
:1. Introduction
2. Materials and Methods
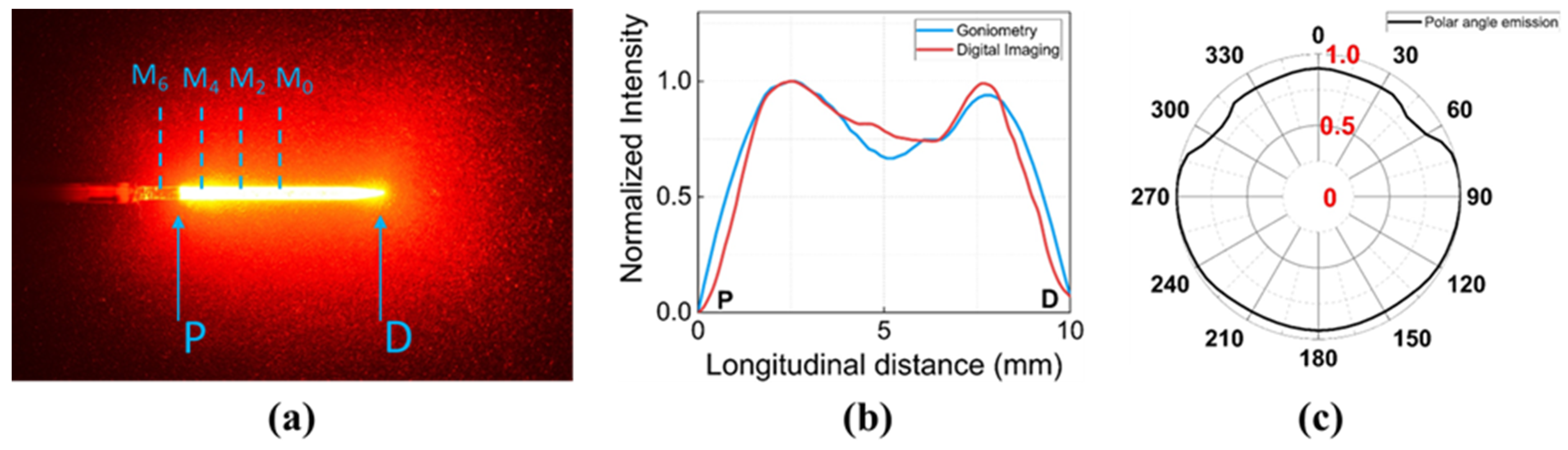
2.1. Light Delivery
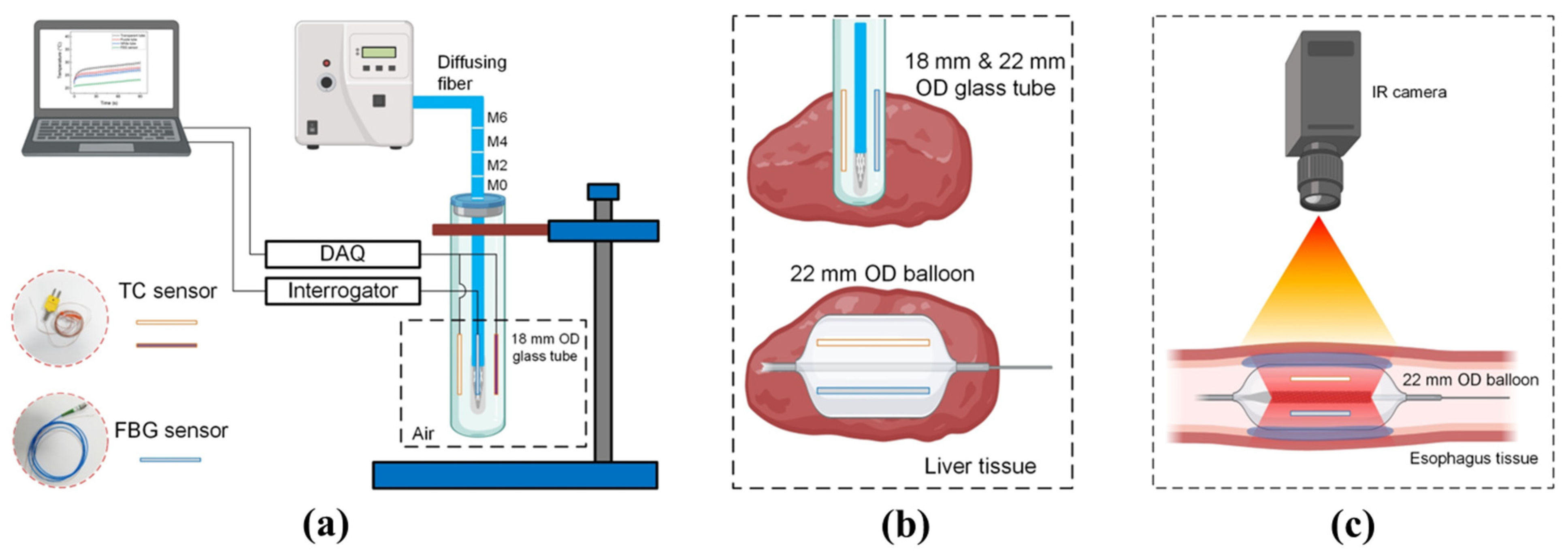
2.2. Temperature Sensors
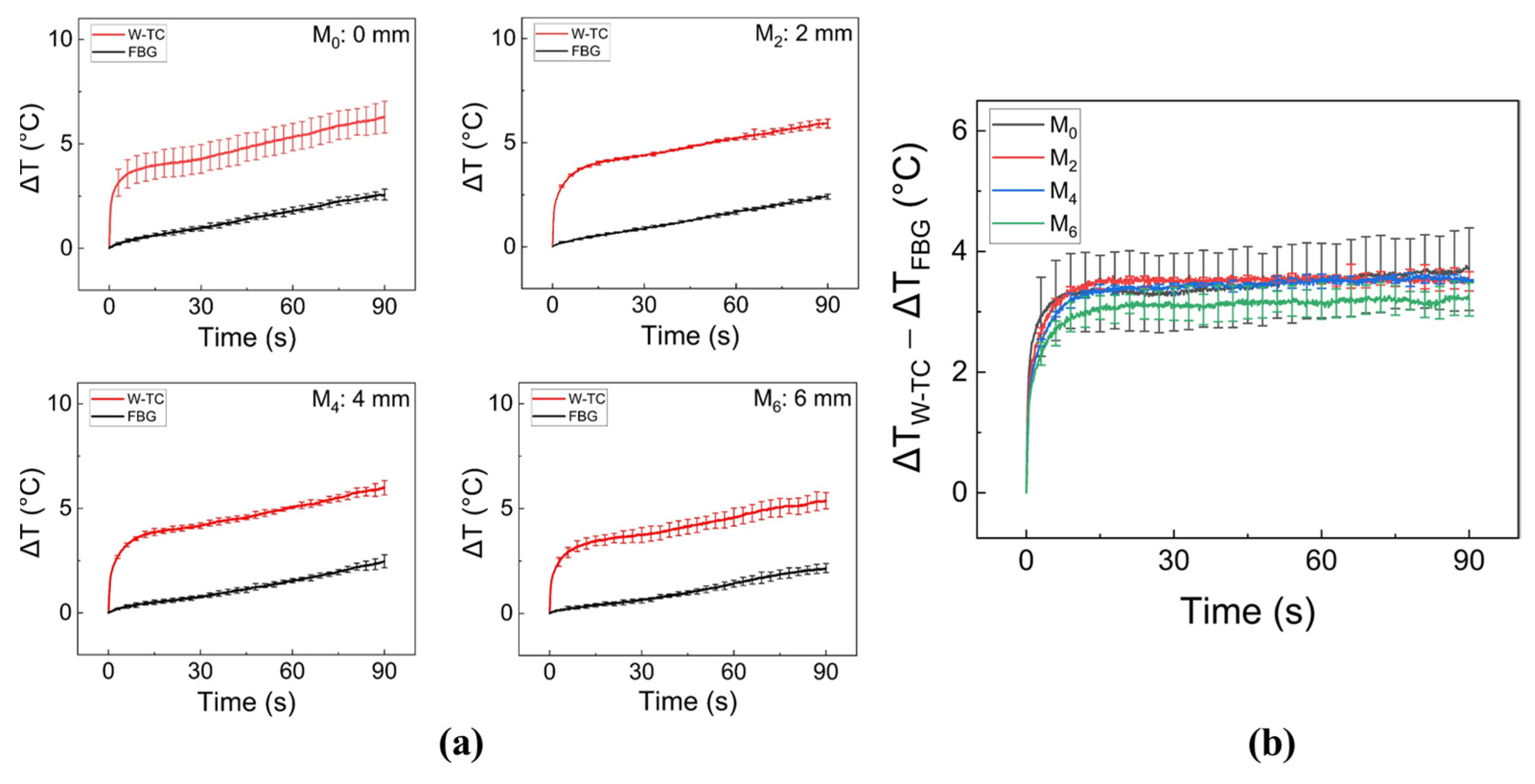
2.3. Evaluation of Thin Plastic Tubes and Sensor Positions
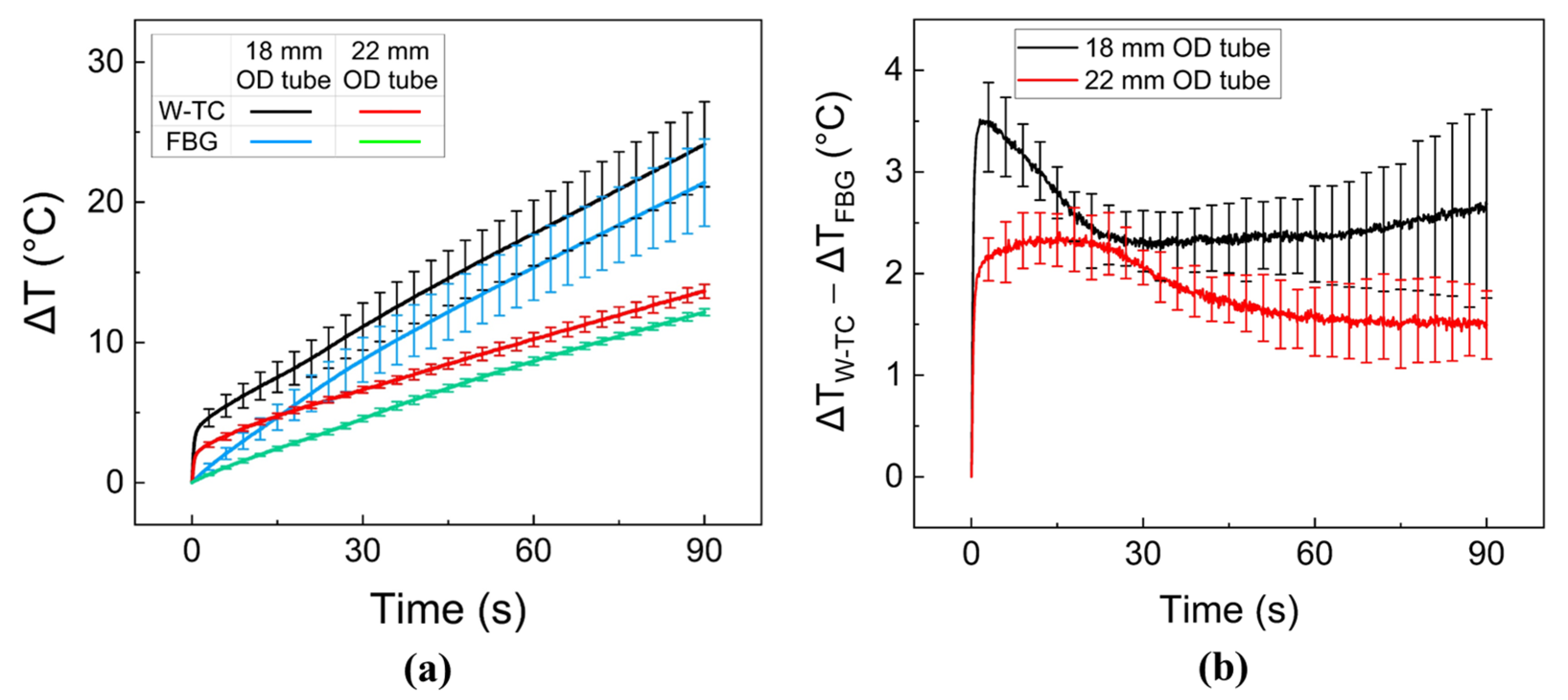
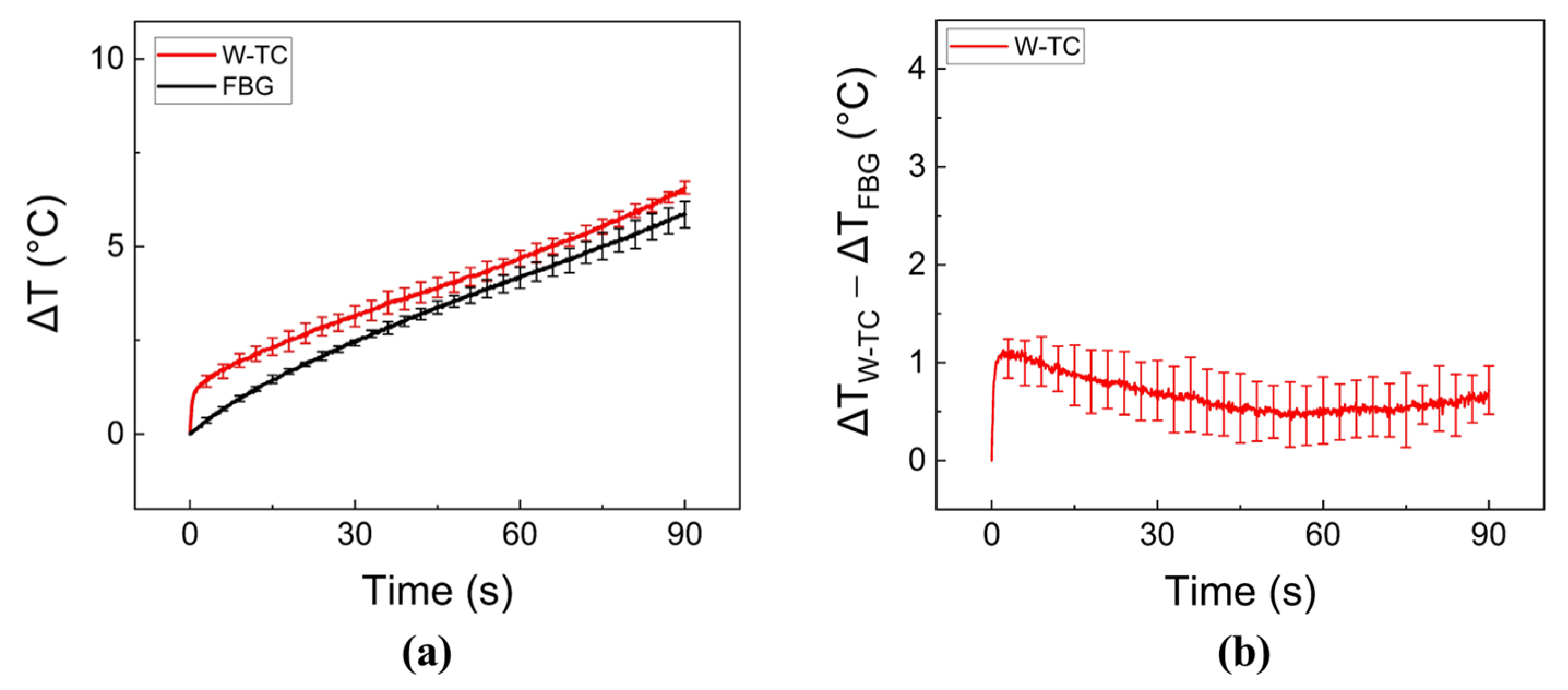
2.4. Ex Vivo Liver Tissue Test
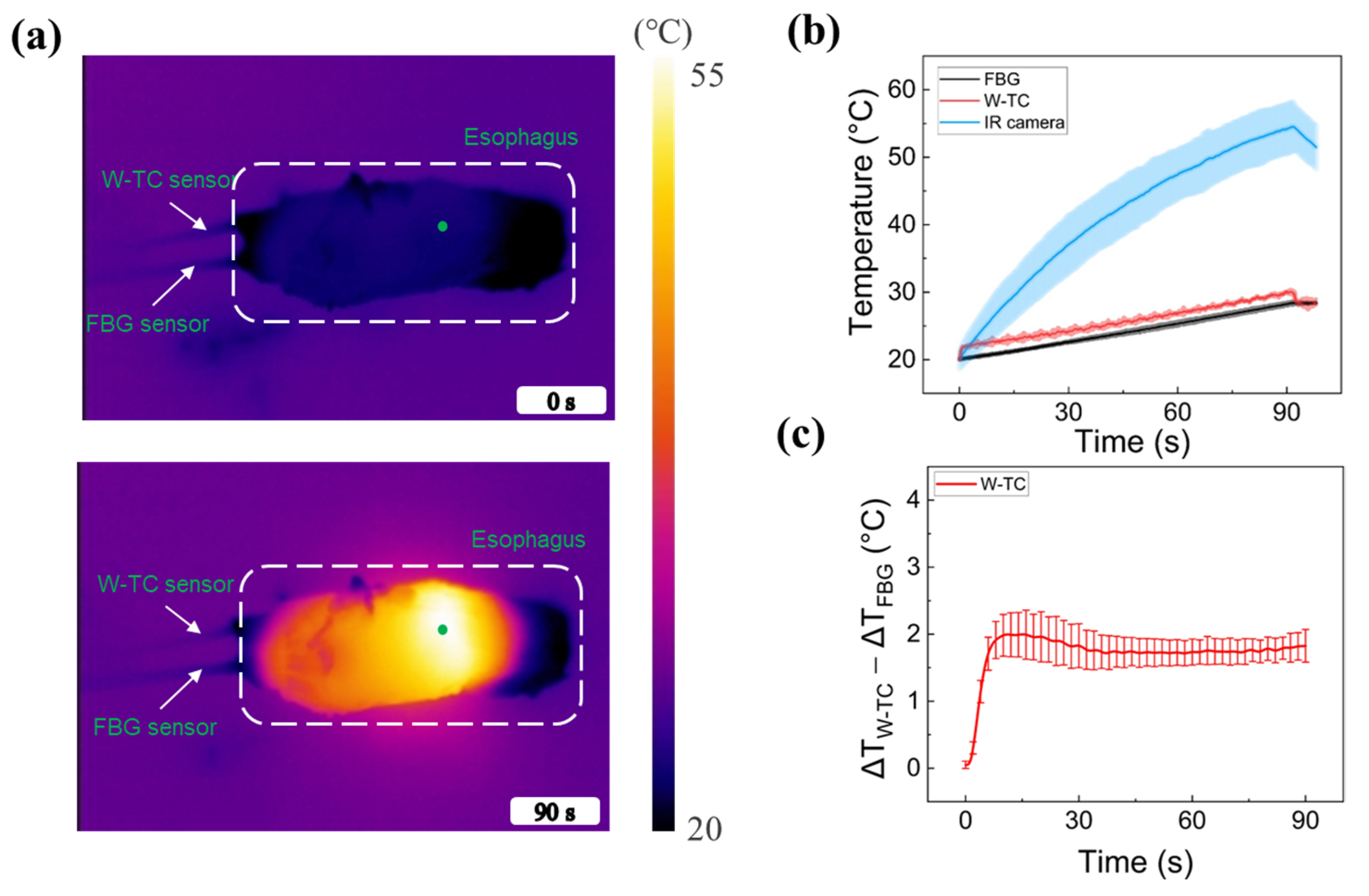
2.5. Ex Vivo Esophagus Tissue Test
2.6. Data and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gyawali, C.; Bredenoord, A.; Conklin, J.; Fox, M.; Pandolfino, J.; Peters, J.; Roman, S.; Staiano, A.; Vaezi, M. Evaluation of esophageal motor function in clinical practice. Neurogastroenterol. Motil. 2013, 25, 99–133. [Google Scholar] [CrossRef] [PubMed]
- Oezcelik, A.; DeMeester, S.R. General anatomy of the esophagus. Thorac. Surg. Clin. 2011, 21, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Köksoy, F.N.; Gönüllü, D. The Benign Strictures of the Esophagus. JAREM J. Acad. Res. Med. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Schraufnagel, D.E.; Michel, J.C.; Sheppard, T.J.; Saffold, P.C.; Kondos, G.T. CT of the normal esophagus to define the normal air column and its extent and distribution. Am. J. Roentgenol. 2008, 191, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Murat Ferhat, F.; Taner, K. Anatomy of Esophagus. In Esophageal Abnormalities; Chai, J., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 1. [Google Scholar]
- Egan, J.V.; Baron, T.H.; Adler, D.G.; Davila, R.; Faigel, D.O.; Gan, S.-L.; Hirota, W.K.; Leighton, J.A.; Lichtenstein, D.; Qureshi, W.A. Esophageal dilation. Gastrointest. Endosc. 2006, 63, 755–760. [Google Scholar] [CrossRef]
- Levine, M.S.; Rubesin, S.E. Diseases of the esophagus: Diagnosis with esophagography. Radiology 2005, 237, 414–427. [Google Scholar] [CrossRef]
- Min, Y.W.; Lee, J.H.; Min, B.-H.; Lee, J.H.; Kim, J.J.; Rhee, P.-L. Association between gastroesophageal reflux disease after pneumatic balloon dilatation and clinical course in patients with achalasia. J. Neurogastroenterol. Motil. 2014, 20, 212. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P. Endoscopic mucosal resection as the primary treatment for Barrett esophagus with dysplasia. Gastroenterol. Hepatol. 2015, 11, 703. [Google Scholar]
- Ota, K.; Takeuchi, T.; Kojima, Y.; Sugawara, N.; Nishida, S.; Iwatsubo, T.; Kawaguchi, S.; Harada, S.; Tokioka, S.; Higuchi, K. Outcomes of endoscopic submucosal dissection for gastroesophageal reflux disease (ESD-G) for medication-refractory gastroesophageal reflux disease: 35 cases underwent ESD-G including 15 cases followed more than 5 years. BMC Gastroenterol. 2021, 21, 432. [Google Scholar] [CrossRef]
- Pandolfino, J.E. The use of endoscopy and radiofrequency ablation for the treatment of GERD. Gastroenterol. Hepatol. 2015, 11, 847. [Google Scholar]
- Jeong, S.; Bak, J.; Kim, S.M.; Kang, H.W. Feasibility study of endoscopic thermal coagulation with circumferential laser irradiation for treating esophageal tissue. Lasers Med. Sci. 2020, 35, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.; Hwang, J.; Park, S.; Kang, H.W. Integration of optical applicator with balloon catheter for photothermal treatment of biliary stricture. Lasers Surg. Med. 2017, 49, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.G.; Jeong, S.; Lee, Y.; Kim, S.M.; Kang, H.W. Novel endoscopic laser treatment of common bile duct stenosis using balloon catheter-integrated diffusing applicator (BCDA). In Proceedings of the SPIE Advanced Biophotonics Conference (SPIE ABC 2021), Busan, Republic of Korea, 4–6 November 2021; SPIE: Bellingham, DC, USA, 2022; pp. 15–17. [Google Scholar]
- Cha, B.; Kim, H.; Truong, V.G.; Oh, S.-J.; Jeong, S.; Kang, H.W. Feasibility Study on Endoscopic Balloon-Assisted Laser Treatment (EBLT) of Gastroesophageal Reflux Disease (GERD) in In Vivo Porcine Model. Biomedicines 2023, 11, 1656. [Google Scholar] [CrossRef]
- Truong, V.G.; Kim, H.; Lee, B.-I.; Cha, B.; Jeong, S.; Oh, S.-J.; Kang, H.W. Development of Novel Balloon-Integrated Optical Catheter for Endoscopic and Circumferential Laser Application. Ann. Biomed. Eng. 2023, 1–14. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Silvestri, S. Techniques for temperature monitoring during laser-induced thermotherapy: An overview. Int. J. Hyperth. 2013, 29, 609–619. [Google Scholar] [CrossRef]
- Larina, I.V.; Larin, K.V.; Esenaliev, R.O. Real-time optoacoustic monitoring of temperature in tissues. J. Phys. D Appl. Phys. 2005, 38, 2633. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Zhang, H. Review of fiber Bragg grating sensor technology. Front. Optoelectron. China 2011, 4, 204–212. [Google Scholar] [CrossRef]
- Hong, C.-Y.; Zhang, Y.-F.; Zhang, M.-X.; Leung, L.M.G.; Liu, L.-Q. Application of FBG sensors for geotechnical health monitoring, a review of sensor design, implementation methods and packaging techniques. Sens. Actuators A Phys. 2016, 244, 184–197. [Google Scholar] [CrossRef]
- Presti, D.L.; Massaroni, C.; Leitão, C.S.J.; Domingues, M.D.F.; Sypabekova, M.; Barrera, D.; Floris, I.; Massari, L.; Oddo, C.M.; Sales, S. Fiber bragg gratings for medical applications and future challenges: A review. IEEE Access 2020, 8, 156863–156888. [Google Scholar] [CrossRef]
- Kim, S.-W.; Jeong, M.-S.; Lee, I.; Kwon, I.-B. Static mechanical characteristics of tin-coated fiber Bragg grating sensors. Sens. Actuators A Phys. 2014, 214, 156–162. [Google Scholar] [CrossRef]
- Li, X.; Shen, Z.; Zheng, M.; Hou, D.; Wen, Q. Great increase of the tensile strength in the lamellar PI-coated FBG sensors. Opt. Contin. 2023, 2, 110–118. [Google Scholar] [CrossRef]
- Pham, N.T.; Lee, S.L.; Park, S.; Lee, Y.W.; Kang, H.W. Real-time temperature monitoring with fiber Bragg grating sensor during diffuser-assisted laser-induced interstitial thermotherapy. J. Biomed. Opt. 2017, 22, 045008. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.M. Advantages and Disadvantages of Using Thermocouples. 2019. Available online: https://sciencing.com/advantages-disadvantages-using-thermocouples-6153729.html (accessed on 4 September 2019).
- Duff, M.; Towey, J. Two ways to measure temperature using thermocouples feature simplicity, accuracy, and flexibility. Analog Dialogue 2010, 44, 1–6. [Google Scholar]
- Nguyen, T.H.; Rhee, Y.-H.; Ahn, J.-C.; Kang, H.W. Circumferential irradiation for interstitial coagulation of urethral stricture. Opt. Express 2015, 23, 20829–20840. [Google Scholar] [CrossRef]
- Lim, S.; Truong, V.G.; Kang, H.W. Impact of residual air trap in balloon on laser treatment of tubular tissue. Lasers Surg. Med. 2022, 54, 767–778. [Google Scholar] [CrossRef]
- Ströbl, S.; Domke, M.; Rühm, A.; Sroka, R. Investigation of non-uniformly emitting optical fiber diffusers on the light distribution in tissue. Biomed. Opt. Express 2020, 11, 3601–3617. [Google Scholar] [CrossRef]
- Schena, E.; Saccomandi, P.; Massaroni, C.; Frauenfelder, G.; Giurazza, F.; Peroglio, G.M.; Silvestri, S.; Caponero, M.A.; Polimadei, A. Thermocouples for temperature monitoring during pancreatic laser ablation: Analysis of the measurement error. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings, Torino, Italy, 7–9 May 2015; IEEE: Piscataway, NJ, USA; pp. 219–223. [Google Scholar]
- Reid, A.D.; Gertner, M.R.; Sherar, M.D. Temperature measurement artefacts of thermocouples and fluoroptic probes during laser irradiation at 810 nm. Phys. Med. Biol. 2001, 46, N149. [Google Scholar] [CrossRef]
- Hübner, F.; Bazrafshan, B.; Roland, J.; Kickhefel, A.; Vogl, T.J. The influence of Nd: YAG laser irradiation on Fluoroptic® temperature measurement: An experimental evaluation. Lasers Med. Sci. 2013, 28, 487–496. [Google Scholar] [CrossRef]
- Van Nimwegen, S.; L’eplattenier, H.; Rem, A.; Van Der Lugt, J.; Kirpensteijn, J. Nd: YAG surgical laser effects in canine prostate tissue: Temperature and damage distribution. Phys. Med. Biol. 2008, 54, 29. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, Y.; Hwang, D.; Longtin, J.P. Thermocouple-tip-exposing temperature assessment technique for evaluating photothermal conversion efficiency of plasmonic nanoparticles at low laser power density. Rev. Sci. Instrum. 2019, 90, 094902. [Google Scholar] [CrossRef]
- Manns, F.; Milne, P.J.; Gonzalez-Cirre, X.; Denham, D.B.; Parel, J.M.; Robinson, D.S. In situ temperature measurements with thermocouple probes during laser interstitial thermotherapy (LITT): Quantification and correction of a measurement artifact. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 1998, 23, 94–103. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Wang, Q.; Li, H.; Xiao, S.; Li, F. Effects of thermal relaxation on temperature elevation in ex vivo tissues during high intensity focused ultrasound. IEEE Access 2020, 8, 212013–212021. [Google Scholar] [CrossRef]
- Triadafilopoulos, G. Stretta: A valuable endoscopic treatment modality for gastroesophageal reflux disease. World J. Gastroenterol. WJG 2014, 20, 7730. [Google Scholar] [CrossRef] [PubMed]
- McGouran, R.; Galloway, J. A laser-induced scar at the cardia increases the yield pressure of the lower esophageal sphincter. Gastrointest. Endosc. 1990, 36, 439–443. [Google Scholar] [CrossRef]
- de Miranda Chaves, R.C.; Suesada, M.; Polisel, F.; de Sá, C.C.; Navarro-Rodriguez, T. Respiratory physiotherapy can increase lower esophageal sphincter pressure in GERD patients. Respir. Med. 2012, 106, 1794–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, R.A.; Gostout, C.J.; Grudem, J.; Swanson, W.; Berg, T.; DeMeester, T.R. Use of a magnetic sphincter for the treatment of GERD: A feasibility study. Gastrointest. Endosc. 2008, 67, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Protsiv, M.; Ley, C.; Lankester, J.; Hastie, T.; Parsonnet, J. Decreasing human body temperature in the United States since the industrial revolution. eLife 2020, 9, e49555. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ta, M.D.; Truong, V.G.; Lim, S.; Lee, B.-I.; Kang, H.W. Comparative Evaluations on Real-Time Monitoring of Temperature Sensors during Endoscopic Laser Application. Sensors 2023, 23, 6069. https://doi.org/10.3390/s23136069
Ta MD, Truong VG, Lim S, Lee B-I, Kang HW. Comparative Evaluations on Real-Time Monitoring of Temperature Sensors during Endoscopic Laser Application. Sensors. 2023; 23(13):6069. https://doi.org/10.3390/s23136069
Chicago/Turabian StyleTa, Minh Duc, Van Gia Truong, Seonghee Lim, Byeong-Il Lee, and Hyun Wook Kang. 2023. "Comparative Evaluations on Real-Time Monitoring of Temperature Sensors during Endoscopic Laser Application" Sensors 23, no. 13: 6069. https://doi.org/10.3390/s23136069
APA StyleTa, M. D., Truong, V. G., Lim, S., Lee, B.-I., & Kang, H. W. (2023). Comparative Evaluations on Real-Time Monitoring of Temperature Sensors during Endoscopic Laser Application. Sensors, 23(13), 6069. https://doi.org/10.3390/s23136069







