Abstract
Analytical devices for bacterial detection are an integral part of modern laboratory medicine, as they permit the early diagnosis of diseases and their timely treatment. Therefore, special attention is directed to the development of and improvements in monitoring and diagnostic methods, including biosensor-based ones. A promising direction in the development of bacterial detection methods is optical sensor systems based on colorimetric and fluorescence techniques, the surface plasmon resonance, and the measurement of orientational effects. This review shows the detecting capabilities of these systems and the promise of electro-optical analysis for bacterial detection. It also discusses the advantages and disadvantages of optical sensor systems and the prospects for their further improvement.
1. Introduction
Bacterial contamination of food and water and the growth of infectious diseases are forcing humanity to look for new pathogen identification methods. In addition, constantly growing threats to personal and territorial security make bacterial detection a priority. Infections are a leading cause of premature mortality, because infectious diseases account for up to 70% of cases [1,2]. The identification and quantification of microorganisms has become key to biodefense, food safety research, diagnostics, and drug discovery. The detection of pathogens and indicator microorganisms in water and food samples is vital to public health and environmental protection. For example, the Department of Disease, Control and Prevention has estimated that each year in the United States, foodborne illnesses cause about 325,000 hospitalizations and 5000 deaths [3].
The accurate detection and identification of bacteria in food and water are very important not only for public safety and health but also for timely and successful treatment. The rapid diagnosis of bacterial infections may ultimately reduce the incidence of diseases and prevent the spread of dangerous epidemics. Traditional laboratory diagnosis methods are lengthy and labor-intensive, and they require expensive equipment and professional personnel. In recent years, substantial progress has been made in the design of accurate, rapid, specific, and cheap methods for the detection of bacteria [4].
An alternative to traditional bacterial detection methods is biosensor systems, which combine the diagnostic capabilities of biomedicine with advances in microelectronics, optoelectronics, and nanotechnology. Of special note are optical biosensors, in which analytical signals are determined by the measurement of physical variables (the intensity of light absorption and reflection, intensity of the object’s luminescence, etc.), rather than by the chemical interaction of the component being determined with the sensitive element. The operating principle of optical sensor systems is based on recording changes in the optical properties of a medium that result from the presence of a detectable biological agent (bacteria or viruses). The optical properties include absorbance (densitometric biosensors), color (colorimetric biosensors), turbidity (turbidimetric biosensors), refractive indices of the medium (refractometric biosensors), and so on.
Although several excellent reviews have been published on the use of optical sensors for bacterial detection [5,6], optical sensor systems based on photonic crystals and ionophores, planar biosensors, fiber optic sensors, and optical sensor systems based on the measurement of orientational effects have not been sufficiently covered in a single review. The purpose of this article is presents an overview of the main directions in the development of optical sensor systems and the use of such systems for bacterial detection.
2. Classical Bacterial Detection Methods and Approaches
Usually, bacteria are detected using traditional methods such as culture and colony counting, which in most cases require several processing steps. Traditional bacterial detection methods take at least 72 h to produce a reliable result [7].
Many rapid methods have been proposed to overcome these difficulties, including polymerase chain reaction (PCR) and immunoassays [8,9]. Immunoassay methods, based on the specific antigen–antibody reaction, are used widely for the rapid detection of bacteria. These include
- -
- Enzyme-linked immunosorbent assay.
- -
- Immunomagnetic separation, an analytic tool used to detect bacteria and based on the capture of target bacteria with antibody-coated magnetic beads. It is combined with various detection methods such as PCR [10].
- -
- Methods based on fluorophore-labeled secondary antibodies [11].
- -
- Enzyme-labeled antibodies [12].
- -
- Quantum-dot-labeled antibodies [13,14].
Although immunoassays are quite sensitive, they involve many incubation steps and are, therefore, inconvenient. Biosensors, which combine biological materials such as antibodies, peptides, and nucleic acids with a physicochemical transducer, have become important in the rapid, sensitive, and selective detection of microorganisms.
3. Biosensors: Operating Principle and Classification
Three generations of biosensors are available on the market. In the first type, the product comes into contact with the sensor and gives an electric response. In the second type, the sensor includes certain intermediaries between the sensor and the response to obtain a better response. In the third type, the sensor itself gives a response, without an intermediary participating.
Biosensors include a combination of biological detection elements such as a sensor system and a transducer. Different types of biosensors are available depending on the sensor design and the biological material used. On the basis of the transducer system used, biosensors can be classified into optical, electrochemical, thermometric, piezoelectric, and magnetic [15,16,17,18].
In a biosensor, a specific enzyme or a desired biological material is deactivated using a conventional method, and the deactivated material is placed in direct contact with the transducer. In this case, the converter can change the signal associated with it, converting it into electric signals that can be changed and calculated.
Biosensors are applicable in medicine, the food industry, and environmental monitoring, because their sensitivity and stability are better than those of conventional methods. Biosensors are used mostly in environmental pollution monitoring, agriculture, and food processing, and they have become very popular in recent years (Figure 1).
Figure 1.
Main applications of biosensors.
Biosensors are characterized by stability, cheapness, sensitivity, and reproducibility. In a biosensor, the core element may be an enzyme, a nucleic acid, or an antibody. The biological element interacts with the analyte, and the biological response can be converted into an electrical signal with a transducer. Biosensors consist of three parts:
- -
- Bioselective element (biological material, (e.g., tissues, microorganisms, organelles, cellular receptors, enzymes, antibodies, and nucleic acids), material of biological origin, or a biomimic material). The sensing element can be made using bioengineering.
- -
- An optical, piezoelectric, or electrochemical transducer, which converts the signal resulting from the interaction of the analyte with the bioselective element into a signal that is easier to measure.
- -
- Associated electronics, primarily responsible for displaying results in a user-friendly manner [18]. Figure 2 shows the basic scheme for a biosensor. Modern technologies in infectious diagnostics and epidemiology have made label-free biosensors increasingly widespread. This allows intermolecular interactions and cellular reactions to be screened, yielding details of the selectivity of bacterial exotoxins, the specificity of antibacterial agents, the antigen–antibody interaction, the kinetics of inflammation, and immunological and serological responses [19,20]. This type of biosensor requires only one recognition element, which simplifies the analysis and reduces its duration and reagent costs. The modern generation of label-free biosensors enables the real-time measurement of the products of biomolecular reactions, continuous recording of data, and kinetic monitoring of the recognition of the ligand–receptor interaction [3,21].
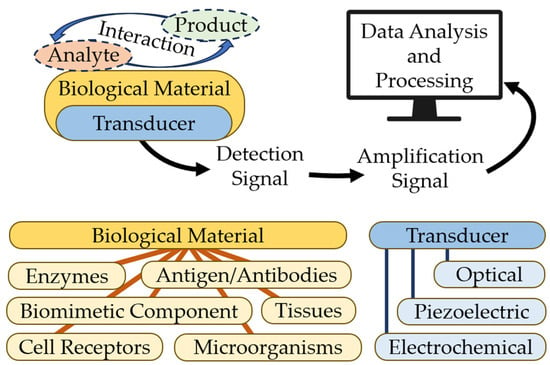 Figure 2. Basic scheme for a biosensor.
Figure 2. Basic scheme for a biosensor.
An important advantage of label-free biosensors is that target analytes are detected in their natural form, without labeling or chemical modification, and therefore can be stored for further analysis.
Optical, (piezo)electrical, or (micro)mechanical transducers are promising for recognizing ligand–receptor interaction signals in label-free biosensors used to diagnose infectious diseases. They are enhanced using surface plasmon resonance (SPR) [3], surface-enhanced Raman scattering (SERS) [22,23,24], and nanoparticles [25,26].
4. Optical Sensor Systems for Bacterial Detection
The fundamental phenomena underlying the operation of optical sensors are these:
- -
- Absorption (the ability of a substance to absorb optical radiation).
- -
- Reflection (when a stream of light falls on the interface between two media, part of its radiation is reflected back).
- -
- Luminescence (the glow of a substance that occurs after it absorbs excitation energy and is excess radiation, as compared with the thermal radiation of the body at a given temperature).
- -
- Photoluminescence (the emission of photons by a substance that occurs when the substance is excited by electromagnetic radiation in the ultraviolet, visible, and infrared wavelength ranges). Photoluminescence is characterized by absorption and luminescence spectra, polarization, energy yield (the ratio of the energy emitted by a substance in the form of luminescence to the absorbed energy), quantum yield (the ratio of the number of emitted quanta to the number of absorbed ones), and kinetics.
An optical biosensor uses an optical measurement principle, fiber optics, and optoelectronic converters. The term “optrode” is a compression of two terms, “optical” and “electrode”. Aptamers, antibodies, and bacteriophages have highly specific abilities to recognize bacteria and are used as specific biorecognition elements. These elements become important components of the sensor, as they can ensure high specificity and sensitivity of detection [27]. In optical biosensors, antibodies and enzymes are used mainly as transducers, enabling safe, nonelectric, and noncontact sensing. An additional advantage is that they often do not require reference sensors, because a reference signal can be obtained with a light source similar to the sampling sensor. Optical biosensors are divided into two types—direct and labeled.
Biosensors using an optical transducer as the main signal-sensing tool are among the most powerful tools used for bacterial identification. Optical transducers are based on measuring changes in optical properties such as absorption, reflectance, emission, or interferometric patterns, which can be detected with a photodetector. They are immune to electromagnetic interference, capable of remote sensing, and offer a number of advantages, including high sensitivity, direct real-time measurement, and multiplexing capability (detection of multiple analytes at once). Microbial biosensors that detect interactions between microorganisms and target ligands are no exception [28].
In optical biosensors, the analytical signal is determined not by the chemical interaction between the components being measured but by the measured physical parameters, including (but not limited to) the intensity of light absorption and reflection and the intensity of the object’s luminescence. The operating principle of optical biosensors is based on recording changes in the optical properties of the medium that result from the presence of a biological agent. These properties include absorbance (densitometric biosensors), color (colorimetric biosensors), turbidity (turbidimetric biosensors), the refractive index (refractometric biosensors), and other properties. Biosensors are compact analytical devices consisting of two components: a sensitive biological element and a detection system. The detector includes an amplifier, which is known as a signal conditioning circuit, a display unit, and a processor (Figure 3).
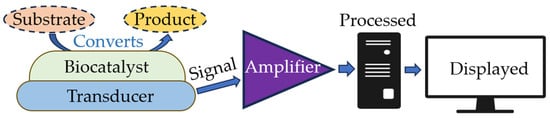
Figure 3.
Main components of a biosensor.
Advanced technologies in the design of label-free optical biosensors with an emphasis on the diagnosis of bacterial infections are associated with the development of modern transduction methods (fiber optics and evanescent electromagnetic field systems, surface plasmon resonance, Raman spectroscopy, or interferometry) and new recognition elements (molecular-imprinted polymers) [28].
4.1. Colorimetric Sensor System for Bacterial Detection
Colorimetric analysis (from Latin color and Greek μετρώ ‘measure’) is a physical method of chemical analysis based on determining the concentration of a substance by the intensity of the color of solutions (more precisely, by the absorption of light by solutions). This method has received great development in biomolecule analysis owing to its advantages, including simplicity, stability, and cheapness. ELISA is a classical method for detecting bacteria, which uses an enzyme to catalyze the chromophore and produce a color change in the solution that can be observed with the naked eye or quantified with a spectrometer. This principle is used widely for the rapid identification of bacteria, including in sensor technologies. However, its detection sensitivity is limited by the low extinction coefficient of the organic chromophore. Recently, plasmonic nanomaterials, specifically gold nanoparticles (AuNPs) and silver nanoparticles (AgNPs), have been commonly used in the colorimetric recognition of biomolecules owing to their special optical properties, including high extinction coefficients [29]. For example, a colorimetric method was described that detects Gram-negative bacteria (Escherichia coli) on the basis of inhibition of aggregation of AgNPs functionalized with a boronic acid derivative [29]. The degree of color change from yellow to brownish-red depends on the bacterial density. Bacteria were detected in a dynamic range from 5 × 104 CFU mL−1 to 1 × 107 CFU mL−1, with a limit of detection of 0.9 × 104 CFU mL−1. The sensor’s response was fast (20 min). The bacterial density could be determined by the color of the resulting solution in the UV–visible range or with the naked eye.
The capabilities of a colorimetric method for detecting bacteria (E. coli) in water that is based on the magnetic separation of proteins of the tail fibers of a T7 phage were shown in [30]. The capture efficiency for E. coli ranged from 88.70 to 95.65%, and 102 CFU/mL of E. coli could be detected by the naked eye. The authors pointed out that colorimetric changes detected by visual inspection may provide an effective platform for point-of-care detection of E. coli in resource-limited areas.
Another study [31] presented a colorimetric sensor system for the detection of E. coli O157:H7 in sausage by using magnetic separation and enzymatic signal amplification on a paper disk. The authors pointed out that it is readily applicable to real food (sausage) with an exceptionally low limit of detection (~30 CFU/mL). Problems associated with the nonspecific binding of the sensing components, which lead to high background signal and low sensitivity of colorimetric detection, were overcome by using hyaluronic acid as a blocking agent [31].
Although colorimetry greatly simplifies the detection of bacteria, very often it is characterized by low accuracy and difficulties in determining individual components in the mixture being analyzed.
4.2. Fluorescence Sensor Systems for Bacterial Analysis
Fluorescence has found wide application in applied biological and biomedical research. The essence of fluorescence is the short-term absorption of a quantum of light by a fluorophore (a substance capable of fluorescence), followed by the rapid emission of another quantum, whose properties differ from those of the original one (Figure 4) [32].
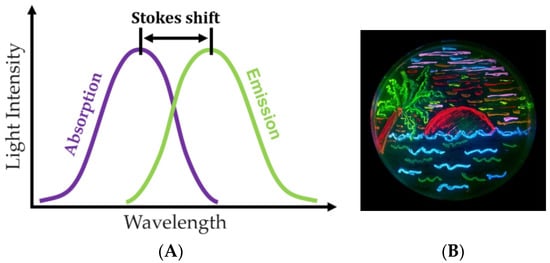
Figure 4.
(A) Principle of fluorescence analysis and (B) Petri dish with bacteria expressing eight fluorescent proteins (figure taken from the Wikipedia website) [33].
Compared with traditional bacterial detection methods, fluorescence probing offers the additional advantage of rapidity of bacterial detection at a relatively low cost.
Among the optical technologies used for bacterial detection, fluorescence sensors have a wide range of applications owing to their hypersensitivity, specificity, and accuracy [34]. Three main strategies for bacterial detection with a fluorescence sensor can be distinguished:
- (1)
- Fluorescence imaging to identify the bacteria;
- (2)
- Enhancement or changing of the method’s characteristics when targets are combined;
- (3)
- Quenching of fluorescent signals through energy receptors, such as quenching agents [35].
The main trends in the development of fluorescence sensors for bacterial detection are these:
- -
- Use of appropriate fluorescent materials;
- -
- Use of DNA, bacteria, and metabolites as the response targets;
Each fluorophore has its own specific absorption and emission spectra, depending on the structure of the molecule and its environment. Initially, researchers used traditional fluorescent dyes as fluorescent agents. However, these dyes have significant disadvantages, including a high background, a narrow excitation spectrum, a wide and asymmetric fluorescence spectrum, and ease of photobleaching, which limit their application in the biomedical field. It is expected that with rapid developments in nanomaterials and nanotechnology, new fluorescent nanomaterials will gradually replace traditional luminescent materials owing to their high fluorescence quantum yield and excellent light stability. The main advantages of using fluorescent nanomaterials for bacterial detection are their small size, high adsorption capacity, and high surface reaction activity. Bacteria can simultaneously combine with multiple nanoparticles, thus permitting a higher fluorescence intensity to be achieved in the labeled target bacteria. Owing to the high stability of fluorescence, bacteria can be monitored in real time from minutes to hours. In addition, multicolored fluorescent nanoparticles can be used to simultaneously detect different strains. The toxicity of fluorescent nanoparticles is strongly reduced after their surface is coated with inert substances.
When used in sensors, fluorescent nanomaterials can be conjugated with biomolecules (enzymes and nucleic acids), which results in a high sensor performance. In recent years, several new materials have been developed, including QDs, CDs, UCNPs, and MOFs. These materials possess improved chemical and physical properties and can be used to identify, transmit, and amplify signals for bacterial detection [35,36,37,38,39]. The potential application of two-level column-like 3D nanofilms in optical sensing technologies was described in [40].
Several different modifications of fluorescence assay methods for detection have been described, as follows:
Escherichia coli: detection limit, 1.0 CFU/mL [41]; detection limit, 4.0 × 101 CFU mL−1; concentration range, 1.3 × 102 CFU mL−1–6.5 × 104 CFU mL−1 [42]; concentration range, 1.3 × 102–1.3 × 108 CFU mL−1; detection limit, 3 CFU mL−1 in [43]; concentration range, 10–105 CFU/mL; detection limit, 8 CFU/mL in [44]; detection limit, 50 cells/mL [45]; concentration range, 103–105 cells mL−1 [46]; concentration range, 103–106 CFU mL−1 [47]; and detection limit, 105 cell/mL [48].
Salmonella typhimurium: detection limit, 50 cells/mL [45]; concentration range, 103–105 cells mL−1 [46,49]; concentration range, 103–106 CFU mL−1; detection limits, 100 and 138 CFU mL−1 in a fresh-cut vegetable washing solution and a lettuce sample, respectively [50].
Staphylococcus aureus: detection limit, 105 cell/mL [48]; and concentration range, 1 × 103–1 × 1011 CFU mL−1; detection limit, 1 × 103 CFU mL−1 [51]; concentration range, not given [52]; concentration range, 47–4.7 × 107 CFU mL−1; and detection limit, 10.7 CFU mL−1 [53].
In Klebsiella pneumonia, the detection limit is 105 cell/mL; in [48] P. aeruginosa, B. cereus 103–105 cells per mL in [46]; etc.
An important direction in the development of fluorescent sensors is the so-called remote detection of pathogens. For example, near-infrared (NIR) fluorescence nanosensors were used for the remote fingerprinting of clinically important bacterial detection [54]. Multiple nanosensors based on NIR fluorescent single-walled carbon nanotubes were synthesized in such a way that they changed their fluorescence signal in response to bacterial metabolites and virulence factors (cell wall components, chelating iron molecules, and enzymatic activity). Using several sensors with different selectivities allowed the authors to clinically distinguish between relevant bacteria on the basis of their metabolic fingerprints. Multiplexing was achieved through spatial or spectral coding, which emphasizes the potential for remote pathogen detection (Figure 5) [54].
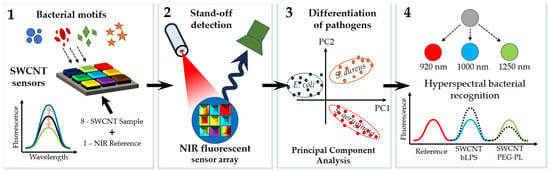
Figure 5.
Remote detection of pathogens. (1) Multiple nanosensors based on NIR fluorescent single-walled carbon nanotubes are synthesized in such a way that they change their fluorescence signal in response to bacterial metabolites and virulence factors (cell wall components, iron chelating molecules, and secretory enzymatic activity). (2) Eight fluorescent nanosensors and one NIR fluorescent reference are incorporated into a polyethylene glycol hydrogel array that is remotely monitored in the NIR. (3) Bacteria growing on top of this hydrogel release molecules that change the (spatial) sensor array fingerprint, which allows one to differentiate between important pathogens. (4) By using chirality-purified single-walled carbon nanotubes, multiple sensors can be spectrally encoded and used for hyperspectral differentiation of important bacteria such as S. aureus [54], with modifications.
Hydrogels were exposed to clinical isolates of six important bacteria, and remote (≥25 cm) NIR imaging allowed the authors to identify and distinguish between the bacteria. Sensors were also spectrally encoded (900, 1000, and 1250 nm) to differentiate between the two major pathogens, P. aeruginosa and S. aureus, and penetrate into tissue (>5 mm). In the future, the remote near-infrared detection of bacteria will enable faster diagnosis and individualized antibiotic treatment, ultimately leading to better clinical outcomes and lower mortality rates.
A fluorescence and an optoelectric recording device were combined for automatic real-time photoelectric microbial sensor analysis [55]. The system enabled the ultra-sensitive (1 CFU/mL) and specific (99%) detection of E. coli and P. aeruginosa. This system can possibly be extended to detect other microorganisms.
Recent advances in fluorescent dyes capable of detecting bacteria and fluorescent sensor assay strategies for identifying bacteria, including specific interactions with the bacterial cell wall, have been described in the reviews [38,56,57].
A separate mention should be made for fluorescence polarization immunoassay, based on the fluorescence and polarization of light [58]. Fluorescence is the process of transition of an electron from an excited singlet state to the ground state. This process is accompanied by a release of energy in the form of radiation with a certain wavelength. If fluorescent molecules are excited by plane-polarized light, the emitted radiation must be polarized in the same plane, but only if the molecule is stationary. If the molecule rotates while in an excited state (the period between the absorption of light and the emission of fluorescence), then fluorescence is emitted in a plane different from that used for excitation. This leads to depolarization of the emitted light (Figure 6). For small molecules that undergo rapid Brownian rotation in solution (e.g., a fluorescently labeled low-molecular-weight antigen (tracer) labeled with a fluorescent label), the fluorescence polarization value is low. Larger molecules (e.g., fluorophores) bound to antibodies are less mobile in solution and have higher fluorescence polarization values (Figure 6). The molecular volume of a fluorescently labeled substance can be changed as a result of cleavage (dissociation), binding to another molecule, or conformational changes.
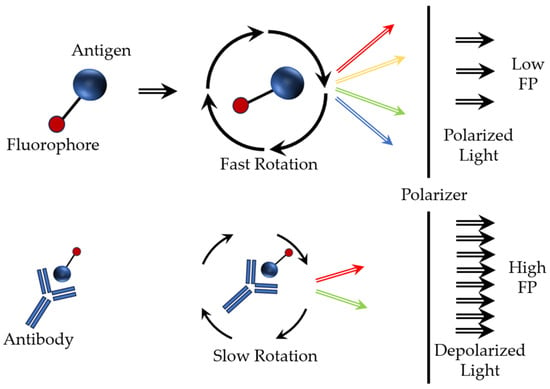
Figure 6.
Basic operating diagram of the fluorescence polarization immunoassay.
The history and application prospects of this method, including its use in the diagnosis of diseases, were described in [59]. The capabilities of fluorescence polarization analysis using fluorescein-labeled ESAT-6 protein were shown in the detection of antibodies to Mycobacterium bovis in bovine serum [60] and antibodies against Brucella melitensis in goat milk [61].
Fluorescence polarization immunoassay is highly specific, sensitive, and productive, and it is simpler, faster, and more reproducible than ELISA. Equilibrium for the antigen–antibody reaction in solution is established within 1–2 min, and the fluorescently labeled tracers remain stable for many years of storage. In addition, the method can be easily automated; in particular, Ellie (Milwaukee, WI, USA) [62] produces the portable polarization fluorometer Sentry-300, which is used widely in diagnostic laboratories and in non-laboratory settings. Despite these advantages, fluorescence polarization immunoassay has several limitations: specifically, it is applicable only to the determination of low-molecular-weight compounds. Because the analysis does not involve separation of components, the proteins, endogenous fluorophores, inhibitors and activators of protein binding, and other substances present in the reaction mixture will always affect the results of the determination.
With account taken of the great progress made in nanomaterials and sensor research, fluorescence-based sensors for bacterial detection show great promise and will play a key part in bacterial detection.
4.3. Chemiluminescence
Chemiluminescence (CL) is another optical method alternative to the colorimetric approach, which is triggered by the transition of an atom or a molecule from an excited state to a steady state with the emission of photons as a by-product of a chemical reaction [63]. CL-using biosensors are typically based on enzyme indicators able to catalyze CL in the presence of a suitable substrate. The measurement of the produced photons can be related to the concentration of the target analyte, allowing quantitative analysis to be conducted, but this implies the use of an external detector capable of detecting luminescent signals. Currently, there is a wide range of miniaturized and compact CL detectors that can be used in portable analytical devices [64].
CL biosensors have been developed and widely applied to mammalian cells and the study of intracellular signaling networks. With respect to bacteria, biosensors relied heavily on fluorescence-based systems to quantify signaling molecules, but in challenging environments, such designs can face problems owing to their dependence on ambient light. To circumvent these problems, ratiometric CL biosensors were developed to study cyclic di-GMP, the key bacterial secondary messenger [65]. For combining the advantages of electrochemical and optical bacterial detection, electrochemiluminescence is widely used for routine analytical operations in the laboratory. It is triggered by an electrochemical stimulus on a specific molecule and ensures high control of the emitted light, a low background signal, and high sensitivity. These aspects make electrochemiluminescence particularly suitable for the development of portable biosensor devices [66]. Compared with fluorescence, electrochemiluminescence does not require an excitation light source, thereby avoiding autofluorescence or a diffuse light background. Moreover, the use of potential to initiate and regulate the output signal provides electrochemiluminescent sensors with high repeatability and accuracy [67].
4.4. Sensors Based on the Surface Plasmon Resonance
Currently, the best developed optical biosensors are those based on changes in the direction of propagation of a light flux through an optical fiber or a triangular prism coated with a thin metal film. Biosensors of this type are based on surface plasmon resonance (SPR). The intermolecular interaction is detected by the change in the refractive index of the medium as a result of the formation of an antigen–antibody complex on the resonance layer of a measuring cell or a flow cell [68,69]. SPR-based biosensors are sensitive and use a special mode of electromagnetic welding, the surface plasmon polariton, to detect the binding of the analyte molecules from a liquid sample with biomolecular recognition elements on the sensor surface [70].
SPR-based optical affinity biosensors are some of the most advanced [71]. Their ability to control the interaction between a molecule immobilized on the sensor surface and an interacting molecular partner in solution has made SPR sensors very powerful for analyzing biomolecular interactions. In recent years, SPR biosensors have been increasingly used to detect chemical and biological substances related to medical diagnostics, environmental monitoring, and safety. SPR biosensors consist of three main subsystems: sensor equipment (optical reader), a biorecognition element, and a sample preparation and delivery system (Figure 7).

Figure 7.
Principal components of an SPR affinity biosensor.
In the optical reader of the SPR sensor, a light wave excites a special mode of the electromagnetic field called a surface plasmon. The surface plasmon propagates along a thin metal film, and its field probes the medium adjacent to the metal surface. Any change in the refractive index near the metal surface leads to a change in the velocity of the surface plasmon. This change can be determined by the characteristics of the light wave associated with the surface plasmon. Biorecognition elements are immobilized on the metal surface. If a liquid sample comes into contact with the sensor surface, the analyte molecules are captured by the biorecognition molecules (Figure 8). This binding leads to a refraction index change near the sensor surface, which is recorded by the sensor [72].
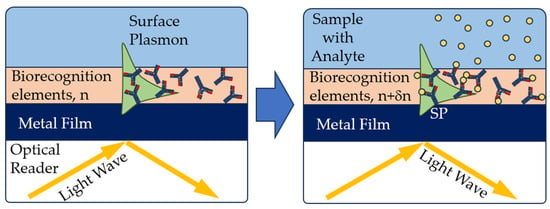
Figure 8.
Principle of operation of SPR affinity biosensors [71], with modification.
A surface plasmon is an electromagnetic wave propagating along the boundary between a dielectric and a metal [73]. Most SPR sensors are based on the Kretschmann attenuated total reflection configuration.
In this geometry, the light wave passes through a prism with a high refractive index and is completely reflected from the base of the prism, covered with a thin gold film (Figure 9). Light tunnels fleetingly through a thin metal film and can excite a surface plasmon at the outer edge of the metal. The interaction of the incident light wave with the surface plasmon is accompanied by a transfer of energy and leads to a decrease in the intensity of the reflected light wave. Because anchoring occurs only over a narrow range of incidence angles (or wavelengths), surface-plasmon excitation causes a narrow dip in the angular (or wavelength) spectrum of the reflected light. On the basis of these characteristics, the reflected light wave is measured with the SPR sensor equipment [71].
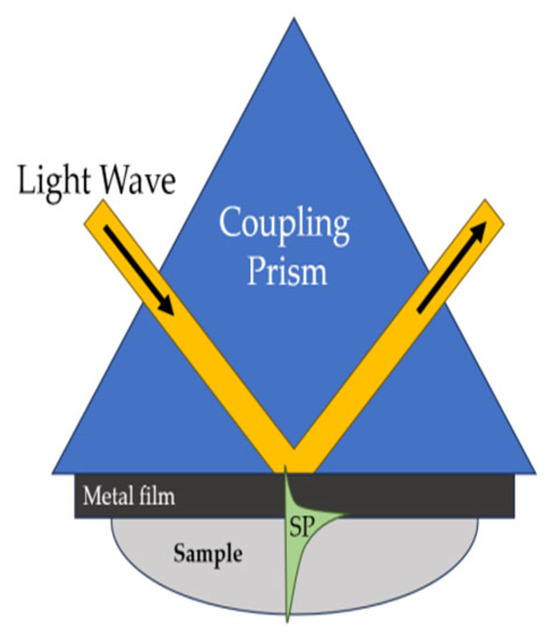
Figure 9.
Surface plasmon excitation in the Kretschmann geometry of the attenuated total reflection method [71], with modification.
In the field of optical or photonic sensor technology, other approaches are based on plasmonic resonator structures [74], optoplasmonics [75,76], met surfaces, strip-waveguide ring resonators, and strip-waveguide resonators [77,78,79], which offer attractive possibilities for bacterial detection.
Photonic crystal plates such as those used in photonic crystal-enhanced microscopy are a special form of structured surfaces used in sensors [80,81,82]. Measurement methods based on some of these structures include surface-enhanced Raman scattering (SERS) [83,84] and surface-enhanced infrared absorption (SEIRA) spectroscopy [85,86,87]. SEIRA allows identification of specific biomolecules or classes of biomolecules by generating specific chemical resonances at mid-infrared wavelengths in organic materials. Photonic crystal-enhanced microscopy typically generates spectrally encoded images, such as color coding of the depth of penetration of light into a cell or local changes in the refractive index of the cell, for which various spectroscopic and spectrometric methods are used [85,86,87].
An obvious alternative to the refractive index approach is to use a fluorescent tag to analyze the bioanalyte of interest. In particular, fluorescent labeling has been used in the “classical” immunosensory competition reaction between antibodies and antigens, in which the presence of specific antigens in the analyte suppresses the fluorescence of identical antigens that have been fluorescently labeled and added to the analyte. For example, Mathias et al. [88] showed that, as compared with a plain glass substrate situation (i.e., glass slide), there is a noticeable (60-fold) increase in fluorescence from the fluorophore cyanine-5 deposited on a streptavidin-conjugated photonic crystal substrate.
The rapid development of SERS in the past 20 years has been facilitated by advances in the targeted synthesis of nanostructured materials and the development of and improvements in Raman spectrometers. SERS offers unprecedented possibilities for lowering the detection limits for relevant analytes and their multiplex determination. SPR biosensor technology has been successfully used to detect various analytes such as proteins, drugs, DNA, and microorganisms [89]. This technology shows promise for the detection of specific pathogens through the use of nanosized materials, which ensure the performance and high selectivity of SPR-based sensors [89].
SPR-based immunosensors coupled with a specific antigen–antibody reaction detect bacteria in a sensitive, specific, rapid, and label-free manner. The integration of new technologies in materials science and molecular biology with the SPR should permit the design of biosensors with detection limits compatible with those offered by traditional methods [24]. In a direct detection format, the analyte molecule in the sample binds to biorecognition elements immobilized on the sensor surface (e.g., antibodies; Figure 10), and the sensor’s response is proportional to the concentration of the captured analyte [71].
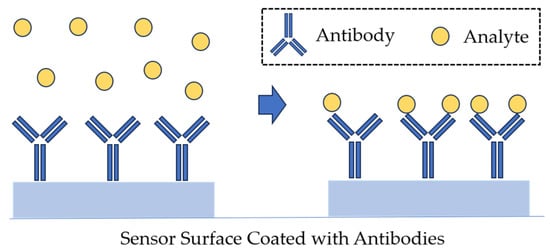
Figure 10.
Direct detection, as used in SPR biosensors.
In the direct detection format, the flow cell of the SPR sensor is initially loaded with a buffer (both sensing and reference channels). As soon as the base line is established, the sample is injected and incubated with the sensor surface for a specified period. The lowest detection limits of the direct detection sensors can be improved using sandwich assay. In such an assay, secondary antibodies bind to the analyte on the sensor surface that has been previously captured by antibodies. For example, the detection limit of an immunosensor for E. coli O157:H7 decreased from 106 to 103 CFU/mL owing to the sandwich assay procedure [90]. Sandwich assays are typically used not only to improve the detection limit beyond what is available in the direct detection format but also to improve specificity and detection. This approach is also suitable for detection in complex matrices [91]. In a sandwich assay, a primary biorecognition element immobilized on the sensor surface captures the analyte from the sample, and a secondary biorecognition element binds to the previously captured analyte [71] (Figure 11).

Figure 11.
Sandwich assay detection, as used in SPR affinity biosensors.
Competitive assays are typically used to detect low concentrations of analytes whose binding to surface-immobilized recognition molecules does not produce a measurable sensor response. In the binding inhibition assay (Figure 12), analyte molecules or their derivatives are fixed to the sensor surface [71].

Figure 12.
Binding inhibition assay detection, as used in SPR affinity biosensors.
A known number of biorecognition elements are incubated and allowed to bind to the analyte in the sample. Then, the mixture is washed off the sensor surface, and unreacted biorecognition elements are fixed. In this case, the sensor response is inversely proportional to the analyte concentration. This approach increases the sensitivity of the assay but also increases the cost and time of analysis owing to the use of secondary antibodies and multistep manipulations.
SPR-based immunosensors have great potential for the rapid detection of microorganisms in food and water samples. However, the detection limits achieved with these sensors cannot compete with those offered by traditional methods. Efforts have been made to increase the sensitivity of immunosensors on the basis of the sandwich assay [91,92,93] and the subtractive inhibition assay (SIA) [94,95].
To develop new biomolecular recognition elements with higher stabilities and higher affinities for target bacteria, researchers have recently integrated phage display with molecular imprinting in SPR biosensors [96]. Bacteriophages are a useful alternative to antibodies in the detection of bacteria. For example, S. aureus was detected with an SPR sensor by using a lytic phage as the recognition element, and the detection limit was 104 CFU/mL [97].
Phage display technology is another tool for developing single-chain antibodies to bacteria. Phage-like antibodies have advantages such as in vitro production, a low cost, a low molecular weight, and high stability [98]. Phage display antibodies have been used to detect Listeria monocytogenes in an SPR biosensor (detection limit, 2 × 106 CFU/mL), which indicates the promise of non-antibody recognition elements [98].
In the past few decades, SPR-based sensors have been used for the simultaneous detection of multiple analytes, but they are limited by the number of analytes. The use of multianalyte sensors will depend on the needs of specific applications. Primary applications of such sensors include pharmaceutical research (high-throughput drug screening systems), medical diagnostics (high-throughput diagnostic tools), food safety (systems for the rapid detection of pathogens and foodborne agents), and security (devices for the early detection and identification of biological warfare and chemical agents) [70].
In SPR-based sensors, molecular imprinting technology is as effective as antibody- or phage-based technology. Idil et al. (2021) described a variation in the SPR-based sensor that uses micro-contact imprinted chips to detect S. aureus within the range 1.0 × 102–2.0 × 105 CFU/mL. The authors showed that this platform is applicable to actual samples (milk) [99]. A few commercial SPR biosensor systems (Biacore, Uppsala, Sweden) for bacterial detection are described in [100].
As already mentioned, SPR-based detection methods are not always highly sensitive. Sensitivity can be increased by introducing magneto-optical SPR (MO–SPR). This method uses the magneto-optical Kerr effect (TMOKE), which results from the use of a magnetic field perpendicular to the plane of propagation of incident p-polarized light [101,102]. When the TMOKE is combined with plasmonic effects, new SPR-based detection configurations emerge, leading to the MO–SPR method (Figure 13). With such a change in the refractive index, the sensitivity of the method can be improved by 10 or even 20 times.

Figure 13.
Principle of operation of MO–SPR sensors.
Chen et al. (2021) used a magneto-optical biochip based on the Cotton–Mouton effect of γ-Fe2O3@Au core/shell magnetic nanoparticles to determine spike glycoprotein S in the diagnosis of SARS-CoV-2. The sensor can be tailored to be used for diagnosing diseases other than SARS-CoV-2. Importantly, the analysis takes 50 min and the detection limit of our method for the spike glycoprotein S of SARS-CoV-2 is estimated to be as low as 0.27 ng/mL (3.4 pM) [103]. The prospects for such sensor systems have been described in [101,102].
4.5. Fiber Optic Sensors
The use of optical fibers as bio- and immunosensors is promising, because they are cheap and offer rapid and easy analysis. Typically, radiation of various wavelengths of the infrared (IR) spectrum is supplied through the fiber. Fiber optic biosensors use the property of the total internal reflection of light as it passes through a waveguide and creates an evanescent wave boundary at the waveguide surface. Antibody- or immunoassay-based fiber optic sensors provide increased sensitivity, selectivity, and speed, as compared with conventional immunoassay methods [104]. The general diagram of such a sensor is shown in Figure 14.
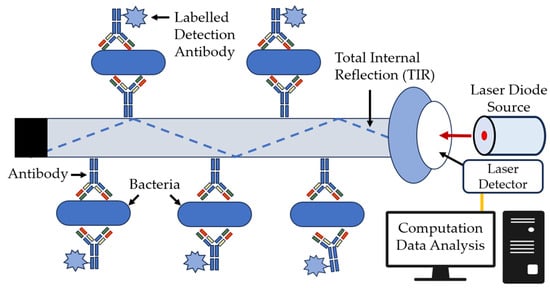
Figure 14.
Bacterial detection with a fiber optic biosensor [104], with modifications.
Optical biosensor fibers can be combined with various spectroscopic methods. Chemi-, bio-, and electroluminescent sensors provide the very sensitive detection of specific substrates. Because many microorganisms have specific IR spectra, fiber IR spectroscopy can be used to detect changes in the properties of biological objects. Immobilization of antibodies inside the fiber ensures specific indication.
Antibody-based fluorescent waveguide biosensors are typically used in the detection of bacteria. First, a specific fluorescently tagged antibody (e.g., Cy-5 or Alexa-Fluor 647) covalently binds to an optical fiber that captures the bacteria of interest, and the detecting antibody specifically binds to the bacteria (Figure 14). Portable sensors such as the Analyte 2000 and RAPTOR from Research International (Monroe, WA, USA) are used widely for such applications [105,106,107,108]. These systems allow the qualitative detection of the target object, and the signal is proportional to the antigen or hapten amount present in the sample [109].
A separate direction in the development of optical fiber sensors is Mach–Zehnder Interferometers (MZIs), which create a picture of interference fringes inside the attenuation bands when bacteria are detected. For example, a sensor system was described that is based on label-free optical fiber made of two gratings of the same type (intergrating space, 1 cm) and immobilized antibodies against E. coli. The detection limit was 7 CFU/mL [110]. Another study presented the potential of sensors based on long-period, label-free fiber arrays for detecting bacteria [111].
Polyelectrolyte functional coating is used for the specific detection of S. aureus. The detection limit for an E. coli sensor was 100 CFU/mL, with MS2 phage as the receptor, and that for a sensor based on peptide aptamers was 10 CFU/mL [111]. A dual-taper microfiber-based MZI biosensor for the detection of S. aureus and the use of porcine immunoglobulin to functionalize the microfiber for the detection of S. aureus bacteria were reported. The maximum wavelength shift of 1.408 nm was achieved when the biosensor was immersed into an S. aureus suspension (cell density, 7 × 101 CFU/mL). The detection limit was as low as 11 CFU/mL [112].
Immunosensors have been developed on the basis of optical fiber to detect the capsular antigen of the plague microbe [113,114,115] and antibodies to it [116].
Fiber optic biosensors are reflected in the RAPTOR™ detection system (Rapid Automatic and Portable Fluorometer Assay System; Research International, USA) [105,117,118]. The RAPTOR™ biosensor allows one to simultaneously detect more than four types of dangerous agents, including Francisella tularensis, ricin, and Staphylococcus enterotoxin [105]. A total of 203 blind samples were tested for staphylococcal enterotoxin B, ricin, Francisella tularensis, and Bacillus globigii. The sensitivities obtained were 10 ng/mL, 50 ng/mL, 5 × 105 CFU/mL, and 5 × 104 CFU/mL, respectively. The advantages of optical biosensors include high sensitivity, a short response time (1–3 min), a non-contact nature, and the low influence of electric noise.
Fiber optic biosensors can detect microorganisms without the use of specific molecules. They are easy to use, small, and cheap, and so they are most promising in the signal detection of biological pathogens.
In recent years, the use of fiber optic sensors for the detection of pathogenic bacteria and their toxins has increased. For example, such sensors have been successfully used to detect as low as 103 CFU/mL of pure-cultured E. coli O157:H7 cells [107]. The Listeria monocytogenes sensitivity threshold was about 4.3 × 103 CFU/mL in [106] and 5 × 105 CFU/mL in [108]. The Salmonella detection limit was ≥105 CFU/100 mL [118], and that for Streptococcus was not given by the authors [119].
Geng et al. (2006) [109] detected E. coli O157:H7 with an initial inoculation of 1 CFU/g ground beef after only 4 h of enrichment. By using RAPTOR™, 5 × 105 CFU/mL of Salmonella typhimurium was detected in sprout rinse water [120]. Geng et al. (2004) reported a fiber optic method for L. monocytogenes detection with a limit of 103–104 CFU/mL in hot dogs and bologna, and this was later confirmed with RAPTOR™ [106].
Combining a fiber optic biosensor with PCR increases the detection sensitivity and strongly improves the detection rate—from the 10 h required for conventional fiber optic sensors to 2 h [104].
Separate mention should be made of an optical fiber sensor based on quantum-dot immunofluorescence for S. aureus detection (detection limit, 103–104 CFU/mL). The sensor combines the specificity of the antigen–antibody interaction with the stability of quantum-dot fluorescence [121]. The application of label-free fiber optic biosensors in bacterial detection has been described in [5,122,123].
It is also necessary to mention optical waveguide light spectroscopy [124] and interferometric methods, for example, those based on the Mach–Zehnder interferometer [125,126], which can detect bacteria or other pathogens in liquid samples. A common feature of these methods is that they use specific adsorption on the waveguide surface and measure the corresponding changes in the intensity of the directional light with photodetectors and carried out quantitative measurements by observing the light scattered by living E. coli located in the vicinity of the waveguide with a detection limit of 102 CFU × mL−1 [127].
Recent advances in nanomaterial-based fiber optic biosensors, with the possibility of their practical application, are presented in the review [127,128,129,130]. Binding nanomaterials to biological receptors improves their sensitivity, detection rate, specificity, and sensor response time, thereby providing many of the performance benefits of fiber optic biosensors. These advantages make fiber optic sensors ideal for commercialization.
4.6. Optical Sensors Based on Ionophores and Planar Biosensors
A special place among the active substances that determine the sensor selectivity is occupied by ionophores. These are neutral or charged substances that selectively bind to the analyte. A traditional example of an ionophore-based optode is a hydrophobic volumetric optical sensor. This is a plasticized poly(vinyl chloride) matrix or another polymer matrix with similar properties that contains lipophilic active components (ionophore, an indicator that changes its optical properties). The analytical signal in optodes is a change in a certain optical property (light absorption, luminescence intensity, etc.) in response to the quantitative content of the analyte in the contacting sample. The optical signal can be recorded even with a color camera, and information about the analyte content in the solution can be obtained using digital image processing. It is also possible to record changes in optical properties with spectral instruments (a spectrophotometer for recording the absorption of visible light, a spectrofluorimeter for recording luminescence, etc.). Although optodes are promising in the making of non-invasive flexible sensor platforms for use in situ, their capabilities have not been sufficiently developed. Optical sensor membranes can be placed on optical fibers or on planar substrates and can also be manufactured in the form of micro- and nanoparticles. Optical sensor platforms based on planar substrates make it possible to integrate sensors selective for different analytes (multianalyte sensor arrays) on a single substrate. Optodes, especially luminescent optodes, are characterized by high-signal amplitudes. This allows them to be used for visualization and sensing to solve practical problems in biology and biotechnology. For example, by immobilizing optodes inside an agarose membrane (a medium for bacterial growth), Wang et al. [131] visualized the acidity of the medium during the life cycle of microorganisms.
Current optode research is active and is a promising and rapidly developing direction of non-destructive analysis [132,133,134]. Sensor arrays of optodes have been described that can be used for in situ analysis. These include well-proven planar arrays of optodes, which make it possible to determine changes in the biofilm oxygen concentration online using the detection of luminescence decay [135,136]. A planar optical waveguide was proposed for gas and humidity sensing by measuring the change in the refractive index of the material in the waveguide cap [137]. This has led to tremendous progress in optical technology. Guided wave sensors are used to measure humidity, heavy metals, and biological objects, including microbial cells [138].
The combination of evanescent field sensing-based measurements and optical phase difference has led to the development of an integrated planar optical waveguide interferometry biosensor. The signal created by interfering fields is detected at the sensor output and is related to the analyte concentration [139]. This biosensor requires a few samples to measure binding interactions, is easy to use, and is cheaper than other optical biosensors. Other studies used a similar sensor with a structured fiber optic coupler to detect S. aureus with a detection limit of 3.1 CFU/mL [140] (Figure 15). Such a biosensor is applicable in the analysis of cellular processes [141]. This method is also very useful for detecting avian influenza virus [142].
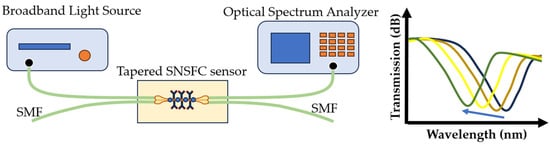
Figure 15.
Experimental setup of the sensor and transmission versus wavelength for S. aureus detection.
Considerable efforts have been made to make biosensors based on the evanescent field interaction. Horváth et al. [123] described a planar grating-coupled optical waveguide sensor that uses evanescent waves for bacterial detection. The waveguide design ensures an increased penetration depth into the sample, making the sensor suitable for detecting micrometer-sized biological objects. Using the sensor, the authors monitored the adhesion of E. coli K12 to the sensor surface.
Of note are biosensors based on evanescent wave fluorescence, in which biological recognition and the manifestation of subsequent binding to the analyte occur within the boundaries determined by fleeting waves. Detection occurs on the basis of excitation of fluorescent molecules near the surface (Figure 16), which helps reduce background signals arising from sample analysis [102,143].

Figure 16.
Scheme for an evanescent-wave planar optical waveguide chip [140], with modification.
An example of a possible practical application of a waveguide-based optical sensor is the miniature PEGASUS device, which can detect toxins, bacterial signatures, viral signatures, and much more in samples such as blood, water, cerebrospinal fluid, and food. PEGASUS does not require trained personnel or laboratory equipment and can easily be used in remote areas of the world. The device can differentiate between bacterial and viral signatures, ensuring the selection of appropriate treatment to improve patient outcomes and reduce the spread of antimicrobial resistance.
Detection occurs in two main steps. First, the sample is processed in a microfluidic device, which requires only a small sample volume, and second, the processed sample is loaded into a miniature detecting sensor. For bacterial infections, it takes the researcher 15–30 min to distinguish between Gram-positive, Gram-negative, and uncertain sources without prior knowledge of the type of infection. To create a sensitive field, a laser at a critical angle of incidence is connected to a planar waveguide, and the total internal reflection of light occurs between the layers of the waveguide owing to their different refractive indices. This causes an evanescent field to be emitted from the wave guide surface, where the fluorescent molecules are detected. The sensor includes a built-in, low-hands sample processing device designed to ensure that every sample is of the quality needed for detection [144].
4.7. Photonic Crystal Biosensors
Of particular interest are biosensor devices using photonic crystals, the operating principles of which can be found in [145]. The spatial periodicity of optical properties is a characteristic feature of photonic crystal structures. The optical waves measured in these sensors are tangible manifestations of Maxwell’s equations, and frequency or wavelength plays a direct role in relation to the characteristics of the medium through which light or, more generally, electromagnetic waves propagate. The transport of information and energy in a photonic crystal is controlled by the total group velocity of Bloch modes at a given frequency of the electromagnetic wave (that is, light at optical frequencies) that impinges on the photonic crystal medium. The speed of the Bloch mode in a periodic medium is determined by coherent multiple (Bragg) scattering on a regular arrangement of photonic “atoms” of a photonic crystal. Threm et al. [146] reviewed the application of various types of photonic crystal structures for use as biosensors. Fan et al. [21] examined optical biosensors that use the principles of cell-free analysis, i.e., changes in the local refractive index associated with the specific binding of the target biomaterial, and not, for example, fluorescent labeling.
Optoelectronics has seen many potential applications of photonic crystals in biosensors [147]. A fundamentally new class of optical biosensor is a sensor based on photonic crystal fibers with a hollow core [28]. The operating principle of photonic crystal waveguides is based on the detection and identification of biological objects by using the spectra of light passing through a hollow core filled with the material under study in the wavelength range 200–1100 nm. Photonic crystal waveguides have channels that can be filled with working solutions. The total working surface area of a photonic crystal fiber is hundreds and thousands of times larger than that of a conventional optical fiber and a plasmon resonance cell. This improves the sensitivity of the method and the speed of the intermolecular interaction. Currently, photonic crystal fibers are considered some of the most promising elements of waveguide optical sensors. Their main advantages include immunity from exposure to electromagnetic fields, high sensitivity and reliability, reproducibility and a wide dynamic range of measurements, the possibility of spectral and spatial multiplexing of sensitive elements, a short response time to changes in the measured value (1 min), a small sample volume, and a small size.
Various modifications, achievements of photonic crystal biosensors and the possibilities of their use for bacterial detection are described in [148,149,150]. Despite the inherent attractiveness of photonic methods, alternative approaches are based on the effect of an electric field on the suspension under study.
5. Optical Sensor Systems Based on Measurement of Orientational Effects
Measuring variations in the optical properties of a suspension during the orientation of anisotropic cells has at least two applications. In the first area, the study of the relaxation part of a signal in the analysis of the orientation of cells under some physical influence and their disorientation can be used directly to determine the cell size. For orientation/disorientation, the start–stop mode of movement of the suspension as a flat film or the start–stop mode of the suspension in a cylindrical layer is used. In either case, this type of fluid movement creates a hydrodynamic effect of Tailor–Quette orientation [151,152]. The orientation and disorientation of cells are described by relaxation curves, in the form of which a rotational diffusion coefficient is manifested, depending on the size and geometry of the cells.
The undeniable advantage of this method for the morphometric validation of biotechnological processes is the absence of need for sample preparation during measurements.
The second area of application of the orientation processes of anisotropic cells is their use as an integral part of electro-optical measurements, which will be discussed below.
5.1. Optical Sensor Systems Based on Measurements of Bacterial Electrical Characteristics
Yet another direction in the development of optical sensors is their use to determine the electrical properties of cellular structures. The probing effect that causes cells to manifest electrophysical properties is an alternating electric field. Under its influence, electrical charges appear at the boundaries of contact between cellular structures. Their magnitude and sign depend on the complex dielectric properties of the adjacent cellular structures.
The electric properties of a sample of any substance are described by the complex dielectric permittivity ἑ:
where σ is the specific conductivity of a substance, ω is the electric field frequency, and ε is the dielectric permittivity.
ἑ = σ + 1/jωε
For example, the magnitude of the induced charges at the boundary between the membrane and the cytoplasm is determined by the function
where εc and εm are the respective dielectric permittivities of the cytoplasm and the membrane, σc and σm are the specific electric conductivities of the cytoplasm and the membrane, and ω is the frequency of the electric field E.
F((εc + σc/jω)/(εm + σm/jω))
The integral effect of the summation of the induced charges will be a superposition of the functions Fi over all contacting cellular structures [153].
Interaction of the induced charges with an electric field gives rise to a torque (an ordered change in cellular orientation and a change in the optical properties of the suspension). Because complex influence of the electric field frequency on the magnitude of the induced charges is present in the F-type functions, the change in the optical properties will have frequency dispersion. This, ultimately, makes it possible to ascertain correspondence between changes in the optical properties of the suspension and some electrophysical variables of cellular structures [154]. Depending on the type of measurement and the electric field frequency, the integral specific conductivity of the cellular sample or its dielectric constant can be determined by using the probing action. Accordingly, these will be the methods of high-frequency conductometry or dielectrometry.
Depending on the spatial structure of the electric field, the electric field vector may be in a stationary or switched position parallel or orthogonal to the direction of the light flux. In the first case, one measures the change in the absorbance of the suspension owing to the transition of cells from random orientation to that in the direction of the field. In the second case, an electrorotational effect of cell rotation occurs [155]. The dielectric model for bacterial cells, the mechanisms of cell polarization under the effect of an electric field, and the capabilities of microfluidic sensor systems for identifying bacteria were described in detail in [156].
5.1.1. Electrorotational Sensors
The essence of electrorotational analysis is that a rotating electric field is created by four electrodes in a square arrangement, fed by four signals of equal amplitude and frequency, with phase shifts of 0°, 90°, 180° and 270°, respectively. The general diagram of electrorotational technology is shown in Figure 17.

Figure 17.
General diagram of electrorotational technology.
Various modifications to the method have been proposed on the basis of both direct microscopic control of electrorotation and light-scattering methods [157]. To unambiguously determine the rotation of bacterial cells with direct microscopic control, one needs a microscope with high resolution and a small working distance. Therefore, an extremely thin polypropylene spacer with a height of 0.2 mm is installed on the electrodes and the glass substrate. The installation of a microscope on an electrorotational device was described in [158], and the theoretical and experimental significance of the electrorotational technique was described in [153,156,157,158,159]. Electrorotation makes it possible to accurately determine changes in the cytoplasm and membranes [159,160,161,162]. Dielectrophoresis and electrorotation have been developed for the analysis of individual cells [156,158,162,163,164]. However, electrorotation has serious disadvantages, including that measurements are made on single cells and that reliable results can be produced only after a large number of parallel experiments.
5.1.2. Electro-Optical Sensors with a Fixed Direction of the Electric Field Vector
Electro-optical analysis, based on the study of cells as electrophysical objects with a layered structure and on the measurement of the polarization characteristics of cellular structures, is a new approach to assessing the vital physiological characteristics of cells and their heterogeneity. The method is based on using particle polarizability in an electric field and measuring the optical manifestation of the polarizability results.
The electric analysis of cells, supplemented with optical recording, allows selective analysis of the electric conductivity of the cell cytoplasm and other variables of cellular structures. Different versions of electro-optical analysis are based on a chain of the following physical processes. The probing electric field gives rise to induced charges (polarization) at the cytoplasm–membrane–cell wall–external low-conducting medium interfaces. The interaction of these charges with the field that generated them leads to partial orientation of the cells. The change in orientation is recorded optically by the change in light scattering, optical turbidity, or light birefringence.
The cell polarizability tensor can be calculated for a cell structure by using electrodynamic equations. The electro-optical signal is defined, up to constants, as the difference between the longitudinal and transverse components of this tensor [165,166].
Actually, the electrophysical model for the cell, connecting the electrophysical properties and morphometry of cellular structures with the cell polarizability tensor, provides a way to solve inverse problems of restoring the required variable from the measured frequency dispersion of the electro-optical signal.
In most cases, it is not the absolute value of the variable that is of interest but its change during cell growth or various physicochemical effects.
The low-frequency portion of the frequency dispersion of the electro-optical signal is associated with changes in the surface properties of cells (Figure 18). It is used to detect changes in cells after exposure to monoclonal, polyclonal, and phage antibodies in the range of cell densities being detected 108–104 cells mL−1 [167,168,169].
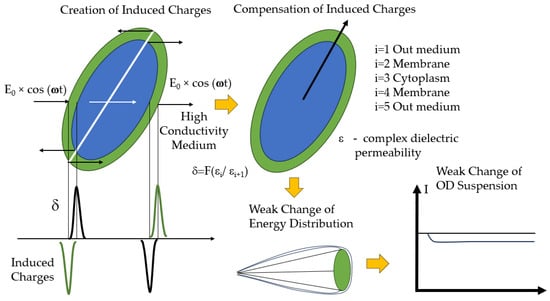
Figure 18.
Main mechanisms of electro-optical analysis.
The frequency dispersion and absolute value of the signal before any effect serves as a standard for evaluating changes in the state of the cell surface.
The frequency dispersion of the electro-optical signal in the frequency range of approximately 50 to 800 kHz directly reflects the specific value of the cell cytoplasm. The high-frequency portion of the frequency dispersion of the signal makes it possible to determine the specific electrical conductivity of the cell membrane and the efficiency of transport processes when cell growth is monitored [170].
As in the case of hydrodynamic particle orientation, the electro-optical signal of cell disorientation after termination of the electric field represents a relaxation curve. Its shape can be used to determine the distribution of cell sizes in a population. But in batch cultivation, as established experimentally, it is sufficient to determine the average cell size. The shape of the size distribution remains unchanged, but the sizes of all age cell groups change synchronously.
In a certain sense, electro-optics complements and expands the capabilities of the optical method for analyzing cellular structures with different refractive indices. From polarizability theory, it follows that the value of the refractive index for a substance in the optical range corresponds to the asymptotic behavior of the real part of the value of the complex dielectric constant. Electrophysical analysis makes it possible to additionally analyze the imaginary part of the complex dielectric permittivity, called the electric conductivity of the medium, and to use the functional dependence of the change in these variables on the electric field frequency.
The main disadvantage of electro-optical measurements is the sample preparation process. Cells must be transferred to a medium with a specific electrical conductivity below 10–15 mS cm−1. Only in this case do the induced charges at the cell surface–membrane–cytoplasm interface remain unsuppressed by the high induced charge of another sign at the interface between the external environment and the cell surface.
6. Conclusions
Biosensors are a good alternative to classical techniques. According to Markets and Markets, the biosensor market was valued at USD 25.5 billion in 2021 and is forecast to reach USD 36.7 billion by 2026 [171,172]. Recently, there has been a growing trend towards the use of smartphone-based optical bacterial analysis systems (smartphone-based photo- and spectrometers and smartphone-based fluorimeters [66,173]. The use of optical biosensors may strongly increase the possibility of early pathogen detection and, as a result, may facilitate disease screening. Of the available detection methods, optical biosensors offer many advantages, especially in terms of increased analytical speed. Optical biosensors instantaneously provide interactive information through real-time analysis.
Developing a biosensor with the necessary properties for reliable and efficient use in everyday applications is an important task for researchers, because in bacterial detection, it is difficult to meet standards. Fluorescence and SERS sensors are the ones developed most actively for the design of sensor systems for the sensitive, selective and reproducible detection of bacteria and their metabolites as markers for diagnosing a wide range of diseases [174].
This review has described different types of optical biosensors and their application for bacterial detection. The biosensors are diverse, as each one is designed according to the needs and objectives of a specific application. It should be noted that each method presented has advantages and disadvantages, which are summarized in Table 1.

Table 1.
Main advantages and disadvantages of optical sensor systems for bacterial detection.
An important direction in current research on optical sensor systems is to make electro-optical platforms based on the effect of an electric field on a cell suspension, without changing the vital biochemical and physiological characteristics of cells.
Recent advances allow optical biosensors to be considered promising diagnostic tools that combine the speed of detection of specific molecular markers, simplicity, user-friendliness, efficiency, accuracy, and cheapness with the tendency to make portable platforms. These qualities exceed the generally accepted standards for microbiological and immunological diagnostics and open up broad prospects for the use of these analytical systems in clinical practice directly at the point of care. In the design of new biosensors for practical applications, properties such as reliability, reproducibility, simplicity, and shelf life should be considered. Further standardization and automation of optical sensor systems will expand the range of their application in microbiology, biotechnology, veterinary medicine, medicine, and environmental protection.
Author Contributions
Conceptualization and design: O.I.G.; data curation and methodology O.I.G., A.V.S., S.A.E., O.A.K. and V.D.B.; formal analysis, O.I.G., S.A.E., V.D.B.; project administration, O.I.G.; resources, O.I.G., A.V.S., V.D.B. and O.A.K.; software O.I.G. and V.D.B.; visualization, A.V.S.; writing—original draft, O.I.G. and V.D.B.; writing—review and editing, O.I.G., O.A.K., V.D.B., A.V.S. and S.A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ministry of Science and Higher Education of the Russian Federation as a state assignment for the Saratov Scientific Centre of the Russian Academy of Sciences, Kotelnikov Institute of Radio Engineering and Electronics, Russian Academy of Sciences, and Department of Chemistry, M. V. Lomonosov Moscow State University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author Viktor D. Bunin was employed by the company EloSystems GbR. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Dudak, F.C.; Boyacı, I.H. Rapid and label-free bacteria detection by surface plasmon resonance (SPR) biosensors. Biotechnol. J. 2009, 4, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Zourob, M.; Elwary, S.; Turner, A. (Eds.) Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Springer Science + Business Media, LLC: Berlin, Germany, 2008. [Google Scholar]
- Wang, P.; Sun, H.; Yang, W.; Fang, Y. Optical methods for label-free detection of bacteria. Biosensors 2022, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Kaura, B.; Kumar, S.; Kaushik, B.K. Trends, challenges, and advances in optical sensing for pathogenic bacteria detection (PathoBactD). Biosens. Bioelectron. X 2023, 14, 100352. [Google Scholar] [CrossRef]
- Manafi, M. Fluorogenic and chromogenic enzyme substrates in culture media and identification tests. Int. J. Food Microbiol. 1996, 31, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Rantsiou, K.; Alessandria, V.; Urso, R.; Dolci, P.; Cocolin, L. Detection, quantification, and vitality of Listeria monocytogenes in food as determined by quantitative PCR. Int. J. Food Microbiol. 2008, 121, 99–105. [Google Scholar] [CrossRef]
- Hobson, N.S.; Tothill, I.; Turner, A.P.F. Microbial detection. Biosens. Bioelectron. 1996, 11, 455–477. [Google Scholar] [CrossRef]
- Docherty, L.; Adams, M.R.; Patel, P.; McFadden, J. The magnetic immunopolymerase chain reaction assay for the detection of Camplyobacter in milk and poultry. Lett. Appl. Microbiol. 1996, 37, 288–292. [Google Scholar] [CrossRef]
- Yu, L.S.L.; Reed, S.A.; Golden, M.H. Time-resolved fluorescence immunoassay (TRFIA) for the detection of Escherichia coli O157:H7 in apple cider. J. Microbiol. Meth. 2002, 49, 63–68. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y. Detection of Escherichia coli O157:H7 using immunomagnetic separation and absorbance measurement. J. Microbiol. Meth. 2002, 51, 369–377. [Google Scholar] [CrossRef]
- Su, X.L.; Li, Y. Quantum dot biolabeling coupled with immunomagnetic separation for detection of Escherichia coli O157:H7. Anal. Chem. 2004, 76, 4806–4810. [Google Scholar] [CrossRef] [PubMed]
- Dudak, F.C.; Boyacı, I.H. Enumeration of immunomagnetically captured Escherichia coli in water samples using quantum dot-labeled antibodies. J. Rapid Methods Autom. Microbiol. 2008, 16, 122–131. [Google Scholar] [CrossRef]
- Lazcka, O.; Del Campo, F.J.; Munoz, F.X. Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.V.; Cordeiro, T.A.R.; e Freitas, G.R.O.; Ferreira, L.F.; Franco, D.L. Biosensors for the detection of respiratory viruses: A review. Talanta Open 2020, 2, 100007. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dkhar, D.S.; Chandra, P.; Azad, U.P. Nanobiosensors design using 2D materials: Implementation in infectious and fatal disease diagnosis. Biosensors 2023, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Purohit, B.; Vernekar, P.R.; Shetti, N.P.; Chandra, P. Biosensor nanoengineering: Design, operation, and implementation for biomolecular analysis. Sens. Int. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Liu, X.; Marrakchi, M.; Xu, D.; Dong, H.; Andreescu, S. Biosensors based on modularly designed synthetic peptides for recognition, detection and live/dead differentiation of pathogenic bacteria. Biosens. Bioelectron. 2016, 80, 9–16. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Hu, Z.; Yu, G.; Yang, D.; Zhao, J. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131–140. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef]
- Wu, X.; Xu, C.; Tripp, R.A.; Huang, Y.W.; Zhao, Y. Detection and differentiation of foodborne pathogenic bacteria in mung bean sprouts using field deployable label-free SERS devices. Analyst 2013, 138, 3005–3012. [Google Scholar] [CrossRef] [PubMed]
- Marks, H.; Schechinger, M.; Garza, J.; Locke, A.; Coté, G. Surface enhanced Raman spectroscopy (SERS) for in vitro diagnostic testing at the point of care. Nanophotonics 2017, 6, 681–701. [Google Scholar] [CrossRef]
- Kahraman, M.; Mullen, E.R.; Korkmaz, A.; Wachsmann-Hogiu, S. Fundamentals and applications of SERS-based bioanalytical sensing. Nanophotonics 2017, 6, 831–852. [Google Scholar] [CrossRef]
- Wu, R.; Ma, Y.; Pan, J.; Lee, S.H.; Liu, J.; Zhu, H.; Gu, R.; Shea, K.J.; Pan, G. Efficient capture, rapid killing and ultrasensitive detection of bacteria by a nano-decorated multi-functional electrode sensor. Biosens. Bioelectron. 2018, 101, 52–59. [Google Scholar] [CrossRef]
- Kimuda, S.G.; Biraro, I.A.; Bagaya, B.S.; Raynes, J.G.; Cose, S. Characterising antibody avidity in individuals of varied Mycobacterium tuberculosis infection status using surface plasmon resonance. PLoS ONE 2018, 13, e0205102. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.X.; Sun, Y.; Li, X.; Wang, L.; Xu, Y.; He, L.L.; Li, G.L. Recent advances in dual recognition based surface enhanced Raman scattering for pathogenic bacteria detection: A review. Anal. Chim. Acta 2021, 1157, 338279. [Google Scholar] [CrossRef] [PubMed]
- Andryukov, B.G.; Lyapun, I.N.; Matosova, E.V.; Somova, L.M. Biosensor technologies in medicine: From detection of biochemical markers to research into molecular targets (Review). Mod. Technol. Med. 2020, 12, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Qi, P.; Zhang, D. A simple, rapid and cost-effective colorimetric assay based on the 4-mercaptophenylboronic acid functionalized silver nanoparticles for bacteria monitoring. Sens. Actuators B Chem 2018, 260, 983–989. [Google Scholar] [CrossRef]
- Hong, B.; Li, Y.; Wang, W.; Ma, Y.; Wang, J. Separation and colorimetric detection of Escherichia coli by phage tail fiber protein combined with nano-magnetic beads. Mikrochim. Acta 2023, 190, 202. [Google Scholar] [CrossRef]
- You, S.M.; Jeong, K.B.; Luo, K.; Park, J.S.; Park, J.W.; Kim, Y.R. Paper-based colorimetric detection of pathogenic bacteria in food through magnetic separation and enzyme-mediated signal amplification on paper disc. Anal. Chim. Acta 2021, 1151, 338252. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence Principles and Applications; Wiley: Weinheim, Germany, 2012; ISBN 9783527328376. [Google Scholar] [CrossRef]
- Fluorescence in Biological Research. Available online: https://ru.wikipedia.org/wiki/%D0%A4%D0%BB%D1%83%D0%BE%D1%80%D0%B5%D1%81%D1%86%D0%B5%D0%BD%D1%86%D0%B8%D1%8F_%D0%B2_%D0%B1%D0%B8%D0%BE%D0%BB%D0%BE%D0%B3%D0%B8%D1%87%D0%B5%D1%81%D0%BA%D0%B8%D1%85_%D0%B8%D1%81%D1%81%D0%BB%D0%B5%D0%B4%D0%BE%D0%B2%D0%B0%D0%BD%D0%B8%D1%8F%D1%85 (accessed on 19 November 2023).
- Chen, L.Y.; Hwang, E.; Zhang, J. Fluorescent nanobiosensors for sensing glucose. Sensors 2018, 18, 1440. [Google Scholar] [CrossRef]
- Fernando, K.A.S.; Sahu, S.; Liu, Y.M.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Infer. 2015, 7, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Lv, F.; Qi, R.; Senthilkumar, T.; Zhao, H.; Chen, Y.; Li, L.; Wang, S. Förster resonance energy transfer mediated rapid and synergistic discrimination of bacteria over fungi using a cationic conjugated glycopolymer. ACS Appl. Bio Mater. 2020, 3, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Zhang, J.; Liu, J.L.; Zhang, Y. Recent progress of rare-earth doped upconversion nanoparticles: Synthesis, optimization, and applications. Adv. Sci. 2019, 6, 1901358. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Li, X.; Fan, Y.; Li, J.; Lu, K.; Wen, H.; Ren, J. Recent advances of fluorescent sensors for bacteria detection-A review. Talanta 2023, 254, 124133. [Google Scholar] [CrossRef] [PubMed]
- Kourti, D.; Angelopoulou, M.; Petrou, P.; Kakabakos, S. Optical immunosensors for bacteria detection in food matrices. Chemosensors 2023, 11, 430. [Google Scholar] [CrossRef]
- Pligovka, A.; Lazavenka, A.; Turavets, U.; Hoha, A.; Salerno, M. Two-level 3D column-like nanofilms with hexagonally–packed tantalum fabricated via anodizing of Al/Nb and Al/Ta layers—A potential nano-optical biosensor. Materials 2023, 16, 993. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Liang, L.; Ye, F.; Zhao, S. An integrated platform for label-free fluorescence detection and inactivation of bacteria based on boric acid functionalized Zr-MOF. Sens. Actuators B Chem. 2021, 345, 130345. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Zhang, L.; Liu, S.; Zhang, M.; Wang, J.; Ning, B.; Peng, Y.; He, J.; Hu, Y.; et al. CRISPR-Cas9 triggered two-step isothermal amplification method for E. coli O157:H7 detection based on a metal–organic framework platform. Anal. Chem. 2020, 92, 3032–3041. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, M.; Singh, S.; Deep, A.; Sharma, A.L. Highly sensitive optical detection of Escherichia coli using terbium-based metal–organic framework. ACS Appl. Mater. Interfaces 2020, 12, 48198–48205. [Google Scholar] [CrossRef]
- Jenie, S.N.A.; Kusumastuti, Y.; Krismastuti, F.S.H.; Untoro, Y.M.; Dewi, R.T.; Udin, L.Z.; Artanti, N. Rapid fluorescence quenching detection of Escherichia coli using natural silica-based nanoparticles. Sensors 2021, 21, 881. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yang, X.; Zhang, B.; Cheng, S.; Han, H.; Jina, Q.; Wang, C.; Xiao, R. Sensitive detection of Escherichia coli O157:H7 and Salmonella typhimurium in food samples using two-channel fluorescence lateral flow assay with liquid Si@quantum dot. Food Chem. 2021, 363, 130400. [Google Scholar] [CrossRef]
- Nandi, S.; Ritenberga, M.; Jelinek, R. Bacterial detection with amphiphilic carbon dots. Analyst 2015, 140, 4232. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.A.; Clapper, J.C. Conjugation of carboxylated graphene quantum dots with cecropin P1 for bacterial biosensing applications. ACS Omega 2020, 5, 26583–26591. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Yang, D.; Yin, X. Rapid detection of three common bacteria based on fluorescence spectroscopy. Sensors 2022, 22, 1168. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zheng, L.; Wang, S.; Huang, F.; Liu, Y.; Jiang, H.; Lin, J. A microfluidic biosensor for rapid and automatic detection of Salmonella using metal-organic framework and Raspberry Pi. Biosens. Bioelectron. 2021, 178, 113020. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Huang, X.; Li, Z.; Shi, J.; Zhai, X.; Hu, X.; Nini, L.; Zou, X. Rapid and highly sensitive detection of Salmonella typhimurium in lettuce by using magnetic fluorescent nanoparticles. Anal. Methods 2020, 12, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Qiao, Y.; Jiang, N.; Li, C.; Zhu, X.; Li, W.; Cai, Q. Water-soluble ZnCuInSe quantum dots for bacterial classification, detection, and imaging. Anal. Bioanal. Chem. 2020, 412, 8379–8389. [Google Scholar] [CrossRef]
- Sur, V.P.; Mazumdar, A.; Ashrafi, A.; Mukherjee, A.; Milosavljevic, V.; Michalkova, H.; Kopel, P.; Richtera, L.; Moulick, A. A novel biocompatible titanium–gadolinium quantum dot as a bacterial detecting agent with high antibacterial activity. Nanomaterials 2020, 10, 778. [Google Scholar] [CrossRef]
- Ouyang, Q.; Yang, Y.; Ali, S.; Wang, L.; Li, H.; Chen, Q. Upconversion nanoparticles-based FRET system for sensitive detection of Staphylococcus aureus. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 255, 119734. [Google Scholar] [CrossRef]
- Nißler, R.; Bader, O.; Dohmen, M.; Walter, S.G.; Noll, C.; Selvaggio, G.; Groß, U.; Kruss, S. Remote near infrared identification of pathogens with multiplexed nanosensors. Nat. Commun. 2020, 11, 5995. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhong, Y.; Li, W.; Wang, W.; Li, C.; Wang, A.; Yan, H.; Wan, Y.; Li, J. Fluorescent and opt-electric recording bacterial identification device for ultrasensitive and specific detection of microbials. ACS Sens. 2021, 6, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.A.; Park, S.Y.; Cha, Y.; Gopala, L.; Lee, M.H. Strategies of detecting bacteria using fluorescence-based dyes. Front. Chem. 2021, 9, 743923. [Google Scholar] [CrossRef] [PubMed]
- Kabiraz, M.P.; Majumdar, P.R.; Mahmud, M.M.C.; Bhowmik, S.; Ali, A. Conventional and advanced detection techniques of foodborne pathogens: A comprehensive review. Heliyon 2023, 9, e15482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, S.; De Ruyck, K.; Beloglazova, N.; Eremin, S.A.; De Saeger, S.; Zhang, S.; Shen, J.; Wang, Z. Fluorescence polarization assays for chemical contaminants in food and environmental analyses. Trends Anal. Chem. 2019, 114, 293–313. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Taranova, N.A.; Zherdev, A.V.; Dzantiev, B.B.; Eremin, S.A. Fluorescence polarization-based bioassays: New horizons. Sensors 2020, 20, 7132. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Narang, D.; Kaur, P.; Chandra, M.; Filia, G.; Singh, S.T. A fluorescence polarization assay using recombinant protein ESAT-6 for the detection of antibodies against pathogenic. Mycobacterium bovis in bovine. Iran. J. Vet. Res. Shiraz Univ. 2022, 23, 204–209. [Google Scholar] [CrossRef]
- Sotolongo-Rodríguez, D.; Gomez-Flores, R.; Navarro-Soto, M.C.; Arellano-Reynoso, B.; Tamez-Guerra, P.; Ramírez-Pfeiffer, C. Evaluation of the fluorescence polarization assay for the diagnosis of brucellosis in goat milk. Vet. Sci. 2022, 9, 303. [Google Scholar] [CrossRef]
- Sentry 300. The Most Accurate and Advanced Fluorescence Polarization Reader. Available online: https://ellielab.com/sentry-300/ (accessed on 19 November 2023).
- Roda, A.; Mirasoli, M.; Michelini, E.; Di Fusco, M.; Zangheri, M.; Cevenini, L.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens. Bioelectron. 2016, 76, 164–179. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Lopreside, A.; Montali, L.; Zangheri, M.; Evangelisti, L.; D’Elia, M.; Michelini, E. Portable light detectors for bioluminescence biosensing applications: A comprehensive review from the analytical chemist’s perspective. Anal. Chim. Acta 2022, 1200, 339583. [Google Scholar] [CrossRef]
- Anderson, W.A.; Dippel, A.B.; Maiden, M.M.; Waters, C.M.; Hammond, M.C. Chemiluminescent sensors for quantitation of the bacterial second messenger cyclic di-GMP. Methods Enzymol. 2020, 640, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Pour, S.R.S.; Calabria, D.; Emamiamin, A.; Lazzarini, E.; Pace, A.; Guardigli, M.; Zangheri, M.; Mirasoli, M. Electrochemical vs. optical biosensors for point-of-care applications: A critical Review. Chemosensors 2023, 11, 546. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, T.; Huang, R. Recent advances in electrochemiluminescence sensors for pathogenic bacteria detection. Micromachines 2019, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Deisingh, A.K.; Thompson, M. Biosensors for the detection of bacteria. Can. J. Microbiol. 2004, 50, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Utkin, D.V.; Ossina, N.A.; Kouklev, V.E.; Erokhin, P.S.; Scherbakova, S.A.; Kutyrev, V.V. Biosensors: Current state and prospects of applying in laboratory diagnostics of particularly dangerous infectious diseases. Probl. Part. Danger. Infect. 2009, 102, 11–14. [Google Scholar] [CrossRef]
- Homola, J.; Vaisocherová, H.; Dostálek, J.; Piliarik, M. Multi-analyte surface plasmon resonance biosensing. Methods 2005, 37, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Piliarik, M.; Vaisocherová, H.; Homola, J. Surface plasmon resonance biosensing. In Methods in Molecular Biology: Biosensors and Biodetection; Rasooly, A., Herold, K.E., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 503, pp. 65–88. [Google Scholar] [CrossRef]
- Homola, J. (Ed.) Surface Plasmon Resonance Based Sensors; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Raether, H. Surface-Plasmons on Smooth and Rough Surfaces and on Gratings; Springer Tracts in Modern Physics; Springer: Berlin/Heidelberg, Germany, 1988; Volume 111, pp. 1–133. [Google Scholar]
- Lahiri, B.; Khokhar, A.Z.; De La Rue, R.M.; McMeekin, S.G.; Johnson, N.P. Asymmetric split ring resonators for optical sensing of organic materials. Opt. Express 2009, 17, 1107. [Google Scholar] [CrossRef]
- De Angelis, F.; Patrini, M.; Das, G.; Maksymov, I.; Galli, M.; Businaro, L.; Andreani, L.C.; Di Fabrizio, E. A hybrid plasmonic–photonic nanodevice for label-free detection of a few molecules. Nano Lett. 2008, 8, 2321–2327. [Google Scholar] [CrossRef]
- Xavier, J.; Vincent, S.; Meder, F.; Vollmer, F. Advances in optoplasmonic sensors—Combining optical nano/microcavities and photonic crystals with plasmonic nanostructures and nanoparticles. Nanophotonics 2018, 7, 1–38. [Google Scholar] [CrossRef]
- Yalçin, A.; Popat, K.C.; Aldridge, J.C.; Desai, T.A.; Hryniewicz, J.; Chbouki, N.; Little, B.E.; King, O.; Van, V.; Chu, S.; et al. Optical sensing of biomolecules using microring resonators. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 148–154. [Google Scholar] [CrossRef]
- Xu, D.X.; Densmore, A.; Delâge, A.; Waldron, P.; McKinnon, R.; Janz, S.; Lapointe, J.; Lopinski, G.; Mischki, T.; Post, E.; et al. Folded cavity SOI microring sensors for high sensitivity and real time measurement of biomolecular binding. Opt. Express 2008, 16, 15137–15148. [Google Scholar] [CrossRef]
- Densmore, A.; Vachon, M.; Xu, D.-X.; Janz, S.; Ma, R.; Li, Y.-H.; Lopinski, G.; Delâge, A.; Lapointe, J.; Luebbert, C.C.; et al. Silicon photonic wire biosensor array for multiplexed real-time and label-free molecular detection. Opt. Lett. 2009, 34, 3598–3600. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Long, K.D.; Lu, M.; Chaudhery, V.; Yu, H.; Choi, J.S.; Polans, J.; Zhuo, Y.; Harley, B.A.C.; Cunningham, B.T. Photonic crystal enhanced microscopy for imaging of live cell adhesion. Analyst 2013, 138, 5886–5894. [Google Scholar] [CrossRef]
- Triggs, G.J.; Fischer, M.; Stellinga, D.; Scullion, M.G.; Evans, G.J.O.; Krauss, T.F. Spatial resolution and refractive index contrast of resonant photonic crystal surfaces for biosensing. IEEE Photonics J. 2015, 7, 6801810. [Google Scholar] [CrossRef] [PubMed]
- Nazirizadeh, Y.; Reverey, J.; Geyer, U.; Lemmer, U.; Selhuber-Unkel, C.; Gerken, M. Material-based three-dimensional imaging with nanostructured surfaces. Appl. Phys. Lett. 2013, 102, 011116. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Choi, C.J.; Cunningham, B.T. Plasmonic nanogap-enhanced Raman scattering using a resonant nanodome array. Small 2012, 8, 2878–2885. [Google Scholar] [CrossRef]
- Yazdi, S.H.; White, I.M. A nanoporous optofluidic microsystem for highly sensitive and repeatable surface enhanced Raman spectroscopy detection. Biomicrofluidics 2012, 6, 14105–141059. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Le, F.; Nordlander, P.; Halas, N.J. Surface enhanced infrared absorption (SEIRA) spectroscopy on nanoshell aggregate substrates. Chem. Phys. Lett. 2008, 452, 115–119. [Google Scholar] [CrossRef]
- Bukasov, R.; Shumaker-Parry, J.S. Silver nanocrescents with infrared plasmonic properties as tunable substrates for surface enhanced infrared absorption spectroscopy. Anal. Chem. 2009, 81, 4531–4535. [Google Scholar] [CrossRef]
- Tittl, A.; Leitis, A.; Liu, M.; Yesilkoy, F.; Choi, D.-Y.; Neshev, D.N.; Kivshar, Y.S.; Altug, H. Imaging-based molecular barcoding with pixelated dielectric metasurfaces. Science 2018, 360, 1105–1109. [Google Scholar] [CrossRef]
- Mathias, P.C.; Wu, H.-Y.; Cunningham, B.T. Employing two distinct photonic crystal resonances to improve fluorescence enhancement. Appl. Phys. Lett. 2009, 95, 021111. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Cho, Y.-W.; Kim, T.-H. Recent advances in surface plasmon resonance sensors for sensitive optical detection of pathogens. Biosensors 2022, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Irudayaraj, J.; Ryan, T. A mixed self-assembled monolayer-based surface plasmon immunosensor for detection of E. coli O157:H7. Biosens. Bioelectron. 2006, 21, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Dostálek, J.; Chen, S.F.; Rasooly, A.; Jiang, S.Y.; and Yee, S.S. Spectral surface plasmon resonance biosensor for detection of staphylococcal enterotoxin B in milk. Int. J. Food Microbiol. 2002, 75, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Nomura, Y.; Zhang, W.; Sakino, M.; Lee, K.H.; Ikebukuro, K.; Karube, I. Simple and rapid detection method using surface plasmon resonance for dioxins, polychlorinated biphenylx and atrazine. Anal. Chim. Acta 2001, 434, 223–230. [Google Scholar] [CrossRef]
- Mazumdar, S.D.; Hartmann, M.; Kampfer, P.; Keusgen, M. Rapid method for detection of Salmonella in milk by surface plasmon resonance (SPR). Biosens. Bioelectron. 2007, 22, 2040–2046. [Google Scholar] [CrossRef]
- Skottrup, P.; Nicolaisen, M.; Justesen, A.F. Rapid determination of Phytophthora infestans sporangia using a surface plasmon resonance immunosensor. J. Microbiol. Meth. 2007, 68, 507–515. [Google Scholar] [CrossRef]
- Skottrup, P.; Hearty, S.; Frøkiaer, H.; Leonard, P.; Hejgaard, J.; O’Kennedy, R.; Nicolaisen, M.; Justesen, A.F. Detection of fungal spores using a generic surface plasmon resonance immunoassay. Biosens. Bioelectron. 2007, 22, 2724–2729. [Google Scholar] [CrossRef]
- Handa, H.; Gurczynski, S.; Jackson, M.P.; Auner, G.; Walker, J.; Mao, G. Recognition of Salmonella typhimurium by immobilized phage P22 monolayers. Surf. Sci. 2008, 602, 1392–1400. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Sorokulova, I.B.; Vodyanoy, V.J.; Simonian, A.L. Lytic phage as a specific and selective probe for detection of Staphylococcus aureus—A surface plasmon resonance spectroscopic study. Biosens. Bioelectron. 2007, 22, 948–955. [Google Scholar] [CrossRef]
- Nanduri, V.; Bhunia, A.K.; Tu, S.I.; Paoli, G.C.; Brewster, J.D. SPR biosensor for the detection of L. monocytogenes using phage-displayed antibody. Biosens. Bioelectron. 2007, 23, 248–252. [Google Scholar] [CrossRef]
- Idil, N.; Bakhshpour, M.; Perçin, I.; Mattiasson, B. Whole cell recognition of Staphylococcus aureus using biomimetic SPR sensors. Biosensors 2021, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Polonschii, C.; Luculescu, C.; Gheorghiu, M.; Gáspár, S.; Gheorghiu, E. Magneto-plasmonic biosensor with enhanced analytical response and stability. Biosens. Bioelectron. 2015, 63, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent advances in optical biosensors for sensing applications: A review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Chen, K.-L.; Yang, Z.-Y.; and Lin, C.-W. A magneto-optical biochip for rapid assay based on the Cotton–Mouton effect of γ-Fe2O3@ Au core/shell nanoparticles. J. Nanobiotechnol. 2021, 19, 301. [Google Scholar] [CrossRef] [PubMed]
- Banada, P.P.; Bhunia, A.K. Antibodies and immunoassays for detection of bacterial pathogens. In Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M., Elwary, S., Turner, A., Eds.; Springer Science + Business Media, LLC: Berlin, Germany, 2008; Chapter 21. [Google Scholar]
- Anderson, G.P.; King, K.D.; Gaffney, K.L.; Johnson, L.H. Multi-analyte interrogation using the fiber optic biosensor. Biosens. Bioelectron. 2000, 14, 771–777. [Google Scholar] [CrossRef]
- Geng, T.; Morgan, M.T.; Bhunia, A.K. Detection of low levels of Listeria monocytogenes cells by using a fiber-optic immunosensor. Appl. Environ. Microbiol. 2004, 70, 6138–6146. [Google Scholar] [CrossRef]
- Geng, T.; Uknalis, J.; Tu, S.I.; Bhunia, A.K. Fiber-optic biosensor employing Alexa-fluor conjugated antibody for detection of Escherichia coli O157: H7 from ground beef in four hours. Sensors 2006, 6, 796–807. [Google Scholar] [CrossRef]
- Nanduri, V.; Kim, G.; Morgan, M.T.; Ess, D.; Hahm, B.K.; Kothapalli, A.; Valadez, A.; Geng, T.; Bhunia, A.K. Antibody immobilization on waveguides using a flow-through system shows improved Listeria monocytogenes detection in an automated fiber optic biosensor: RAPTOR™. Sensors 2006, 6, 808–822. [Google Scholar] [CrossRef]
- Taitt, C.R.; Anderson, G.P.; Ligler, S. Evanescent wave fluorescence biosensors. Biosens. Bioelectron. 2005, 20, 2470–2487. [Google Scholar] [CrossRef]
- Kaushik, S.; Tiwari, U.; Nilima, P.S.; Das, B.; Sinha, R.K. Label-free detection of Escherichia coli bacteria by cascaded chirped long period gratings immunosensor. Rev. Sci. Instrum. 2019, 90, 025003. [Google Scholar] [CrossRef]
- Janik, M.; Brzozowska, E.; Czyszczoń, P.; Celebańska, A.; Koba, M.; Gamian, A.; Bock, W.J.; Śmietana, M. Optical fiber aptasensor for label-free bacteria detection in small volumes. Sens. Actuator. B Chem. 2021, 330, 129316. [Google Scholar] [CrossRef]
- Li, H.C.; Leng, Y.K.; Liao, Y.C.; Liu, B.; Luo, W.; Liu, J.; Shi, J.L.; Yuan, J.; Xu, H.Y.; Xiong, Y.H.; et al. Tapered microfiber MZI biosensor for highly sensitive detection of Staphylococcus aureus. IEEE Sens. J. 2022, 22, 5531–5539. [Google Scholar] [CrossRef]
- Anderson, G.P.; Breslin, K.A.; Ligler, F.S. Assay development for a portable fiberoptic biosensor. ASAIO J. 1996, 42, 942–946. [Google Scholar] [CrossRef]
- Anderson, G.P.; Jacoby, M.A.; Ligler, F.S.; King, K.D. Effectiveness of protein A for antibody immobilization for a fiber optical biosensor. Biosens. Bioelectron. 1997, 12, 329–336. [Google Scholar] [CrossRef]
- Cao, L.K.; Anderson, G.P.; Ligler, F.S.; Ezzell, J. Detection of Yersinia pestis fraction 1 antigen with a fiber optic biosensor. J Clin. Microbiol. 1995, 33, 336–341. [Google Scholar] [CrossRef]
- Anderson, G.P.; King, K.D.; Cao, L.K.; Jacoby, M.; Ligler, F.S.; Ezzell, J. Quantifying serum antiplague antibody with a fiber-optic biosensor. Clin. Diagn. Lab. Immunol. 1998, 5, 609–612. [Google Scholar] [CrossRef]
- Lim, D.V.; Simpson, J.M.; Kearns, E.A.; Kramer, M.F. Current and developing technologies for monitoring agents of bioterrorism and biowarfare. Clin. Microbiol. Rev. 2005, 18, 583–607. [Google Scholar] [CrossRef]
- Leskinen, S.D.; Lim, D.V. Rapid ultrafiltration concentration and biosensor detection of Enterococci from large volumes of Florida recreational water. Appl. Environ. Microbiol. 2008, 74, 4792–4798. [Google Scholar] [CrossRef]
- 121 Kishen, A.; John, M.S.; Lim, C.S.; Asundi, A. A fiber optic biosensor (FOBS) to monitor mutans streptococci in human saliva. Biosens. Bioelectron. 2003, 18, 1371–1378. [Google Scholar] [CrossRef]
- Kramer, M.F.; Lim, D.V. A rapid and automated fiber optic-based biosensor assay for the detection of Salmonella in spent irrigation water used in the sprouting of sprout seeds. J. Food Prot. 2004, 67, 46–52. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, M.; Li, Y.; Liang, Z.; Li, Y.; Yu, L.; Liu, Y.; Liang, Y.; Chen, L.; Yang, C. A new optical fiber probe-based quantum dots immunofluorescence biosensors in the detection of Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2021, 11, 665241. [Google Scholar] [CrossRef]
- Tripathi, S.M.; Bock, W.J.; Mikulic, P.; Chinnappan, R.; Ng, A.; Tolba, M.; Zourob, M. Long period grating based biosensor for the detection of Escherichia coli bacteria. Biosens. Bioelectron. 2012, 35, 308–312. [Google Scholar] [CrossRef]
- Horváth, R.; Pedersen, H.C.; Skivesen, N.; Selmeczi, D.; Larsen, N.B. Optical waveguide sensor for on-line monitoring of bacteria. Opt. Lett. 2003, 28, 1233–1235. [Google Scholar] [CrossRef]
- Cooper, I.R.; Meikle, S.T.; Standen, G.; Hanlon, G.W.; Santin, M. The rapid and specific real-time detection of Legionella pneumophila in water samples using optical waveguide lightmode spectroscopy. J. Microbiol. Methods 2009, 78, 40–44. [Google Scholar] [CrossRef]
- Mathesz, A.; Valkai, S.; Újvárosy, A.; Aekbote, B.; Sipos, O.; Stercz, B.; Kocsis, B.; Szabó, D.; Dér, A. Integrated optical biosensor for rapid detection of bacteria. Optofluid Microfluid. Nanofluid. 2015, 2, 15. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Petrou, P.S.; Makarona, E.; Haasnoot, W.; Moser, I.; Jobst, G.; Goustouridis, D.; Lees, M.; Kalatzi, K.; Raptis, I.; et al. Ultrafast multiplexed-allergen detection through advanced fluidic design and monolithic interferometric silicon chips. Anal. Chem. 2018, 90, 9559–9567. [Google Scholar] [CrossRef]
- Petrovszki, D.; Valkai, S.; Gora, E.; Tanner, M.; Bányai, A.; Fürjes, P.; Dér, A. An integrated electro-optical biosensor system for rapid, low-cost detection of bacteria. Microelectron. Eng. 2021, 239–240, 111523. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Wang, Y.; Marques, C.; Zhang, B.; Kumar, S. Advances in novel nanomaterial-based optical fiber biosensors—A review. Biosensors 2022, 12, 843. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Li, X.; Gong, P.; Zhang, Y.; Zhao, Y. Recent advancements of LSPR fiber-optic biosensing: Combination methods, structure, and prospects. Biosensors 2023, 13, 405. [Google Scholar] [CrossRef]
- Elsherif, M.; Salih, A.E.; Muñoz, M.G.; Alam, F.; AlQattan, B.; Antonysamy, D.S.; Zaki, M.F.; Yetisen, A.K.; Park, S.; Wilkinson, T.D.; et al. Optical fiber sensors: Working principle, applications, and limitations. Adv. Photonics Res. 2022, 3, 210037. [Google Scholar] [CrossRef]
- Wang, X.; Meier, R.; Wolfbeis, O. Fluorescent pH-sensitive nanoparticles in an agarose matrix for imaging of bacterial growth and metabolism. Chem. Int. Ed. 2013, 52, 406. [Google Scholar] [CrossRef] [PubMed]
- Mistlberger, G.; Crespo, G.A.; Bakker, E. Ionophore-based optical sensors. Annu. Rev. Anal. Chem. 2014, 7, 483–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2008–2012). Anal. Chem. 2013, 85, 487–508. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Bakker, E. Ion selective optodes: From the bulk to the nanoscale. Anal. Bioanal. Chem. 2015, 407, 3899–3910. [Google Scholar] [CrossRef] [PubMed]
- Staal, M.; Borisov, S.; Rickelt, L.; Klimant, I.; KuÈhl, M. Ultrabright planar optodes for luminescence life-time based microscopic imaging of O2 dynamics in biofilms. J. Microbiol. Methods 2011, 85, 67–74. [Google Scholar] [CrossRef]
- Bamsey, M.; Berinstain, A.; Dixon, M. Development of a potassium-selective optode for hydroponic nutrient solution monitoring. Anal. Chim. Acta 2012, 737, 72–82. [Google Scholar] [CrossRef]
- Tiefenthaler, K.; Lukosz, W. Integrated optical switches and glass sensor. Opt. Letter. 1984, 10, 137–139. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, D. Theoretical modeling of a metal-clad planar waveguide based biosensors for the detection of Pseudomonas-like bacteria. Prog. Electromagn. Res. M 2009, 6, 167–184. [Google Scholar] [CrossRef]
- Kozma, P.; Kehl, F.; Ehrentreich, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Leng, Y.K.; Liu, B.; Liu, J.; Wan, S.P.; Wu, T.; Yuan, J.; Shao, L.; Gu, G.; Fu, Y.Q.; et al. Ultrahigh-sensitivity label-free optical fiber biosensor based on a tapered singlemode- no core-single mode coupler for Staphylococcus aureus detection. Sens. Actuators B 2020, 320, 128283. [Google Scholar] [CrossRef]
- Zaytseva, N.; Miller, W.; Goral, V.; Hepburn, J.; Fang, Y. Microfluidic resonant waveguide grating biosensor system for whole cell sensing. Appl. Phys. Lett. 2011, 98, 1–4. [Google Scholar] [CrossRef]
- Xu, J.; Suarez, D.; Gottfried, D.S. Detection of avian influenza virus using an interferometric biosensor. Anal. Bioanal. Chem. 2007, 389, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Antiochia, R.; Bollella, P.; Favero, G.; Mazzei, F. Nanotechnology based surface plasmon resonance affinity biosensors for in vitro diagnostics. Int. J. Anal. Chem. 2016, 2016, 2981931. [Google Scholar] [CrossRef] [PubMed]
- New Biosensor Designed to Detect Toxins and More Portable Device Can Differentiate among Bacteria, Viruses, and Other Biothreats. Available online: https://discover.lanl.gov/news/0422-pegasus-biosensor/ (accessed on 19 November 2023).
- De La Rue, R.; Herzig, H.P.; Gerken, M. (Eds.) Biomedical Optical Sensors: Differentiators for Winning Technologies; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Threm, D.; Nazirizadeh, Y.; Gerken, M. Photonic crystal biosensors towards on-chip integration. J. Biophotonics 2012, 5, 601–616. [Google Scholar] [CrossRef]
- Taya, S.A. Highly sensitive nano-sensor based on a binary photonic crystal for the detection of Mycobacterium tuberculosis bacteria. J. Mater. Sci. Mater Electron. 2021, 32, 28406–28416. [Google Scholar] [CrossRef]
- Inana, H.; Poyraza, M.; Incia, F.; Lifsona, M.A.; Badaya, M.; Cunningham, B.T.; Demirci, U. Photonic crystals: Emerging biosensors and their promise for point-of-care applications. Chem. Soc. Rev. 2017, 46, 366–388. [Google Scholar] [CrossRef]
- Gowdhami, D.; Balaji, V.R.; Murugan, M.; Robinson, S. Gopalkrishna Hegde Photonic crystal based biosensors: An overview. ISSS J. Micro Smart Syst. 2022, 11, 147–167. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, M. (Eds.) Nanophotonics in Biomedical Engineering; Springer Nature Singapore Pte Ltd.: Singapore, 2021; ISBN 978-981-15-6136-8. [Google Scholar] [CrossRef]
- Munson, B.R.; Young, D.F.; Okiishi, T.H. Fundamentals of Fluid Mechanics; John Wiley and Sons: New York, NY, USA, 2002; 568p. [Google Scholar]
- Huang, H.; Yang, X.; Krafczyk, M.; Xi-Yun, L. Rotation of spheroidical paricles in couette flow. J. Fluid Mech. 2012, 692, 369–394. [Google Scholar] [CrossRef]
- Bottcher, G.P.F. Theory of Electrical Polarizability; Academic Press: New York, NY, USA, 1982; Volume 1, p. 480. [Google Scholar]
- Bunin, V.D. Cell Suspension and Structures: Electrooptical Analysis. In Encyclopedia of Surface and Colloid Science; Marcel Dekker: New York, NY, USA, 2002; pp. 1–13. [Google Scholar]
- Stoylov, S.P. Colloid Electro-Optics: Theory and Applications; Academic Press: London, UK, 1991. [Google Scholar]
- Fernandez, R.E.; Rohani, A.; Farmehini, V.; Swami, N.S. Review: Microbial analysis in dielectrophoretic microfluidic systems. Anal. Chim. Acta 2017, 966, 11–33. [Google Scholar] [CrossRef]
- Gimsa, J.; Prüger, B.; Eppmann, P.; Donath, E. Electrorotation of particles measured by dynamic light scattering—A new dielectric spectroscopy technique. Colloids Surf. A Physicochem. Eng. Asp. 1995, 98, 243–249. [Google Scholar] [CrossRef]
- Gimsa, J.; Müller, T.; Schnelle, T.; Fuhr, G. Dielectric spectroscopy of single human erythrocytes at physiological ionic strength: Dispersion of the cytoplasm. Biophys. J. 1996, 71, 495–506. [Google Scholar] [CrossRef]
- Maier, H. Electrorotation of colloidal particles and cells depends on surface charge. Biophys. J. 1997, 73, 1617–1626. [Google Scholar] [CrossRef]
- Gimsa, J.; Wachner, D. A unified resistor-capacitor model for impedance, dielectrophoresis, electrorotation, and induced transmembrane potential. Biophys. J. 1998, 75, 1107–1116. [Google Scholar] [CrossRef]
- Georgieva, R.; Neu, B.; Shilov, V.M.; Knippel, E.; Budde, A.; Latza, R.; Donath, E.; Kiesewetter, H.; Bäumler, H. Low frequency electrorotation of fixed red blood cells. Biophys. J. 1998, 74, 2114–2120. [Google Scholar] [CrossRef]
- Kanevskiy, M.V.; Shardin, V.V.; Bunin, V.D.; Guliy, O.I. Electrophysical sensor systems for in vitro monitoring of bacterial metabolic activity. Biosens. Bioelectron. X 2022, 11, 100179. [Google Scholar] [CrossRef]
- Bahrieh, G.; Erdem, M.; Özgür, E.; Gündüz, U.; Külah, H. Assessment of effects of multi drug resistance on dielectric properties of K562 leukemic cells using electrorotation. RSC Adv. 2014, 4, 44879–44887. [Google Scholar] [CrossRef]
- Huang, L.; Tu, L.; Zeng, X.; Mi, L.; Li, X.; Wang, W. Study of a microfluidic chip integrating single cell trap and 3D stable rotation manipulation. Micromachines 2016, 7, 141. [Google Scholar] [CrossRef]
- Guliy, O.I.; Bunin, V.D.; Korzhenevich, V.I.; Ignatov, O.V. Electro-optical assays for immunoindication of microbial cells. Curr. Immunol. Rev. 2017, 13, 153–162. [Google Scholar] [CrossRef]
- Guliy, O.I.; Bunin, V.D. Electrooptical analysis as sensing system for detection and diagnostics bacterial cells. In Biointerface Engineering: Prospects in Medical Diagnostics and Drug Delivery; Chandra, P., Pandey, L.M., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; Chapter 11; pp. 233–254. [Google Scholar] [CrossRef]
- Bunin, V.D.; Ignatov, O.V.; Guliy, O.I.; Voloshin, A.G.; Dykman, L.A.; O’Neil, D.; Ivnitski, D. Studies of Listeria monocytogenes-antibody binding using electro-orientation. Biosens. Bioelectron. 2004, 19, 1759–1761. [Google Scholar] [CrossRef] [PubMed]
- Guliy, O.I.; Matora, L.Y.; Burygin, G.L.; Dykman, L.A.; Ostudin, N.A.; Bunin, V.D.; Ignatov, V.V.; Ignatov, O.V. Electrophysical characteristics of Azospirillum brasilense Sp245 during interaction with antibodies to various cell-surface epitopes. Anal. Biochem. 2007, 370, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Guliy, O.I.; Velichko, N.S.; Fedonenko, Y.P.; Bunin, V.D. Use of an electro-optical sensor and phage-displayed miniantibodies for immunodetection of Herbaspirillum. Talanta 2019, 202, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Bunin, V.D.; Angersbach, A.K.; Mehta, S.K.; Guliy, O.I. Use of an optical sensor to directly monitor the metabolic activity of growing bacteria. Talanta 2021, 234, 122590. [Google Scholar] [CrossRef] [PubMed]
- Biosensors Market Size, Global Forecast, Growth Drivers, Opportunities 2030. Available online: https://www.marketsandmarkets.com/Market-Reports/biosensors-market-798.html (accessed on 10 November 2023).
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical biosensors and their applications for the detection of water pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Di Nonno, S.; Ulber, R. Smartphone-based optical analysis systems. Analyst 2021, 146, 2749–2768. [Google Scholar] [CrossRef]
- Veselova, I.A.; Makedonskaya, M.I.; Eremina, O.E.; Shekhovtsova, T.N. Fluorometric and SERS sensor systems for diagnostics and monitoring of catecholamine-dependent diseases. In Macro, Micro and Nano-Biosensors: Potential Applications and Possible Limitations; Rai, M., Reshetilov, A., Plekhanova, Y., Ingle, A.P., Eds.; Springer: Cham, Switzerland, 2021; Chapter 8; pp. 133–160. [Google Scholar] [CrossRef]
- Hayman, R.B. Fiber optic biosensors for bacterial detection. In Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M., Elwary, S., Turner, A., Eds.; Springer Science + Business Media, LLC: Berlin, Germany, 2008; Chapter 7; pp. 125–137. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).