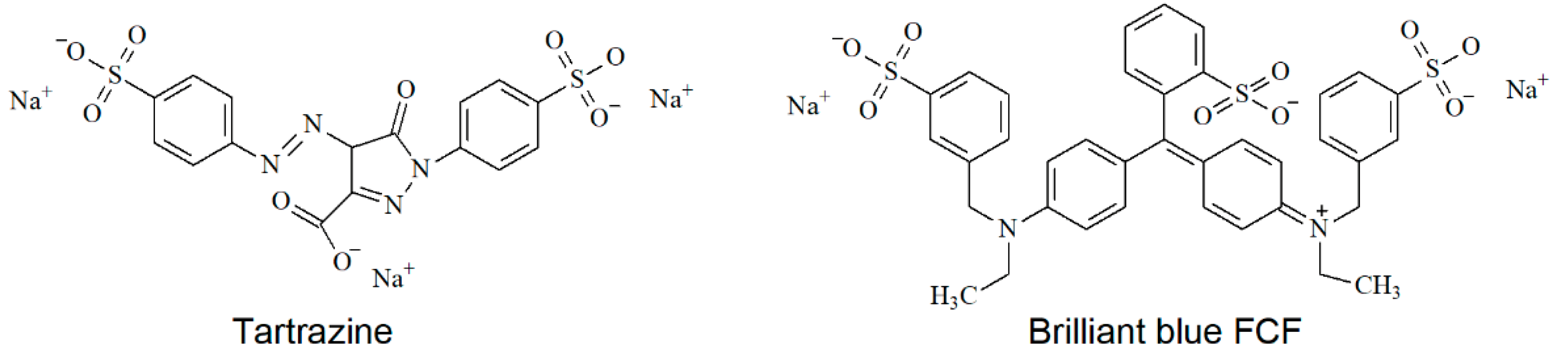
Selective Voltammetric Sensor for the Simultaneous Quantification of Tartrazine and Brilliant Blue FCF
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Equipment
2.3. Procedures
2.3.1. Fabrication of the Modified Electrode
2.3.2. Electrochemical Measurements
2.3.3. Beverages Analysis
2.3.4. Statistical Treatment
3. Results and Discussion
3.1. Voltammetric Behavior of Food Dyes on Bare GCE
3.2. MnO2 Nanorods as Electrode Surface Modifier
3.2.1. Selection of the Dispersing Agent for MnO2 Nanorods
3.2.2. Characterization of MnO2 Nanorods-Based Electrode
- The porous structure of the modified electrode surface is indirectly confirmed by the heterogeneity factor n value [56];
- The increase in the total surface charge is due to the presence of cationic surfactant at the electrode surface.
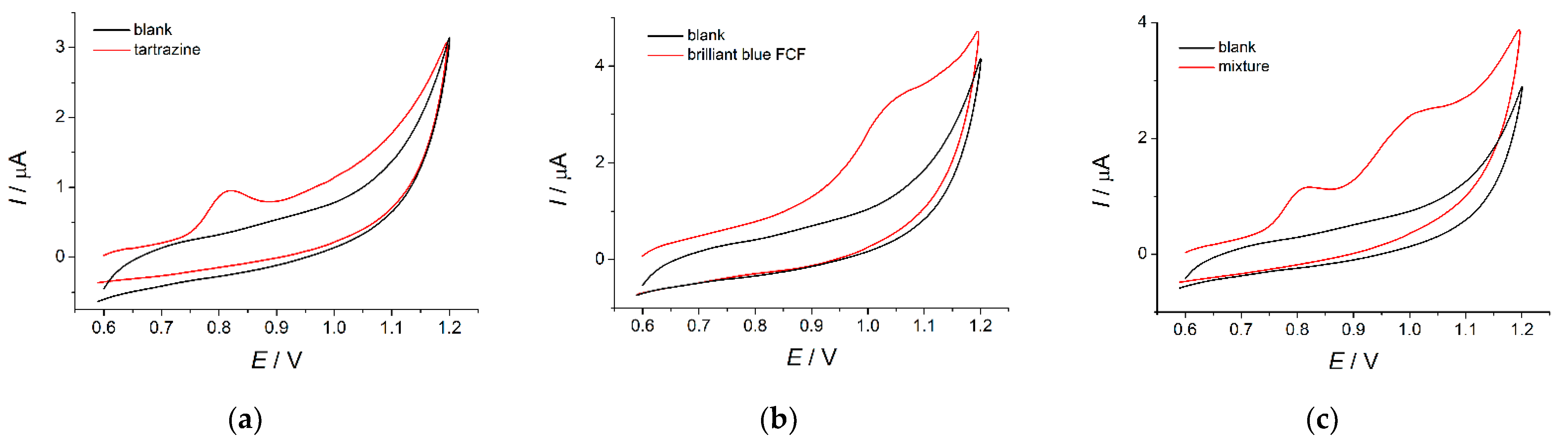
3.3. Cyclic Voltammetry of Dyes on the MnO2 Nanorods-Based Electrode
3.3.1. Voltammetric Characteristics of Dyes
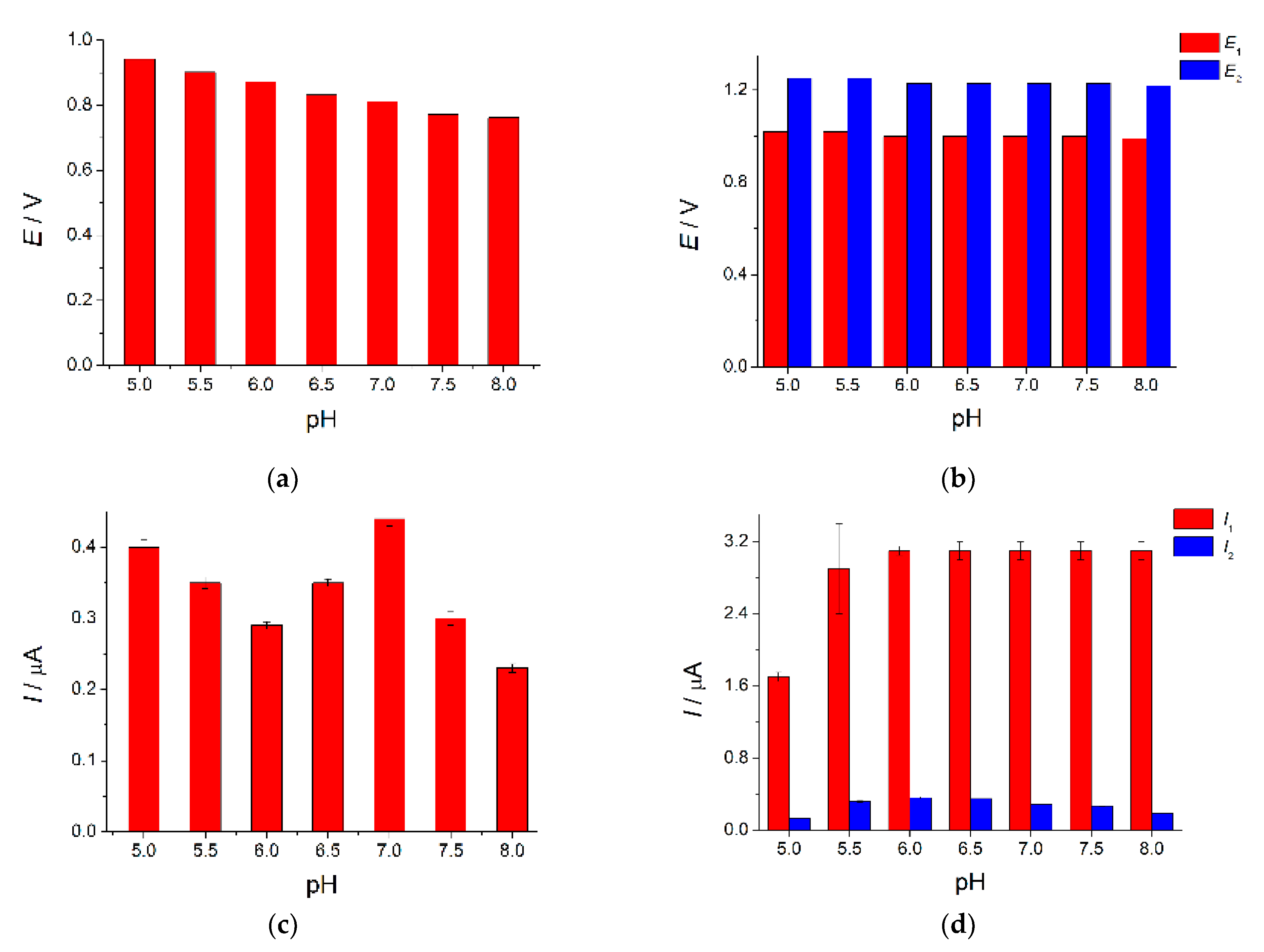
3.3.2. Effect of pH on the Tartrazine and Brilliant Blue FCF Response
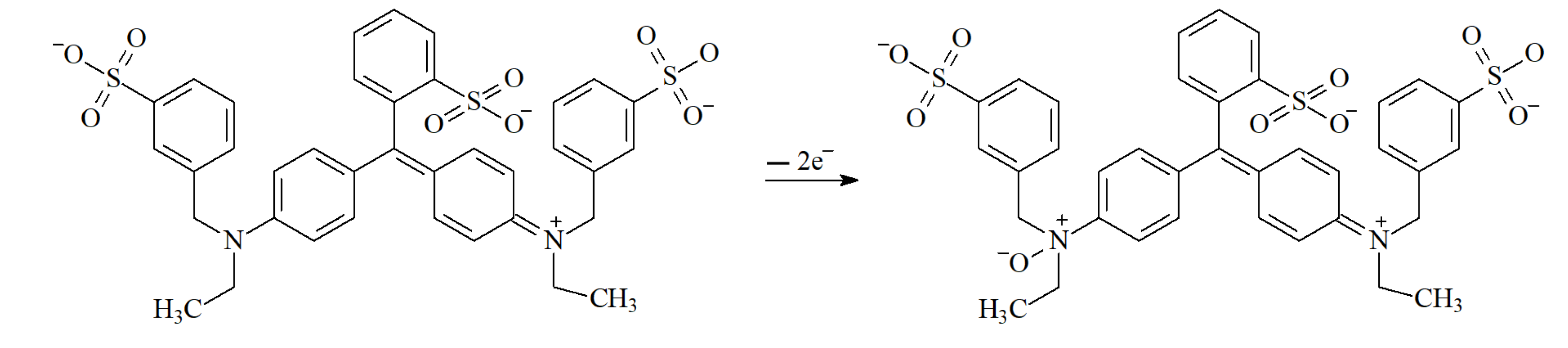
3.3.3. Electrooxidation Parameters of Tartrazine and Brilliant Blue FCF
3.4. Voltammetric Sensor for the Simultaneous Quantification of Dyes
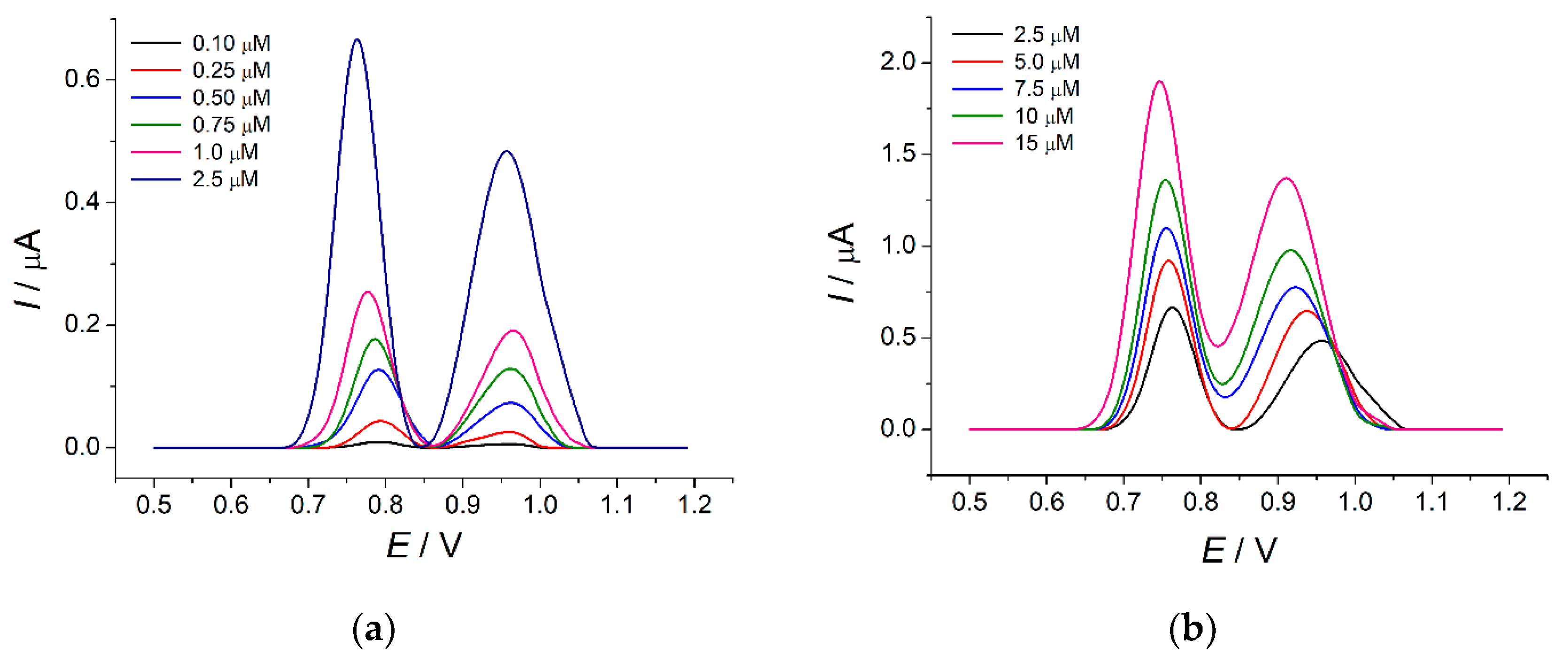
3.4.1. Analytical Performance of the Sensor
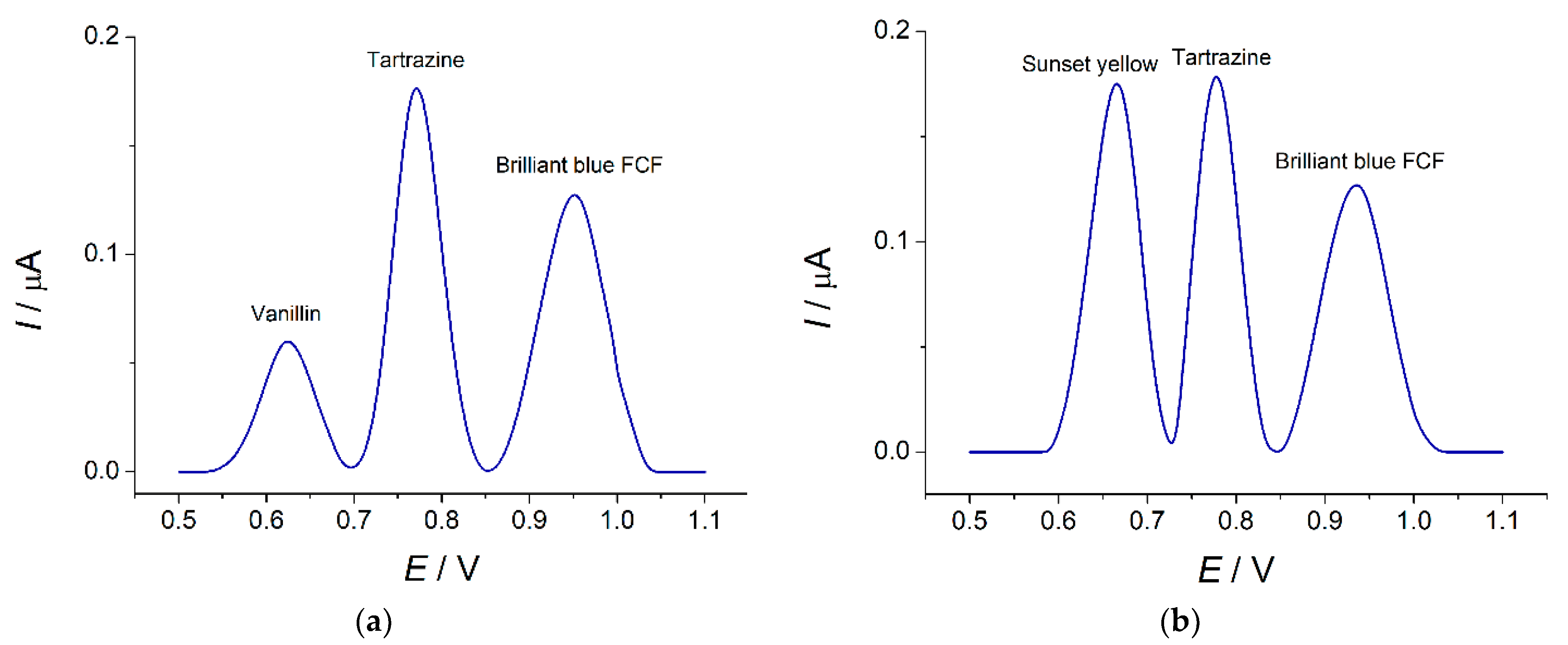
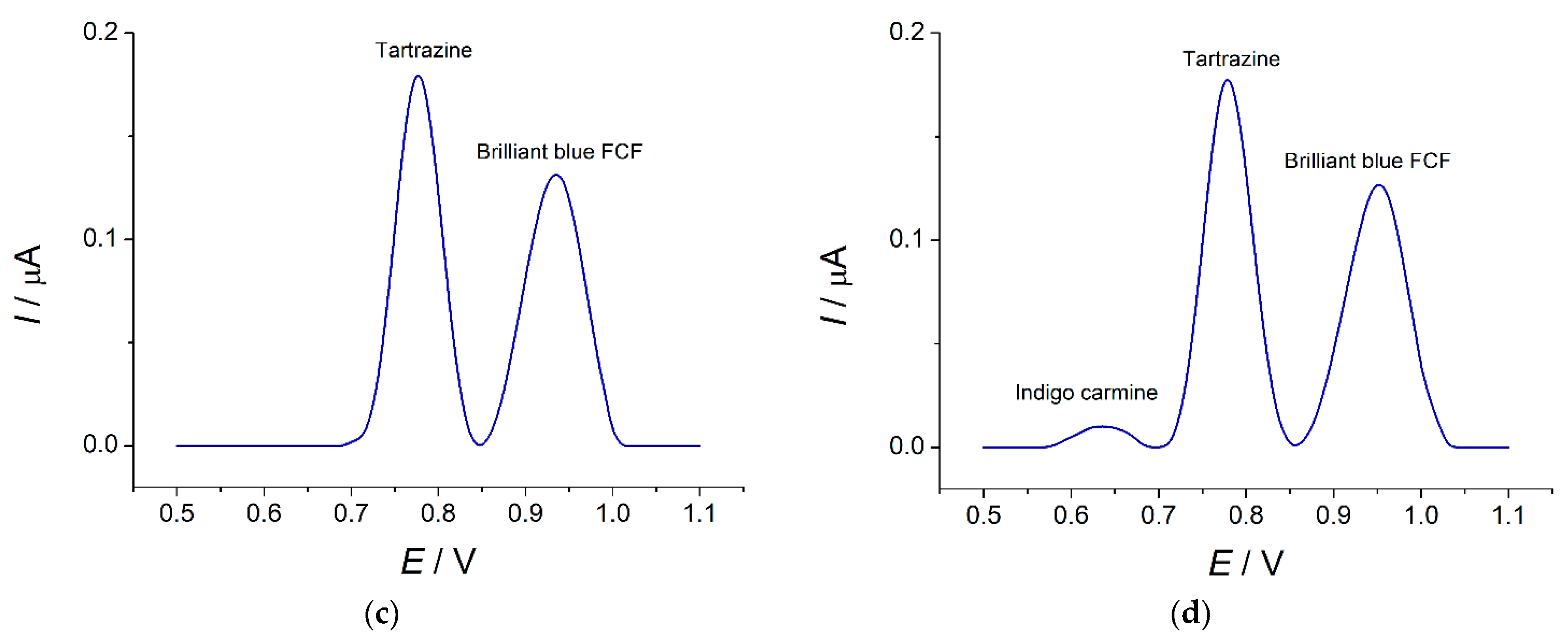
3.4.2. Selectivity Study
3.4.3. Repeatability, Reproducibility and Robustness of the Sensor
3.4.4. Real Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okafor, S.N.; Obonga, W.; Ezeokonkwo, M.A.; Nurudeen, J.; Orovwigho, U.; Ahiabuike, J. Assessment of the health implications of synthetic and natural food colourants. UK J. Pharm. Biosci. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T. Azo dyes and human health. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Reproductive and neurobehavioural toxicity study of tartrazine administered to mice in the diet. Food Chem. Toxicol. 2006, 44, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion. On re-evaluation of Tartrazine (E102) as a food additive. In: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA J. 2009, 7, 1331. [Google Scholar] [CrossRef]
- Scientific Opinion. On the re-evaluation of brilliant blue FCF (E133) as a food additive. In: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA J. 2010, 8, 1853. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food Colour Additives: A synoptical overview on their chemical properties, applications in food products, and health side effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef]
- Yildirim, S.; Yasar, A. A core-shell column approach to fast determination of synthetic dyes in foodstuffs by high-performance liquid chromatography. Food Anal. Methods 2018, 11, 1581–1590. [Google Scholar] [CrossRef]
- Floriano, L.; Ribeiro, L.C.; Saibt, N.; Bandeira, N.M.G.; Prestes, O.D.; Zanella, R. Determination of six synthetic dyes in sports drinks by dispersive solid-phase extraction and HPLC-UV-Vis. J. Braz. Chem. Soc. 2018, 29, 602–608. [Google Scholar] [CrossRef]
- Palianskikh, A.I.; Sychik, S.I.; Leschev, S.M.; Pliashak, Y.M.; Fiodarava, T.A.; Belyshava, L.L. Development and validation of the HPLC-DAD method for the quantification of 16 synthetic dyes in various foods and the use of liquid anion exchange extraction for qualitative expression determination. Food Chem. 2022, 369, 130947. [Google Scholar] [CrossRef]
- Nguyen, N.V.T.; Nguyen, K.N.H.; Dam, K.T.T.; Vo, H.T.T.; Nguyen, K.A.T.; Kim, K.H. Simultaneous determination of 11 water-soluble dyes in food products and beverages by high performance liquid chromatography. Int. Food Res. J. 2021, 28, 120–128. [Google Scholar] [CrossRef]
- Shin, J.Y.; Jung, M.Y. Ultra-high-throughput analytical strategy based on UHPLC-DAD in combination with syringe filtration for the quantitation of nine synthetic colorants in beverages: Impacts of syringe membrane types and sample pH on recovery. J. Agric. Food Chem. 2017, 65, 9916–9922. [Google Scholar] [CrossRef]
- Yi, J.; Zeng, L.; Wu, Q.; Yang, L.; Xie, T. Sensitive simultaneous determination of synthetic food colorants in preserved fruit samples by capillary electrophoresis with contactless conductivity detection. Food Anal. Methods 2018, 11, 1608–1618. [Google Scholar] [CrossRef]
- Liu, F.-J.; Liu, C.-T.; Li, W.; Tang, A.-N. Dispersive solid-phase microextraction and capillary electrophoresis separation of food colorants in beverages using diamino moiety functionalized silica nanoparticles as both extractant and pseudostationary phase. Talanta 2015, 132, 366–372. [Google Scholar] [CrossRef]
- da Silva Neto, G.F.; de Andrade Rodrigues, M.L.; Fonseca, A. A new quantitative gel electrophoresis method with image-based detection for the determination of food dyes and metallic ions. Talanta 2021, 221, 121602. [Google Scholar] [CrossRef]
- Dudkina, A.A.; Volgina, T.N.; Saranchina, N.V.; Gavrilenko, N.A.; Gavrilenko, M.A. Colorimetric determination of food colourants using solid phase extraction into polymethacrylate matrix. Talanta 2019, 202, 186–189. [Google Scholar] [CrossRef]
- Bişgin, A.T. Simultaneous spectrophotometric determination of brilliant blue and tartrazine in diverse sample matrices after solid phase extraction. J. AOAC Intern. 2020, 103, 1478–1485. [Google Scholar] [CrossRef]
- Benvidi, A.; Abbasi, S.; Gharaghani, S.; Tezerjani, M.D.; Masoum, S. Spectrophotometric determination of synthetic colorants using PSO–GA-ANN. Food Chem. 2017, 220, 377–384. [Google Scholar] [CrossRef]
- State, R.G.; van Staden, J.K.F.; Stefan-van Staden, R.-I. Recent trends on the electrochemical sensors used for the determination of tartrazine and sunset yellow FCF from food and beverage products. J. Electrochem. Soc. 2022, 169, 017509. [Google Scholar] [CrossRef]
- Sierra-Rosales, P.; Toledo-Neira, C.; Ortúzar-Salazar, P.; Squella, J.A. MWCNT-modified electrode for voltammetric determination of allura red and brilliant blue FCF in isotonic sport drinks. Electroanalysis 2019, 31, 883–890. [Google Scholar] [CrossRef]
- Wang, M.; Yang, M.; Suin, Q.; Cao, Y.; Zhao, J. Development of a facile sensor for the determination of brilliant blue FCF in beverages. Int. J. Environ. Anal. Chem. 2015, 95, 969–979. [Google Scholar] [CrossRef]
- Lipskikh, O.I.; Korotkova, E.I.; Barek, J.; Vyskocil, V.; Saqib, M.; Khristunova, E.P. Simultaneous voltammetric determination of Brilliant Blue FCF and Tartrazine for food quality control. Talanta 2020, 218, 121136. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, Y.; Zhang, J.; Wang, X.; Chen, Z. Electrochemical determination of brilliant blue and tartrazine based on an ionic liquid-modified expanded graphite paste electrode. J. AOAC Int. 2015, 98, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous voltammetric determination of Brilliant Blue and Tartrazine in real samples at the surface of a multi-walled carbon nanotube paste electrode. Anal. Methods 2011, 3, 2842–2847. [Google Scholar] [CrossRef]
- Agnihotri, A.S.; Varghese, A.; M, N. Transition metal oxides in electrochemical and bio sensing: A state-of-art review. Appl. Surf. Sci. Adv. 2021, 4, 100072. [Google Scholar] [CrossRef]
- Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Leonardi, S.G.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-oxide based nanomaterials: Synthesis, characterization and their applications in electrical and electrochemical sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Gimadutdinova, L. Cerium(IV) and iron(III) oxides nanoparticles based voltammetric sensor for the sensitive and selective determination of lipoic acid. Sensors 2021, 21, 7639. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Yakupova, E.; Davletshin, R. Voltammetric determination of hesperidin on the electrode modified with SnO2 nanoparticles and surfactants. Electroanalysis 2021, 33, 2417–2427. [Google Scholar] [CrossRef]
- Chikere, C.; Faisal, N.H.; Lin, P.K.T.; Fernandez, C. Zinc oxide nanoparticles modified-carbon paste electrode used for the electrochemical determination of gallic acid. In Proceedings of the Applied Nanotechnology and Nanoscience International Conference (ANNIC 2018), Berlin, Germany, 22–24 October 2018; Volume 1310, p. 012008. [Google Scholar] [CrossRef] [Green Version]
- Ziyatdinova, G.; Ziganshina, E.; Romashkina, S.; Budnikov, H. Highly sensitive amperometric sensor for eugenol quantification based on CeO2 nanoparticles and surfactants. Electroanalysis 2017, 29, 1197–1204. [Google Scholar] [CrossRef]
- Pwavodi, P.C.; Ozyurt, V.H.; Asir, S.; Ozsoz, M. Electrochemical sensor for determination of various phenolic compounds in wine samples using fe3o4 nanoparticles modified carbon paste electrode. Micromachines 2021, 12, 312. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Antonova, T.; Vorobev, V.; Osin, Y.; Budnikov, H. Selective voltammetric determination of α-lipoic acid on the electrode modified with SnO2 nanoparticles and cetyltriphenylphosphonium bromide. Monatsh. Chem. 2019, 150, 401–410. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Antonova, T.S.; Mubarakova, L.R.; Budnikov, H.C. An amperometric sensor based on tin dioxide and cetylpyridinium bromide nanoparticles for the determination of vanillin. J. Anal. Chem. 2018, 73, 801–808. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B.; Erkmen, C.; Kurbanoglu, S.; Ozkan, S.A.; Uslu, B. Electrochemical analysis for pharmaceuticals by the advantages of metal oxide nanomaterials. Curr. Anal. Chem. 2021, 17, 1322–1339. [Google Scholar] [CrossRef]
- Su, L. Overview on the sensors for direct electrochemical detection of illicit drugs in sports. Int. J. Electrochem. Sci. 2022, 17, 221260. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Yuan, C.; Zhang, F.; Ma, L.; Qin, D.; Shan, D.; Lu, X. A novel electrochemical sensor based on zirconia/ordered macroporous polyaniline for ultrasensitive detection of pesticides. Analyst 2015, 140, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.A.; Shin, J.H. A novel and highly sensitive electrochemical monitoring platform for 4-nitrophenol on MnO2 nanoparticles modified graphene surface. RSC Adv. 2015, 5, 88996–89002. [Google Scholar] [CrossRef]
- Kavieva, L.; Ziyatdinova, G. Sensitive voltammetric quantification of carminic acid in candies using selenium dioxide nanoparticles based electrode. Food Chem. 2022, 386, 132851. [Google Scholar] [CrossRef]
- Kavieva, L.; Ziyatdinova, G. Voltammetric Sensor Based on SeO2 Nanoparticles and Surfactants for Indigo Carmine Determination. Sensors 2022, 22, 3224. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and selective detection of tartrazine based on tio2-electrochemically reduced graphene oxide composite-modified electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.; Liu, Y.; Wu, J.; Li, G.; Liu, J.; He, Q. A simple but efficient voltammetric sensor for simultaneous detection of tartrazine and ponceau 4R based on TiO2/electro-reduced graphene oxide nanocomposite. Chemosensors 2020, 8, 70. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Voltammetric determination of tartrazine on an electrode modified with cerium dioxide nanoparticles and cetyltriphenylphosphonium bromide. J. Anal. Chem. 2022, 77, 664–670. [Google Scholar] [CrossRef]
- Usui, H.; Suzuki, S.; Domi, Y.; Sakaguchi, H. Impacts of MnO2 crystal structures and fe doping in those on photoelectrochemical charge–discharge properties of TiO2/MnO2 composite electrodes. ACS Sustain. Chem. Eng. 2020, 8, 9165–9173. [Google Scholar] [CrossRef]
- Luo, L.; Li, F.; Zhu, L.; Zhang, Z.; Ding, Y.; Deng, D. Non-enzymatic hydrogen peroxide sensor based on MnO2-ordered mesoporous carbon composite modified electrode. Electrochim. Acta 2012, 77, 179–183. [Google Scholar] [CrossRef]
- Sohal, N.; Maity, B.; Shetti, N.P.; Basu, S. Biosensors based on MnO2 nanostructures: A review. ACS Appl. Nano Mater. 2021, 4, 2285–2302. [Google Scholar] [CrossRef]
- Tehseen, B.; Rehman, A.; Rahmat, M.; Bhatti, H.N.; Wu, A.; Butt, F.K.; Naz, G.; Khan, W.S.; Bajwa, S.Z. Solution growth of 3D MnO2 mesh comprising 1D nanofibres as a novel sensor for selective and sensitive detection of biomolecules. Biosens. Bioelectron. 2018, 117, 852–859. [Google Scholar] [CrossRef]
- Ravi, A.K.; Punnakkal, N.; Vasu, S.P.; Nair, B.G.; Babu, T.G.S. Manganese dioxide based electrochemical sensor for the detection of nitro-group containing organophosphates in vegetables and drinking water samples. J. Electroanal. Chem. 2020, 859, 113841. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Mobin, S.M. Hydrothermally grown α-MnO2 nanorods as highly efficient low cost counter-electrode material for dye-sensitized solar cells and electrochemical sensing applications. Electrochim. Acta 2017, 252, 549–557. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Di Bartolomeo, A. Application of MnO2 nanorod–ionic liquid modified carbon paste electrode for the voltammetric determination of sulfanilamide. Micromachines 2022, 13, 598. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Xia, Y.; Tuo, D.; Deng, P.; Tian, Y.; Wu, Y.; Li, G.; Chen, D. Rapid and sensitive voltammetric detection of rhodamine B in chili-containing foodstuffs using MnO2 nanorods/electro-reduced graphene oxide composite. J. Electrochem. Soc. 2019, 166, B805–B813. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Magesa, F.; Liu, J.; Li, G.; He, Q. Construction of effective electrochemical sensor for the determination of quinoline yellow based on different morphologies of manganese dioxide functionalized graphene. J. Food Compos. Anal. 2019, 84, 103280. [Google Scholar] [CrossRef]
- Harp, B.P.; Miranda-Bermudez, E.; Barrows, J.N. Determination of seven certified color additives in food products using liquid chromatography. J. Agric. Food Chem. 2013, 61, 3726–3736. [Google Scholar] [CrossRef]
- Rahaman, M.N. Ceramic Processing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 150–153. [Google Scholar] [CrossRef]
- Chae, C.; Kim, K.W.; Yun, Y.J.; Lee, D.; Moon, J.; Choi, Y.; Lee, S.S.; Choi, S.; Jeong, S. Polyethylenimine-mediated electrostatic assembly of MnO2 nanorods on graphene oxides for use as anodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 11499–11506. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; 864p. [Google Scholar]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014; 367p. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Khorablou, Z.; Zamani, M.; Dorraji, P.S.; Alamgholiloo, M. Voltammetric sensor for tartrazine determination in soft drinks using poly (p-aminobenzenesulfonic acid)/zinc oxide nanoparticles in carbon paste electrode. J. Food Drug Anal. 2017, 25, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Kolozof, P.-A.; Florou, A.B.; Spyrou, K.; Hrbac, J.; Prodromidis, M.I. In-situ tailoring of the electrocatalytic properties of screen-printed graphite electrodes with sparked generated molybdenum nanoparticles for the simultaneous voltammetric determination of sunset yellow and tartrazine. Sens. Actuators B Chem. 2020, 304, 127268. [Google Scholar] [CrossRef]
- Flury, M.; Fluhler, H. Brilliant blue FCF as a dye tracer for solute transport studies—A toxicological overview. J. Environ. Qual. 1994, 23, 1108–1112. [Google Scholar] [CrossRef]
- Klett, C.; Barry, A.; Balti, I.; Lelli, P.; Schoenstein, F.; Jouini, N. Nickel doped zinc oxide as a potential sorbent for decolorization of specific dyes, methylorange and tartrazine by adsorption process. J. Environ. Chem. Eng. 2014, 2, 914–926. [Google Scholar] [CrossRef]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Velasco, J.G. Determination of standard rate constants for electrochemical irreversible processes from linear sweep voltammograms. Electroanalysis 1997, 9, 880–882. [Google Scholar] [CrossRef]
- Chebotarev, A.; Koicheva, A.; Bevziuk, K.; Pliuta, K.; Snigur, D. Simultaneous determination of sunset yellow and tartrazine in soft drinks on carbon-paste electrode modified by silica impregnated with cetylpyridinium chloride. J. Food Meas. Charact. 2019, 13, 1964–1972. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Selective voltammetric determination of tartrazine in the presence of red 10B by nanogold-modified carbon paste electrode. J. Chin. Chem. Soc. 2013, 60, 120–126. [Google Scholar] [CrossRef]






| Sensor | Method | Dye | Redox Process | Detection Limit (µM) | Linear Dynamic Range (µM) | Ref. |
|---|---|---|---|---|---|---|
| Carbon ink film-modified carbon black-polyethylene composite electrode | First-order derivative LSV 1 | Tartrazine | Reduction | 0.019 | 0.037–1.38 | [22] |
| Brilliant blue FCF | 0.011 | 0.025–2.52 | ||||
| 1-Butyl-3-methylimidazolium hexafluorophosphate-modified expanded graphite paste electrode | Square-wave stripping voltammetry | Tartrazine | Oxidation | 0.0033 | 0.010–1.0 | [23] |
| Brilliant blue FCF | 0.002 | 0.0050–0.10; 0.10–4.0 | ||||
| Multi-walled carbon nanotubes-carbon paste electrode | Differential pulse voltammetry | Tartrazine | Oxidation | – | 0.1–30 | [24] |
| Brilliant blue FCF | – | 0.1–24 |
| Electrode | Rs (Ω) | Ret (kΩ) | Q (µΩ−1) | n | W (µΩ−1) | χ2 |
|---|---|---|---|---|---|---|
| GCE | 245 ± 5 | 72 ± 3 | 3.7 ± 0.2 | 0.789 | – | 0.028 |
| MnO2 nanorods/GCE | 98 ± 3 | 1.0 ± 0.2 | 108 ± 6 | 0.507 | 440 ± 10 | 0.031 |
| Dye | Electrode Reaction Nature | αa 1 | n2 | D (cm2 s−1) 3 | Г (pmol cm−2) 4 | ks (s−1) 5 | k0 (cm s−1) 6 |
|---|---|---|---|---|---|---|---|
| Tartrazine | Surface control | 0.50 | 1.8 | 152 ± 4 | 864 | ||
| Brilliant blue FCF | Diffusion | 0.42 | 2 | (5.1 ± 0.8) × 10−5 | (3.7 ± 0.4) × 10−3 |
| Dye | Linear Dynamic Range (µM) | I = a + bc | ||
|---|---|---|---|---|
| a ± SD (µA) | (b ± SD) × 10−4 (µA M−1) | R2 | ||
| Tartrazine | 0.10–2.5 | −0.024 ± 0.004 | 28.0 ± 0.3 | 0.9994 |
| 2.5–15 | 0.49 ± 0.02 | 7.8 ± 0.2 | 0.9965 | |
| Brilliant blue FCF | 0.25–2.5 | −0.033 ± 0.003 | 22.1 ± 0.2 | 0.9996 |
| 2.5–15 | 0.420 ± 0.005 | 4.02 ± 0.05 | 0.9993 | |
| Added (µM) | Tartrazine | Brilliant Blue FCF | ||||
|---|---|---|---|---|---|---|
| Found (µM) | RSD (%) | R (%) | Found (µM) | RSD (%) | R (%) | |
| 0.25 | 0.25 ± 0.01 | 3.3 | 100 ± 3 | 0.25 ± 0.02 | 2.9 | 100 ± 3 |
| 0.75 | 0.75 ± 0.03 | 1.4 | 100 ± 4 | 0.74 ± 0.02 | 1.0 | 99 ± 3 |
| 2.5 | 2.51 ± 0.09 | 1.4 | 100 ± 4 | 2.5 ± 0.1 | 1.8 | 100 ± 4 |
| 7.5 | 7.5 ± 0.1 | 1.0 | 100 ± 1 | 7.53 ± 0.08 | 0.71 | 100 ± 1 |
| 15 | 15.0 ± 0.5 | 1.9 | 100 ± 3 | 14.9 ± 0.5 | 1.3 | 99 ± 3 |
| Dye | Sample | Dye Contents (mg L−1) | t-Test 1 | F-test 2 | |||
|---|---|---|---|---|---|---|---|
| Voltammetry | RSD (%) | Chromatography | RSD (%) | ||||
| Tartrazine | 1 | 4.6 ± 0.1 | 2.1 | 4.7 ± 0.2 | 1.6 | 2.31 | 1.67 |
| Brilliant blue FCF | 2 | 12.7 ± 0.3 | 2.0 | 13 ± 1 | 3.0 | 2.29 | 2.39 |
| 3 | 5.5 ± 0.1 | 1.6 | 5.5 ± 0.2 | 1.6 | 0.152 | 1.01 | |
| 4 | 3.84 ± 0.09 | 1.8 | 3.88 ± 0.05 | 0.52 | 0.949 | 12.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimadutdinova, L.; Ziyatdinova, G.; Davletshin, R. Selective Voltammetric Sensor for the Simultaneous Quantification of Tartrazine and Brilliant Blue FCF. Sensors 2023, 23, 1094. https://doi.org/10.3390/s23031094
Gimadutdinova L, Ziyatdinova G, Davletshin R. Selective Voltammetric Sensor for the Simultaneous Quantification of Tartrazine and Brilliant Blue FCF. Sensors. 2023; 23(3):1094. https://doi.org/10.3390/s23031094
Chicago/Turabian StyleGimadutdinova, Liliya, Guzel Ziyatdinova, and Rustam Davletshin. 2023. "Selective Voltammetric Sensor for the Simultaneous Quantification of Tartrazine and Brilliant Blue FCF" Sensors 23, no. 3: 1094. https://doi.org/10.3390/s23031094
APA StyleGimadutdinova, L., Ziyatdinova, G., & Davletshin, R. (2023). Selective Voltammetric Sensor for the Simultaneous Quantification of Tartrazine and Brilliant Blue FCF. Sensors, 23(3), 1094. https://doi.org/10.3390/s23031094








