Can MRI Be Used as a Sensor to Record Neural Activity?
Abstract
:1. Introduction
2. MRI Measurement of Activity in the Heart
3. MRI Measurement of Activity in Peripheral Nerves
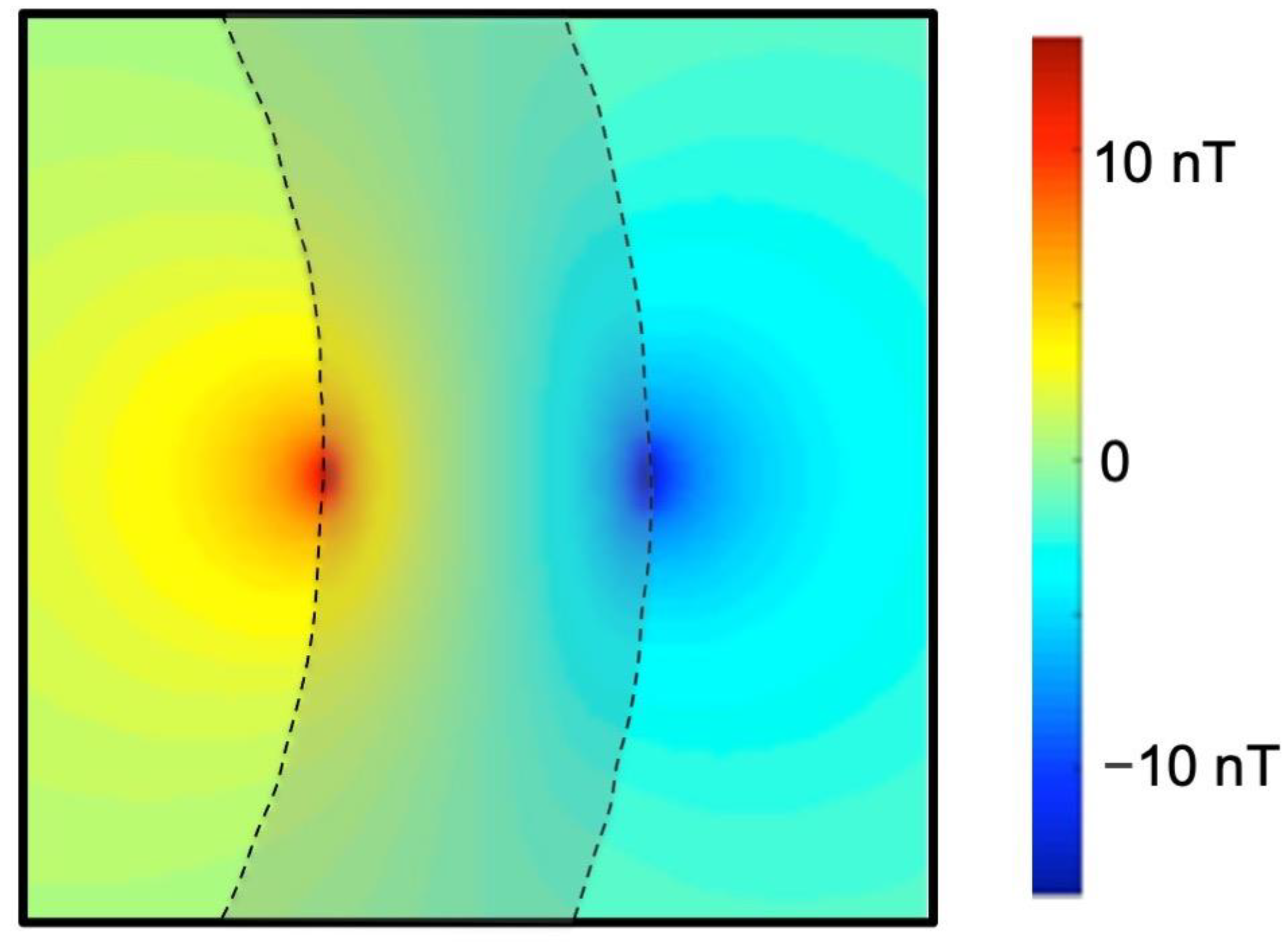
3.1. The Magnetic Field Produced by a Peripheral Nerve
3.2. Lorentz Force Imaging
4. MRI Measurement of Activity in the Brain
4.1. The Magnetic Field Produced by the Brain: Phantom Studies
4.2. The Magnetic Field Produced by the Brain: Calculations
4.3. The Magnetic Field Produced by the Brain: Detection
4.4. Different Mechanisms Responsible for Brain Signals
5. Recent Experimental Results
5.1. Sundaram et al. (2016)
5.2. Truong et al. (2019)
5.3. Toi et al. (2022)
6. Artificial Intelligence
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagel, J.H. NMR imaging of action currents. IEEE Trans. Biomed. Eng. 1984, 31, 569. [Google Scholar]
- Joy, M.; Scott, G.; Henkelman, M. In vivo detection of applied electric currents by magnetic resonance imaging. Magn. Reson. Imaging 1989, 7, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.C.; Joy, M.L.G.; Armstrong, R.L.; Henkelman, R.M. Measurement of nonuniform current density by magnetic resonance. IEEE Trans. Med. Imaging 1991, 10, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.C.; Joy, M.L.G.; Armstrong, R.L.; Henkelman, R.M. Sensitivity of magnetic-resonance current-density imaging. J. Magn. Reson. 1992, 97, 235–254. [Google Scholar] [CrossRef]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [Green Version]
- Kwong, K.K.; Belliveau, J.W.; Chesler, D.A.; Goldberg, I.E.; Weisskoff, R.M.; Poncelet, B.P.; Kennedy, D.N.; Hoppel, B.E.; Cohen, M.S.; Turner, R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. USA 1992, 89, 5675–5679. [Google Scholar] [CrossRef] [Green Version]
- Bandettini, P.A.; Petridou, N.; Boduraka, J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Appl. Magn. Reson. 2005, 29, 65–88. [Google Scholar] [CrossRef]
- Xu, D.; Roth, B.J. The magnetic field produced by the heart and its influence on MRI. Math. Probl. Eng. 2017, 2017, 3035479. [Google Scholar] [CrossRef] [Green Version]
- Wikswo, J.P.; Barach, J.P.; Freeman, J.A. Magnetic field of a nerve impulse: First measurements. Science 1980, 208, 53–55. [Google Scholar] [CrossRef] [Green Version]
- Roth, B.J.; Wikswo, J.P., Jr. The magnetic field of a single axon: A comparison of theory and experiment. Biophys. J. 1985, 48, 93–109. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, R.S.; Roth, B.J. Detection of peripheral nerve and skeletal muscle action currents using magnetic resonance imaging. Ann. Biomed. Eng. 2009, 37, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Paley, M.N.J.; Chow, L.S.; Whitby, E.H.; Cook, G.G. Modeling of axonal fields in the optic nerve for direct MR detection studies. Image Vis. Comput. 2009, 27, 331–341. [Google Scholar] [CrossRef]
- Yang, H.; Cook, G.G.; Paley, M.N.J. Mapping of periodic waveforms using the ghost reconstructed alternating current estimation (GRACE) magnetic resonance imaging technique. Magn. Reson. Med. 2003, 50, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Song, A.W.; Takahashi, A.M. Lorentz effect imaging. Magn. Reson. Imaging 2001, 19, 763–767. [Google Scholar] [CrossRef]
- Truong, T.-K.; Wilbur, J.L.; Song, A.W. Synchronized detection of minute electrical currents with MRI using Lorentz effect imaging. J. Magn. Reson. 2006, 179, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Truong, T.-K.; Song, A.W. Finding neuroelectric activity under magnetic field oscillations (NAMO) with magnetic resonance imaging in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 12598–12601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, B.J.; Basser, P.J. Mechanical model of neural tissue displacement during Lorentz effect imaging. Magn. Reson. Med. 2009, 61, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Roth, B.J.; Luterek, A.; Puwal, S. The movement of a nerve in a magnetic field: Application to MRI Lorentz effect imaging. Med. Biol. Eng. Comput. 2014, 52, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, T.-K.; Avram, A.; Song, A.W. Lorentz effect imaging of ionic currents in solution. J. Magn. Reson. 2008, 191, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, R.S.; Roth, B.J. Lorentz effect imaging of ionic currents in solution using correct values for ion mobility. J. Magn. Reson. 2010, 204, 225–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourtaheri, N.; Truong, T.-K.; Henriquez, C.S. Electromagnetohydrodynamic modeling of Lorentz effect imaging. J. Magn. Reson. 2013, 236, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, M.; Mulkern, R.V.; Wells, W.M.; Sundaram, P.; Orbach, D.B. Magnetic resonance imaging of ionic currents in solution: The effect of magnetohydrodynamic flow. Magn. Reson. Med. 2015, 74, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Bodurka, J.; Jesmanowicz, A.; Hyde, J.S.; Xu, H.; Estkowski, L.; Li, S.-J. Current-induced magnetic resonance phase imaging. J. Mang. Reson. 1999, 137, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Konn, D.; Gowland, P.; Bowtell, R. MRI detection of weak magnetic fields due to an extended current dipole in a conduction sphere: A model for direct detection of neuronal currents in the brain. Magn. Reson. Med. 2003, 50, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Pell, G.S.; Abbott, D.F.; Fleming, S.W.; Prichard, J.W.; Jackson, G.D. Further steps toward direct magnetic resonance (MR) imaging detection of neural action currents: Optimization of MR sensitivity to transient and weak currents in a conductor. Magn. Reson. Med. 2006, 55, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Buracas, G.T.; Liu, T.T.; Buxton, R.B.; Frank, L.R.; Wong, E.C. Imaging periodic currents using alternating balanced steady-state free precession. Magn. Reson. Med. 2008, 59, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Heo, H.-I.; Park, S.-H. Detection of fast oscillating magnetic fields using dynamic multiple TR imaging and Fourier analysis. PLoS ONE 2018, 13, e0189916. [Google Scholar] [CrossRef] [Green Version]
- Singh, M. Sensitivity of MR phase shift to detect evoked neuromagnetic fields inside the head. IEEE Trans. Nucl. Sci. 1994, 41, 349–351. [Google Scholar] [CrossRef]
- Bodurka, J.; Bandettini, P.A. Toward direct mapping of neuronal activity: MRI detection of ultraweak transient magnetic field changes. Magn. Reson. Med. 2002, 47, 1052–1058. [Google Scholar] [CrossRef]
- Hatada, T.; Sekino, M.; Ueno, S. Finite element method-based calculation of the theoretical limit of sensitivity for detecting weak magnetic fields in the human brain using magnetic-resonance imaging. J. Appl. Phys. 2005, 97, 10E109. [Google Scholar] [CrossRef]
- Park, T.S.; Lee, S.Y. Effects of neuronal magnetic fields on MRI: Numerical analysis with axon and dendrite models. Neuroimage 2007, 35, 531–538. [Google Scholar] [CrossRef]
- Blagoev, K.B.; Mihaila, B.; Travis, B.J.; Alexandrov, L.B.; Bishop, A.R.; Ranken, D.; Posse, S.; Gasparovic, C.; Mayer, A.; Aine, C.J.; et al. Modelling the magnetic signature of neuronal tissue. Neuroimage 2007, 37, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Heller, L.; Barrowes, B.E.; George, J.S. Modeling direct effects of neural current on MRI. Hum. Brain Mapp. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Xiong, H.-C.; Yao, D.-Z. Direct MRI detection of the neuronal magnetic field: The effect of the dendrite branch. Phys. Med. Biol. 2010, 55, 5599–5616. [Google Scholar] [CrossRef]
- Luo, Q.; Jiang, X.; Chen, B.; Zhu, Y.; Gao, J.-H. Modeling neuronal current MRI signal with human neuron. Magn. Reson. Med. 2011, 65, 1680–1689. [Google Scholar] [CrossRef] [Green Version]
- Jay, W.I.; Wijesinghe, R.S.; Dolasinski, B.D.; Roth, B.J. Is it possible to detect dendrite currents using presently available magnetic resonance imaging techniques? Med. Biol. Eng. Comput. 2012, 50, 651–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassara, A.M.; Hagberg, G.E.; Bianciardi, M.; Migliore, M.; Maraviglia, B. Realistic simulations of neuronal activity: A contribution to the debate on direct detection of neuronal currents by MRI. Neuroimage 2008, 39, 87–106. [Google Scholar] [CrossRef]
- Kraus, R.H.; Volegov, P.; Matlachov, A.; Espy, M. Toward direct neural current imaging by resonant mechanisms at ultra-low field. Neuroimage 2008, 39, 310–317. [Google Scholar] [CrossRef]
- Cassara, A.M.; Maraviglia, B. Microscopic investigation of the resonant mechanism for the implementation of nc-MRI at ultra-low field MRI. Neuroimage 2008, 41, 1228–1241. [Google Scholar] [CrossRef]
- Hofner, N.; Albrecht, H.-H.; Cassara, A.M.; Curio, G.; Hartwig, S.; Haueisen, J.; Hilschenz, I.; Korber, R.; Martens, S.; Scheer, H.-J.; et al. Are brain currents detectable by means of low-field NMR? A phantom study. Magn. Reson. Imaging 2011, 29, 1365–1373. [Google Scholar] [CrossRef]
- Sveinsson, B.; Koonjoo, N.; Zhu, B.; Witzel, T.; Rosen, M.S. Detection of nanotesla AC magnetic fields using steady-state SIRS and ultra-low field MRI. J. Neural Eng. 2020, 17, 034001. [Google Scholar] [CrossRef]
- Ueda, H.; Ito, Y.; Oida, T.; Taniguchi, Y.; Kobayashi, T. Detection of tiny oscillatory magnetic fields using low-field MRI: A combined phantom and simulation study. J. Magn. Reson. 2020, 319, 106828. [Google Scholar] [CrossRef]
- Ueda, H.; Ito, Y.; Oida, T.; Taniguchi, Y.; Kobayashi, T. Magnetic resonance imaging simulation with spin-lock preparations to detect tiny oscillatory magnetic fields. J. Magn. Reson. 2021, 324, 106910. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, G.E.; Bianciardi, M.; Maraviglia, B. Challenges for detection of neuronal currents by MRI. Magn. Reson. Med. 2006, 24, 483–493. [Google Scholar] [CrossRef]
- Kamei, H.; Iramina, K.; Yoshikawa, K.; Ueno, S. Neuronal current distribution imaging using magnetic resonance. IEEE Trans. Magn. 1999, 35, 4109–4111. [Google Scholar] [CrossRef]
- Xiong, J.; Fox, P.T.; Gao, J.-H. Directly mapping magnetic field effects of neuronal activity by magnetic resonance imaging. Hum. Brain Mapp. 2003, 20, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bianciardi, M.; Di Russo, F.; Aprile, T.; Maraviglia, B.; Hagberg, G.E. Combination of BOLD-fMRI and VEP recordings for spin-echo MRI detection of primary magnetic effects caused by neuronal currents. Magn. Reson. Imaging 2004, 22, 1429–1440. [Google Scholar] [CrossRef]
- Petridou, N.; Plenz, D.; Silva, A.C.; Loew, M.; Bodurka, J.; Bandettini, P.A. Direct magnetic resonance detection of neuronal electrical activity. Proc. Natl. Acad. Sci. USA 2006, 103, 16015–16020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, L.S.; Cook, G.G.; Whitby, E.; Paley, M.N.J. Investigation of MR signal modulation due to magnetic fields from neuronal currents in the adult human optic nerve and visual cortex. Magn. Reson. Imaging 2006, 24, 681–691. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, X.; Grabowski, T.; Xiong, J. Direct MRI mapping of neuronal activity evoked by electrical stimulation of the median nerve at the right wrist. Magn. Reson. Med. 2009, 61, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, P.; Wells, W.M.; Mulkern, R.V.; Bubrick, E.J.; Bromfield, E.B.; Munch, M.; Orbach, D.B. Fast human brain magnetic resonance responses associated with epileptiform spikes. Magn. Reson. Med. 2010, 64, 1728–1738. [Google Scholar] [CrossRef] [Green Version]
- Chu, R.; de Zwart, J.A.; van Gelderen, P.; Fukunaga, M.; Kellman, P.; Holroyd, T.; Duyn, J.H. Hunting for neuronal currents: Absence of rapid MRI signal changes during visual-evoked response. Neuroimage 2004, 23, 1059–1067. [Google Scholar] [CrossRef]
- Mandelkow, H.; Halder, P.; Brandeis, D.; Soellinger, M.; de Zanche, N.; Luechinger, R.; Boesiger, P. Heart beats brain: The problem of detecting alpha waves by neuronal current imaging in joint EEG-MRI experiments. Neuroimage 2007, 37, 149–163. [Google Scholar] [CrossRef]
- Parkes, L.M.; de Lange, F.P.; Fries, P.; Toni, I.; Norris, D.G. Inability to directly detect magnetic field changes associated with neuronal activity. Magn. Reson. Med. 2007, 57, 411–416. [Google Scholar] [CrossRef]
- Tang, L.; Avison, M.J.; Gatenby, J.C.; Gore, J.C. Failure to direct detect magnetic field dephasing corresponding to ERP generation. Magn. Reson. Imaging 2008, 26, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Q.; Lu, H.; Lu, H.; Senseman, D.; Worsley, K.; Yang, Y.; Gao, J.-H. Physiologically evoked neuronal current MRI in a bloodless turtle brain: Detectable or not? Neuroimage 2009, 47, 1268–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodionov, R.; Siniatchkin, M.; Michel, C.M.; Liston, A.D.; Thornton, R.; Guye, M.; Carmichael, D.W.; Lemieux, L. Looking for neuronal currents using MRI: An EEG-fMRI investigation of fast MR signal changes time-locked to frequent focal epileptic discharges. Neuroimage 2010, 50, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Jiang, X.; Gao, J.-H. Detection of neuronal current MRI in human without BOLD contamination. Magn. Reson. Med. 2011, 66, 492–497. [Google Scholar] [CrossRef]
- Huang, J. Detecting neuronal currents with MRI: A human study. Magn. Reson. Med. 2014, 71, 756–762. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, D.C. Exploring human brain neuronal currents with phase MRI. Int. J. Imaging Syst. Technol. 2015, 25, 172–178. [Google Scholar] [CrossRef]
- Konn, D.; Leach, S.; Gowland, P.; Bowtell, R. Initial attempts at directly detecting alpha wave activity in the brain using MRI. Magn. Reson. Imaging 2004, 22, 1413–1427. [Google Scholar] [CrossRef]
- Park, T.S.; Lee, S.Y.; Park, J.-H.; Cho, M.H.; Lee, S.Y. Observation of the fast response of a magnetic resonance signal to neuronal activity: A snail ganglia study. Physiol. Meas. 2006, 27, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lu, H.; Shigeno, S.; Tan, L.-H.; Yang, Y.; Ragsdale, C.W.; Gao, J.-H. Octopus visual system: A functional MRI model for detecting neuronal electric currents without a blood-oxygen-level-dependent confound. Magn. Reson. Med. 2014, 72, 1311–1319. [Google Scholar] [CrossRef] [Green Version]
- Koretsky, A.P. Is there a path beyond BOLD? Molecular imaging of brain function. Neuroimage 2012, 62, 1208–1215. [Google Scholar] [CrossRef] [Green Version]
- Barandov, A.; Bartelle, B.B.; Williamson, C.G.; Loucks, E.S.; Lippard, S.J.; Jasanoff, A. Sensing intracellular calcium ions using a manganese-based MRI contrast agent. Nat. Commun. 2019, 10, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bihan, D.; Urayama, S.-I.; Aso, T.; Hanakawa, T.; Fukuyama, H. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proc. Natl. Acad. Sci. USA 2006, 103, 8263–8268. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.L.; Bulte, D.P.; Devlin, H.; Robson, M.D.; Wise, R.G.; Woolrich, M.W.; Jezzard, P.; Behrens, T.E.J. Evidence for a vascular contribution to diffusion fMRI at high b value. Proc. Natl. Acad. Sci. USA 2007, 104, 20967–20972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, D.; Gil, R.; Shemesh, N. A rapid-onset diffusion functional MRI signal reflects neuromorphological coupling dynamics. Neuroimage 2021, 231, 117862. [Google Scholar] [CrossRef]
- Tsurugizawa, T.; Ciobanu, L.; Le Bihan, D. Water diffusion in brain cortex closely tracks underlying neuronal activity. Proc. Natl. Acad. Sci. USA 2013, 110, 11636–11641. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.J.; Reutens, D.C.; Hocking, J. Influence of BOLD contributions to diffusion fMRI activation of the visual cortex. Front. Neurosci. 2016, 10, 279. [Google Scholar] [CrossRef]
- Bai, R.; Stewart, C.V.; Plenz, D.; Basser, P.J. Assessing the sensitivity of diffusion MRI to detect neuronal activity directly. Proc. Natl. Acad. Sci. USA 2016, 113, E1728–E1737. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, P.; Nummenmaa, A.; Wells, W.; Orbach, D.; Orringer, D.; Mulkern, R.; Okada, Y. Direct neural current imaging in an intact cerebellum with magnetic resonance imaging. Neuroimage 2016, 132, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Truong, T.-K.; Roberts, K.C.; Woldorff, M.G.; Song, A.W. Toward direct MRI of neuro-electro-magnetic oscillations in the human brain. Magn. Reson. Med. 2019, 81, 3462–3475. [Google Scholar] [CrossRef] [PubMed]
- Witzel, T.; Lin, F.-H.; Rosen, B.R.; Wald, L.L. Stimulus-induced rotary saturation (SIRS): A potential method for the detection of neuronal currents with MRI. Neuroimage 2008, 42, 1357–1365. [Google Scholar] [CrossRef] [Green Version]
- Halpern-Manners, N.W.; Bajaj, V.S.; Teisseyre, T.Z.; Pines, A. Magnetic resonance imaging of oscillating electrical currents. Proc. Natl. Acad. Sci. USA 2010, 107, 8519–8524. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Sheng, J.; Li, H.; Chai, Y.; Zhou, X.; Wu, B.; Guo, X.; Gao, J.-H. Detection of subnanotesla oscillatory magnetic fields using MRI. Magn. Reson. Med. 2016, 75, 519–526. [Google Scholar] [CrossRef]
- Ito, Y.; Ueno, M.; Kobayashi, T. Neural magnetic field dependent fMRI toward direct functional connectivity measurements: A phantom study. Sci. Rep. 2020, 10, 5463. [Google Scholar] [CrossRef] [Green Version]
- Unger, D.M.; Wiest, R.; Kiefer, C.; Raillard, M.; Dutil, G.F.; Stein, V.M.; Schweizer, D. Neuronal current imaging: An experimental method to investigate electrical currents in dogs with idiopathic epilepsy. J. Vet. Intern. Med. 2021, 35, 2828–2836. [Google Scholar] [CrossRef]
- Toi, P.T.; Jang, H.J.; Min, K.; Kim, S.-P.; Lee, S.-K.; Lee, J.; Kwag, J.; Park, J.-Y. In vivo direct imaging of neuronal activity at high temporospatial resolution. Science 2022, 378, 160–168. [Google Scholar] [CrossRef]
- van Kerkoerle, T.; Cloos, M.A. Creating a window into the mind. Science 2022, 378, 139–140. [Google Scholar] [CrossRef]
- Pereira, F.; Mitchell, T.; Botvinick, M. Machine learning classifiers and fMRI: A tutorial overview. Neuroimage 2009, 45, S199–S209. [Google Scholar] [CrossRef] [Green Version]
- Wen, D.; Wei, Z.; Zhou, Y.; Li, G.; Zhang, X.; Han, W. Deep learning methods to process fMRI data and their application in the diagnosis of cognitive impairment: A brief overview and our opinion. Front. Neuroinform. 2018, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Li, L.; Wu, F.-X. Deep learning for brain disorder diagnosis based on fMRI images. Neurocomputing 2022, 469, 332–345. [Google Scholar] [CrossRef]
- Abuzaid, M.M.; Tekin, H.O.; Reza, M.; Elhag, I.R.; Elshami, W. Assessment of MRI technologists in acceptance and willingness to integrate artificial intelligence into practice. Radiography 2021, 27, S83–S87. [Google Scholar] [CrossRef]
- Chen, Y.; Schonlieb, C.-B.; Lio, P.; Leiner, T.; Dragotti, P.L.; Wamg, G.; Rueckert, D.; Firmin, D.; Yang, G. AI-based reconstruction for fast MRI: A systematic review and meta-analysis. Proc. IEEE 2022, 110, 224–245. [Google Scholar] [CrossRef]
- Allen, E.J.; St-Yves, G.; Wu, Y.; Breedlove, J.L.; Prince, J.S.; Dowdle, L.T.; Nau, M.; Caron, B.; Pestilli, F.; Charest, I.; et al. A massive 7T fMRI dataset to bridge cognitive neuroscience and artificial intelligence. Nat. Neurosci. 2022, 25, 116–126. [Google Scholar] [CrossRef]












| Reference | Sundaram et al. (2016) [72] | Truong et al. (2018) [73] | Toi et al. (2022) [79] |
|---|---|---|---|
| Field strength (T) | 4.7 | 3 | 9.4 |
| Preparation | Isolated turtle cerebellum | Normal human volunteer | Mouse |
| Pulse sequence | Gradient echo/Echo planar imaging | Spin-lock | 2D gradient echo |
| Temporal resolution (ms) | 100 | 125 spin-lock duration 500 repetition time | 5 |
| Spatial resolution (mm) | 2.8 × 2.8 × 3 | 3.75 × 3.75 × 8 | 0.22 × 0.22 × 1 |
| Stimulation protocol | Electrical stimulation using electrode | Eyes open/eyes close task, Alpha wave | Whisker-pad stimulation |
| Compare to electrical signal | Yes | No | Yes |
| Primarily sensitive to magnitude or phase | Phase | Magnitude | Magnitude |
| Method to eliminate BOLD | Bloodless preparation | Measurement with and without spin-lock, and other control experiments | Compare to conventional BOLD fMRI, use of a short echo time, and controls in reduced oxygen atmosphere |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, B.J. Can MRI Be Used as a Sensor to Record Neural Activity? Sensors 2023, 23, 1337. https://doi.org/10.3390/s23031337
Roth BJ. Can MRI Be Used as a Sensor to Record Neural Activity? Sensors. 2023; 23(3):1337. https://doi.org/10.3390/s23031337
Chicago/Turabian StyleRoth, Bradley J. 2023. "Can MRI Be Used as a Sensor to Record Neural Activity?" Sensors 23, no. 3: 1337. https://doi.org/10.3390/s23031337






