Adherence and Wearing Time of Prescribed Footwear among People at Risk of Diabetes-Related Foot Ulcers: Which Measure to Use?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures and Data Collection
2.3. Measures of Adherence
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Bus, S.A.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Raspovic, A.; Sacco, I.C.N.; van Netten, J.J.; on behalf of the International Working Group on the Diabetic Foot (IWGDF). Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3269. [Google Scholar] [CrossRef]
- Parker, C.N.; Van Netten, J.J.; Parker, T.J.; Jia, L.; Corcoran, H.; Garrett, M.; Kwok, C.F.; Nather, A.; Que, M.T.; Srisawasdi, G.; et al. Differences between national and international guidelines for the management of diabetic foot disease. Diabetes/Metab. Res. Rev. 2019, 35, e3101. [Google Scholar] [CrossRef]
- The National Board of Health and Welfare. Nationella Riktlinjer för Diabetesvård (National Guidlines for Diabetes Care). Available online: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-riktlinjer/2018-10-25.pdf (accessed on 30 May 2022).
- Kaminski, M.R.; Golledge, J.; Lasschuit, J.W.; Schott, K.-H.; Charles, J.; Cheney, J.; Raspovic, A. Australian guideline on prevention of foot ulceration: Part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. J. Foot Ankle Res. 2022, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Jarl, G.; Tranberg, R.; Johansson, U.; Alnemo, J.; Lundqvist, L.-O. Predictors of adherence to wearing therapeutic footwear among people with diabetes. J. Foot Ankle Res. 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.L.; de Haart, M.; Bus, S.A.; Bakker, J.P.; Hacking, H.G.; Nollet, F. Perceived usability and use of custom-made footwear in diabetic patients at high risk for foot ulceration. J. Rehabil. Med. 2014, 46, 357–362. [Google Scholar] [CrossRef]
- Waaijman, R.; Keukenkamp, R.; de Haart, M.; Polomski, W.P.; Nollet, F.; Bus, S.A. Adherence to wearing prescription custom-made footwear in patients with diabetes at high risk for plantar foot ulceration. Diabetes Care 2013, 36, 1613–1618. [Google Scholar] [CrossRef]
- Connor, H.; Mahdi, O.Z. Repetitive ulceration in neuropathic patients. Diabetes Metab. Res. Rev. 2004, 20 (Suppl. S1), S23–S28. [Google Scholar] [CrossRef]
- Waaijman, R.; de Haart, M.; Arts, M.L.; Wever, D.; Verlouw, A.J.; Nollet, F.; Bus, S.A. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care 2014, 37, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Jarl, G.; Lundqvist, L.-O. Adherence to wearing therapeutic shoes among people with diabetes: A systematic review and reflections. Patient Prefer. Adherence 2016, 10, 1521–1528. [Google Scholar] [CrossRef] [Green Version]
- Ehrmann, D.; Spengler, M.; Jahn, M.; Niebuhr, D.; Haak, T.; Kulzer, B.; Hermanns, N. Adherence over time: The course of adherence to customized diabetic insoles as objectively assessed by a temperature sensor. J. Diabetes Sci. Technol. 2018, 12, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Keukenkamp, R.; Merkx, M.J.; Busch-Westbroek, T.E.; Bus, S.A. An explorative study on the efficacy and feasibility of the use of motivational interviewing to improve footwear adherence in persons with diabetes at high-risk of foot ulceration. J. Am. Podiatr. Med. Assoc. 2018, 108, 90–99. [Google Scholar] [CrossRef]
- Jarl, G. Methodological considerations of investigating adherence to using offloading devices among people with diabetes. Patient Prefer. Adherence 2018, 12, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Racaru, S.; Saghdaoui, L.B.; Choudhury, J.R.; Wells, M.; Davies, A.H. Offloading treatment in people with diabetic foot disease: A systematic scoping review on adherence to foot offloading. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102493. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, P.A.; Crews, R.T.; van Netten, J.J.; Bus, S.A.; Fernando, M.E.; Chadwick, P.J.; Najafi, B. Measuring plantar tissue stress in people with diabetic peripheral neuropathy: A critical concept in diabetic foot management. J. Diabetes Sci. Technol. 2019, 13, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Waaijman, R.; Arts, M.; de Haart, M.; Busch-Westbroek, T.; van Baal, J.; Nollet, F. Effect of custom-made footwear on foot ulcer recurrence in diabetes: A multicenter randomized controlled trial. Diabetes Care 2013, 36, 4109–4116. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Waaijman, R.; Nollet, F. New monitoring technology to objectively assess adherence to prescribed footwear and assistive devices during ambulatory activity. Arch. Phys. Med. Rehabil. 2012, 93, 2075–2079. [Google Scholar] [CrossRef]
- Jarl, G.; Tranberg, R. An innovative sealed shoe to off-load and heal diabetic forefoot ulcers—A feasibility study. Diabet. Foot Ankle 2017, 8, 1348178. [Google Scholar] [CrossRef]
- Crews, R.T.; Shen, B.J.; Campbell, L.; Lamont, P.J.; Boulton, A.J.; Peyrot, M.; Kirsner, R.S.; Vileikyte, L. Role and determinants of adherence to off-loading in diabetic foot ulcer healing: A prospective investigation. Diabetes Care 2016, 39, 1371–1377. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; Kimbriel, H.R.; Nixon, B.P.; Boulton, A.J. Activity patterns of patients with diabetic foot ulceration: Patients with active ulceration may not adhere to a standard pressure off-loading regimen. Diabetes Care 2003, 26, 2595–2597. [Google Scholar] [CrossRef] [Green Version]
- Ababneh, A.; Finlayson, K.; Edwards, H.; Lazzarini, P.A. Factors associated with adherence to using removable cast walker treatment among patients with diabetes-related foot ulcers. BMJ Open Diabetes Res. Care 2022, 10, e002640. [Google Scholar] [CrossRef] [PubMed]
- Crews, R.T.; Armstrong, D.G.; Boulton, A.J. A method for assessing off-loading compliance. J. Am. Podiatr. Med. Assoc. 2009, 99, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Crews, R.T.; Wrobel, J.S. Importance of time spent standing for those at risk of diabetic foot ulceration. Diabetes Care 2010, 33, 2448–2450. [Google Scholar] [CrossRef] [PubMed]
- Lutjeboer, T.; Postema, K.; Hijmans, J. Validity and feasibility of a temperature sensor for measuring use and non-use of orthopaedic footwear. J. Rehabil. Med. 2018, 50, 920–926. [Google Scholar] [CrossRef]
- Menz, H.B.; Bonanno, D.R. Objective measurement of adherence to wearing foot orthoses using an embedded temperature sensor. Med. Eng. Phys. 2021, 88, 19–24. [Google Scholar] [CrossRef]
- Jarl, G.; Alnemo, J.; Tranberg, R.; Lundqvist, L.-O. Predictors of Adherence to Using Therapeutic Shoes among People with Diabetic Foot Complications. In Proceedings of the 8th International Symposium on the Diabetic Foot, The Hague, The Netherlands, 22–25 May 2019. [Google Scholar]
- Van Netten, J.J.; Hijmans, J.M.; Jannink, M.J.; Geertzen, J.H.; Postema, K. Development and reproducibility of a short questionnaire to measure use and usability of custom-made orthopaedic shoes. J. Rehabil. Med. 2009, 41, 913–918. [Google Scholar] [CrossRef]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3266. [Google Scholar] [CrossRef]
- Mills, J.L., Sr.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G.; Society for Vascular Surgery Lower Extremity Guidelines Committee. The society for vascular surgery lower extremity threatened limb classification system: Risk stratification based on wound, ischemia, and foot infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234.e222. [Google Scholar] [CrossRef]
- Fokkenrood, H.; Verhofstad, N.; Van Den Houten, M.; Lauret, G.; Wittens, C.; Scheltinga, M.; Teijink, J. Physical activity monitoring in patients with peripheral arterial disease: Validation of an activity monitor. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 194–200. [Google Scholar] [CrossRef]
- Van Schooten, K.S.; Rispens, S.M.; Elders, P.J.; Lips, P.; van Dieën, J.H.; Pijnappels, M. Assessing physical activity in older adults: Required days of trunk accelerometer measurements for reliable estimation. J. Aging Phys. Act. 2015, 23, 9–17. [Google Scholar] [CrossRef]
- Matthews, C.E.; Ainsworth, B.E.; Thompson, R.W.; Bassett, D.R., Jr. Sources of variance in daily physical activity levels as measured by an accelerometer. Med. Sci. Sports Exerc. 2002, 34, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, B.; Kamsma, Y.; Zijlstra, W. Detection of gait and postures using a miniaturised triaxial accelerometer-based system: Accuracy in community-dwelling older adults. Age Ageing 2010, 39, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Storm, F.A.; Heller, B.W.; Mazzà, C. Step detection and activity recognition accuracy of seven physical activity monitors. PLoS ONE 2015, 10, e0118723. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Price, P. How can we improve adherence? Diabetes Metab. Res. Rev. 2016, 32 (Suppl. S1), 201–205. [Google Scholar] [CrossRef]
- Jarl, G.; van Netten, J.J.; Lazzarini, P.A. Fragile feet and trivial trauma: Communicating etiology of diabetes-related foot ulcers with patients. J. Am. Podiatr. Med. Assoc. 2023; in press. [Google Scholar]
- Bus, S.A.; Armstrong, D.G.; Gooday, C.; Jarl, G.; Caravaggi, C.; Viswanathan, V.; Lazzarini, P.A.; On behalf of the International Working Group on the Diabetic Foot (IWGDF). Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, e3274. [Google Scholar] [CrossRef] [Green Version]



| Participants’ Characteristics | Mean (SD) or n (%) | ||
|---|---|---|---|
| Sex, men/women | 43 (81.1)/10 (18.9) | ||
| Age, years | 65.3 (9.4) | ||
| BMI | 29.6 (5.6) | ||
| Diabetes type, type 1/2 | 10 (18.9)/43 (81.1) | ||
| Diabetes duration, years | 18.9 (12.0) | ||
| HbA1c (n = 6 missing) | NGSP, % | 7.7 (3.8) | |
| IFCC, mmol/mol | 60.6 (17.9) | ||
| Foot deformities a | Absent | 0 | |
| Mild | 2 (3.8) | ||
| Moderate | 45 (84.9) | ||
| Severe | 6 (11.3) | ||
| History of foot ulcer | 51 (96.2) | ||
| Amputations b | No | 33 (62.3) | |
| Smaller toes | 8 (15.1) | ||
| Hallux or more proximal partial foot | 10 (18.9) | ||
| Through or above ankle | 2 (3.8) | ||
| Type of prescribed footwear | Prefabricated | 6 (11.3) | |
| Semi-custom-made | 14 (26.4) | ||
| Fully custom-made | 33 (62.3) | ||
| Steps and weight-bearing activities with and without prescribed footwear | |||
| With prescribed footwear | Total, with and without prescribed footwear | Proportion with prescribed footwear, % | |
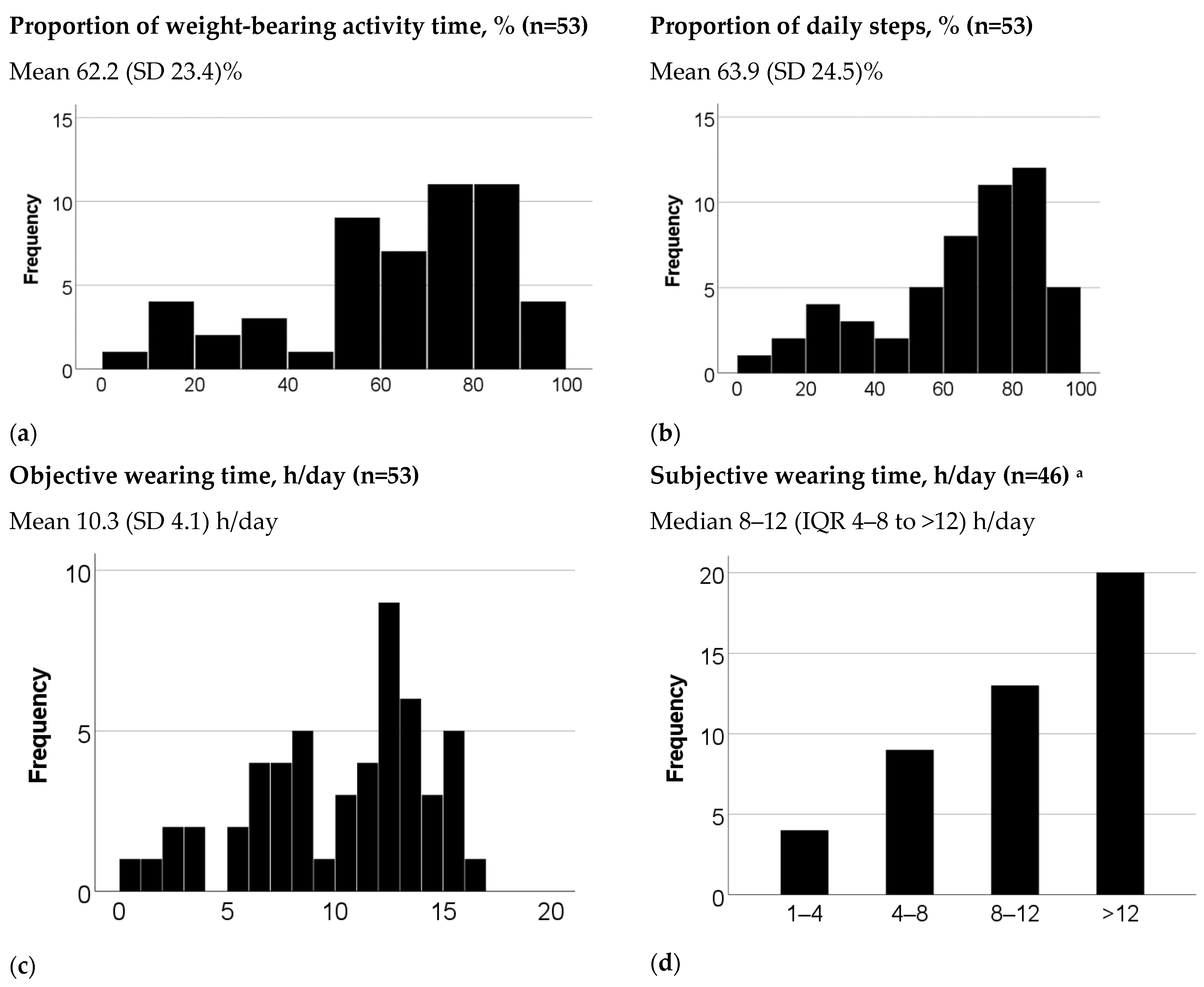
| Number of daily steps, mean (SD) | 3678 (2784) | 5835 (3731) | 63.9 (24.5) |
| Number of hours of daily weight-bearing activity time, mean (SD) | 2.2 (1.3) | 3.5 (1.6) | 62.2 (23.4) |
| Cut-Off for “High Adherence” According to the Reference Measure | ||
| 60% | 70% | |
| Proportion of steps | 1.00 (0.99–1.00) | 0.98 (0.94–1.00) |
| Objective wearing time | 0.99 (0.97–1.00) | 0.97 (0.92–1.00) |
| Subjective wearing time | 0.69 (0.52–0.85) | 0.68 (0.52–0.84) |
| ROC curves |  |  |
| 80% | 90% | |
| Proportion of steps | 0.96 (0.91–1.00) | 0.97 (0.92–1.00) |
| Objective wearing time | 0.97 (0.93–1.00) | 0.89 (0.76–1.00) |
| Subjective wearing time | 0.67 (0.51–0.84) | 0.81 (0.66–0.95) |
| ROC curves |  |  |
| 60% cut-off for “high adherence” | |||||
| Measure | Cut-off a | Sensitivity | Specificity | PPV | NPV |
| Proportion of steps | ≥61.6% | 100% | 95% | 97% | 100% |
| Objective wearing time | ≥10.5 h/day | 91% | 100% | 100% | 87% |
| Subjective wearing time | ≥category “8–12 h/day” | 83% | 50% | 76% | 62% |
| 70% cut-off for “high adherence” | |||||
| Measure | Cut-off a | Sensitivity | Specificity | PPV | NPV |
| Proportion of steps | ≥69.4.0% | 100% | 89% | 90% | 100% |
| Objective wearing time | ≥10.5 h/day | 100% | 85% | 87% | 100% |
| Subjective wearing time | ≥category “8–12 h/day” | 87% | 43% | 61% | 77% |
| 80% cut-off for “high adherence” | |||||
| Measure | Cut-off a | Sensitivity | Specificity | PPV | NPV |
| Proportion of steps | ≥77.4% | 93% | 92% | 82% | 97% |
| Objective wearing time | ≥12.2 h/day | 100% | 82% | 68% | 100% |
| Subjective wearing time | ≥category “ > 12 h/day” | 64% | 66% | 45% | 81% |
| 90% cut-off for “high adherence” | |||||
| Measure | Cut-off a | Sensitivity | Specificity | PPV | NPV |
| Proportion of steps | ≥86.4% | 100% | 90% | 44% | 100% |
| Objective wearing time | ≥12.7 h/day | 100% | 69% | 21% | 100% |
| Subjective wearing time | ≥category “ > 12 h/day” | 100% | 62% | 20% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarl, G.; Hulshof, C.M.; Busch-Westbroek, T.E.; Bus, S.A.; van Netten, J.J. Adherence and Wearing Time of Prescribed Footwear among People at Risk of Diabetes-Related Foot Ulcers: Which Measure to Use? Sensors 2023, 23, 1648. https://doi.org/10.3390/s23031648
Jarl G, Hulshof CM, Busch-Westbroek TE, Bus SA, van Netten JJ. Adherence and Wearing Time of Prescribed Footwear among People at Risk of Diabetes-Related Foot Ulcers: Which Measure to Use? Sensors. 2023; 23(3):1648. https://doi.org/10.3390/s23031648
Chicago/Turabian StyleJarl, Gustav, Chantal M. Hulshof, Tessa E. Busch-Westbroek, Sicco A. Bus, and Jaap J. van Netten. 2023. "Adherence and Wearing Time of Prescribed Footwear among People at Risk of Diabetes-Related Foot Ulcers: Which Measure to Use?" Sensors 23, no. 3: 1648. https://doi.org/10.3390/s23031648





