Performance of a Full-Scale Upstream MAPS-Based Verification Device for Radiotherapy
Abstract
:1. Introduction
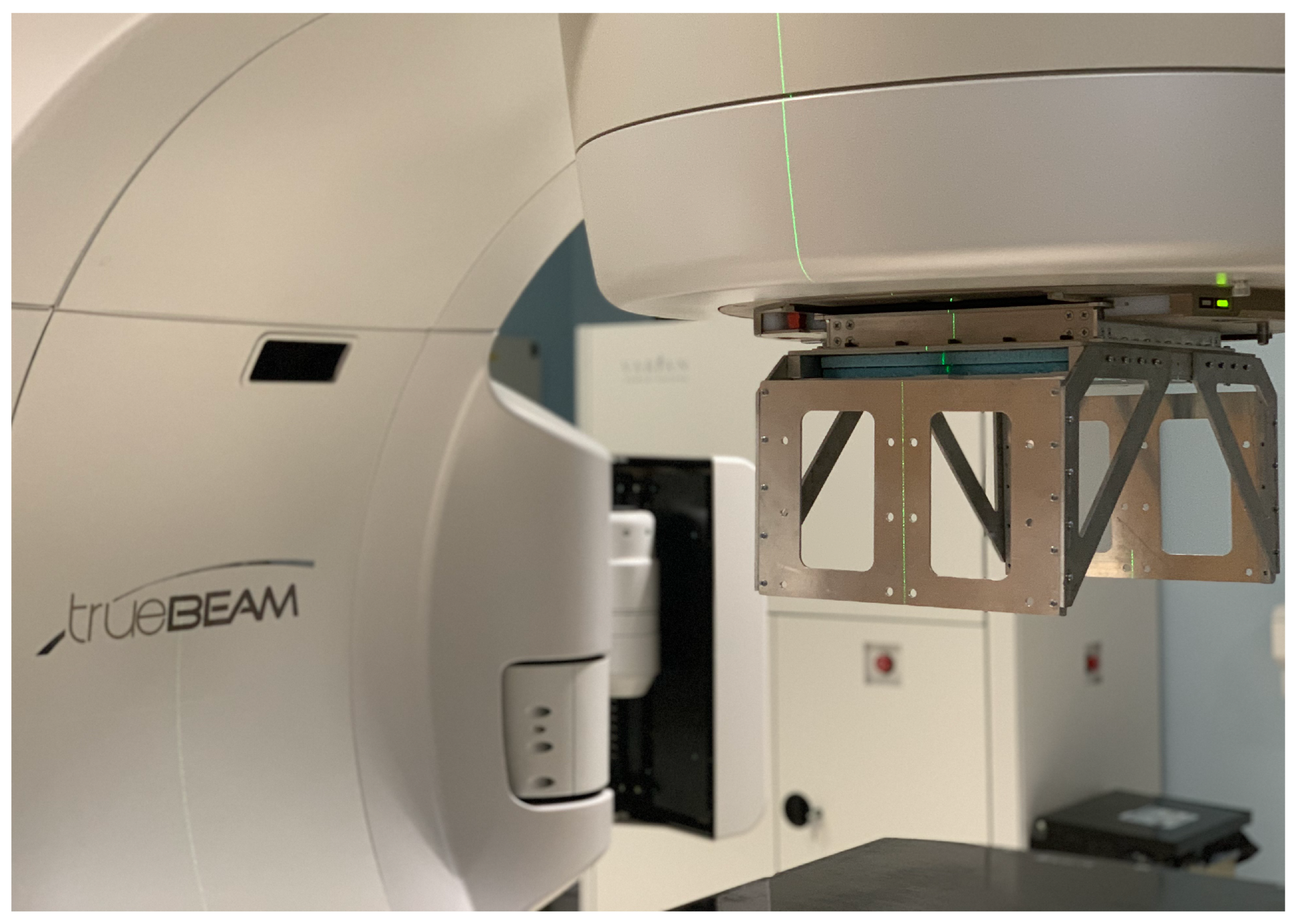
2. A Full-Scale Demonstrator Device
3. Performance of the Full-Scale Demonstrator
3.1. Device Attenuation
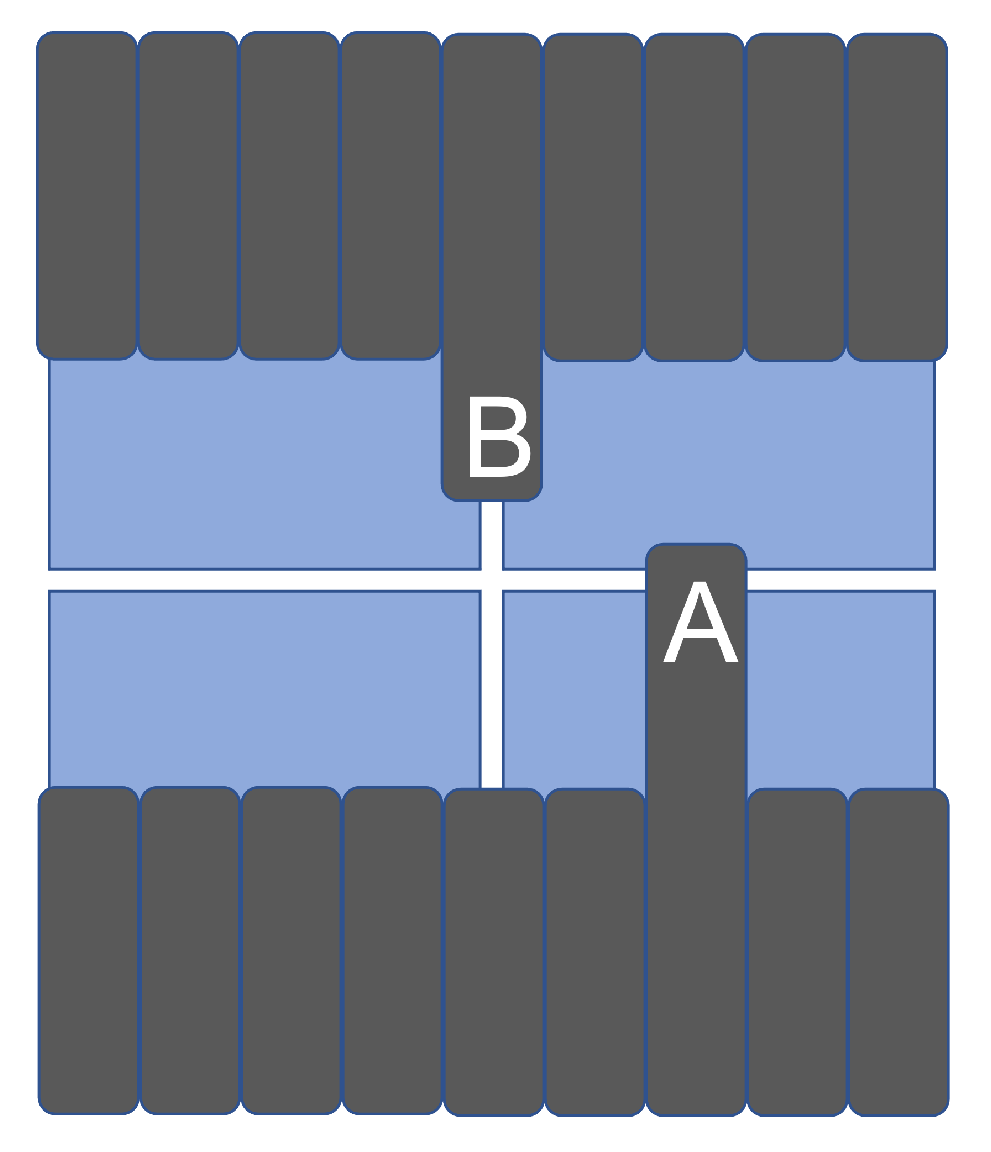
3.2. Leaf Edge Position Resolution with Leaves Perpendicular to the Dead Area
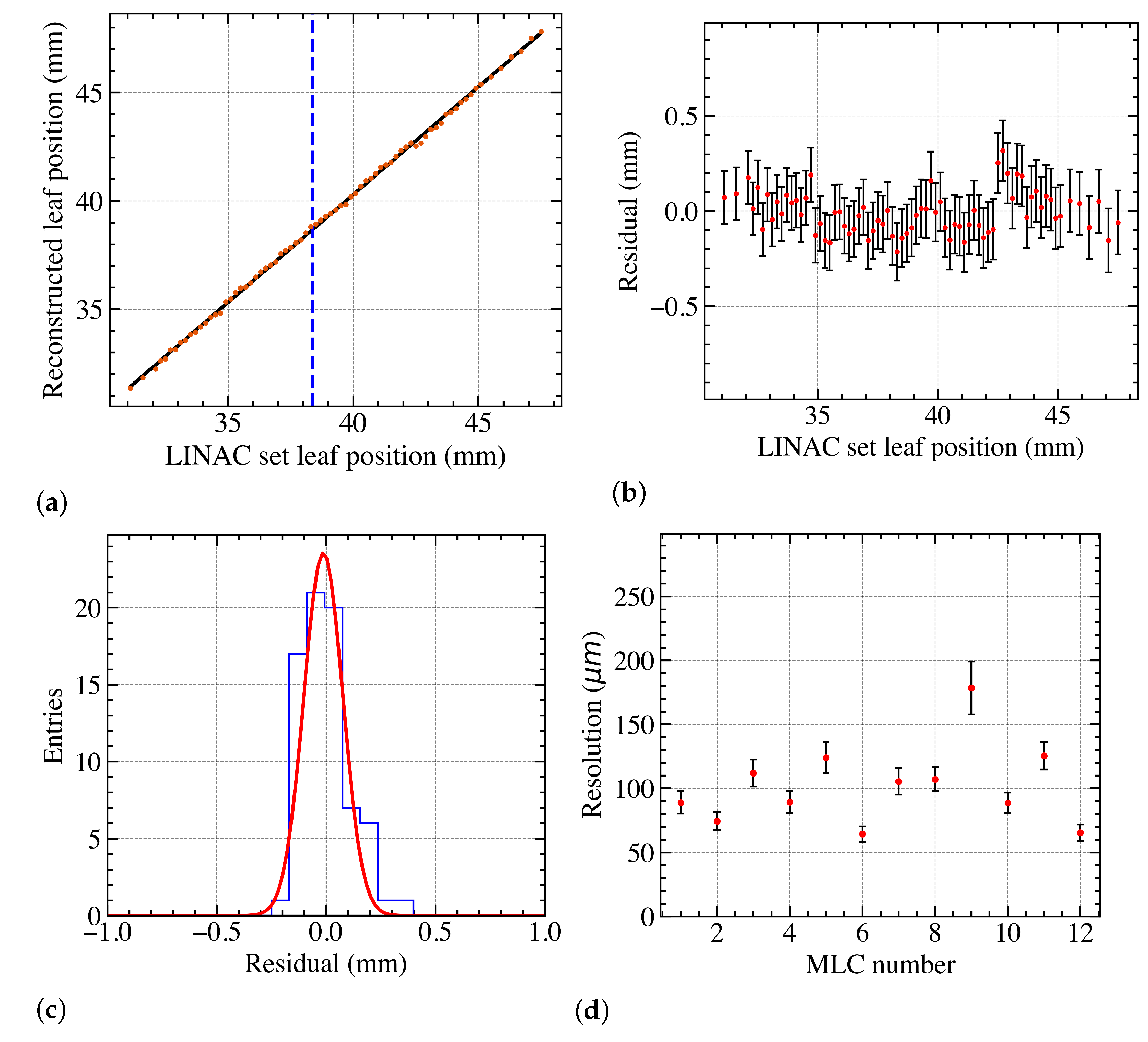
3.2.1. The Matching Index Method
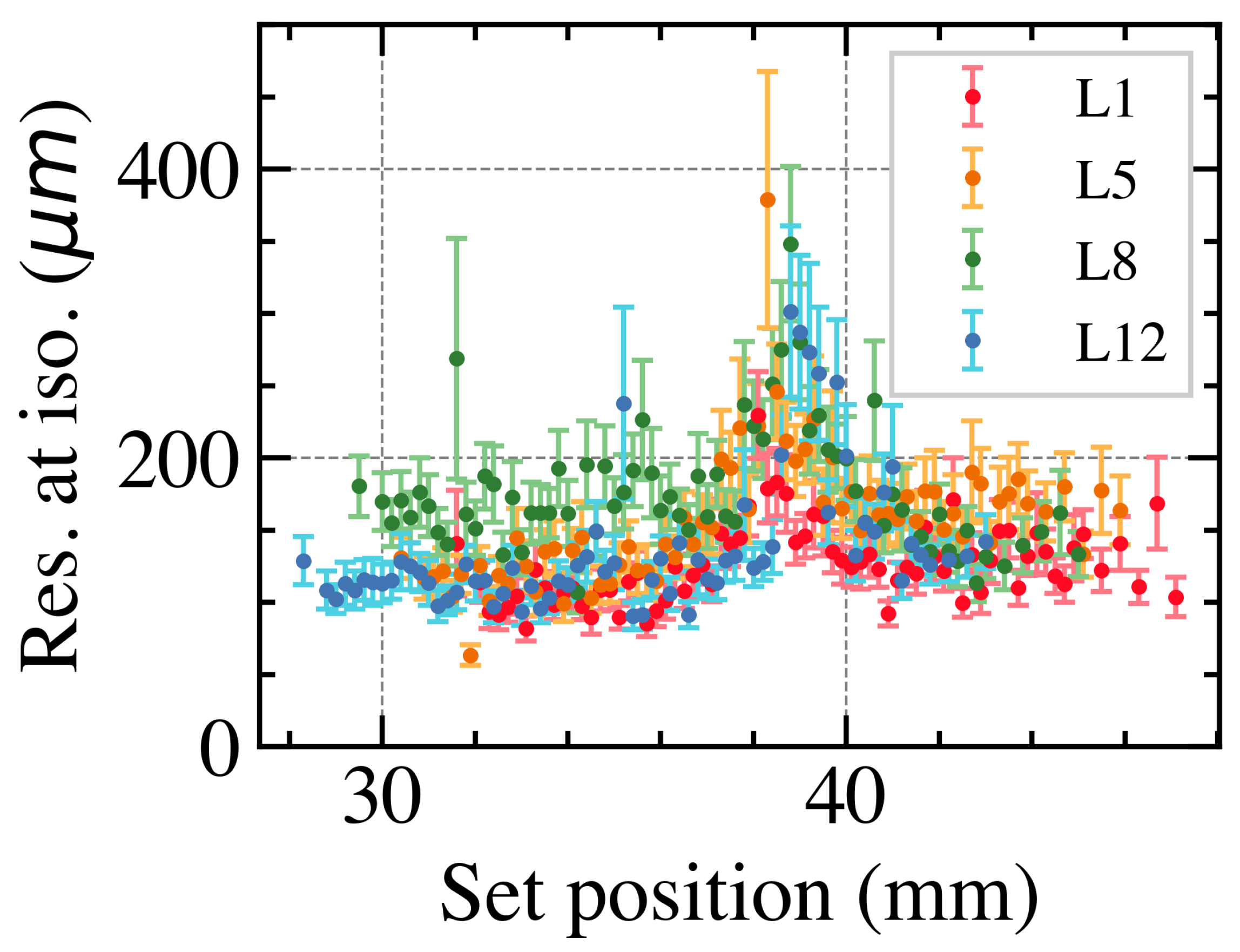
3.2.2. Overall Position Resolution with Leaves Perpendicular to the Dead Area
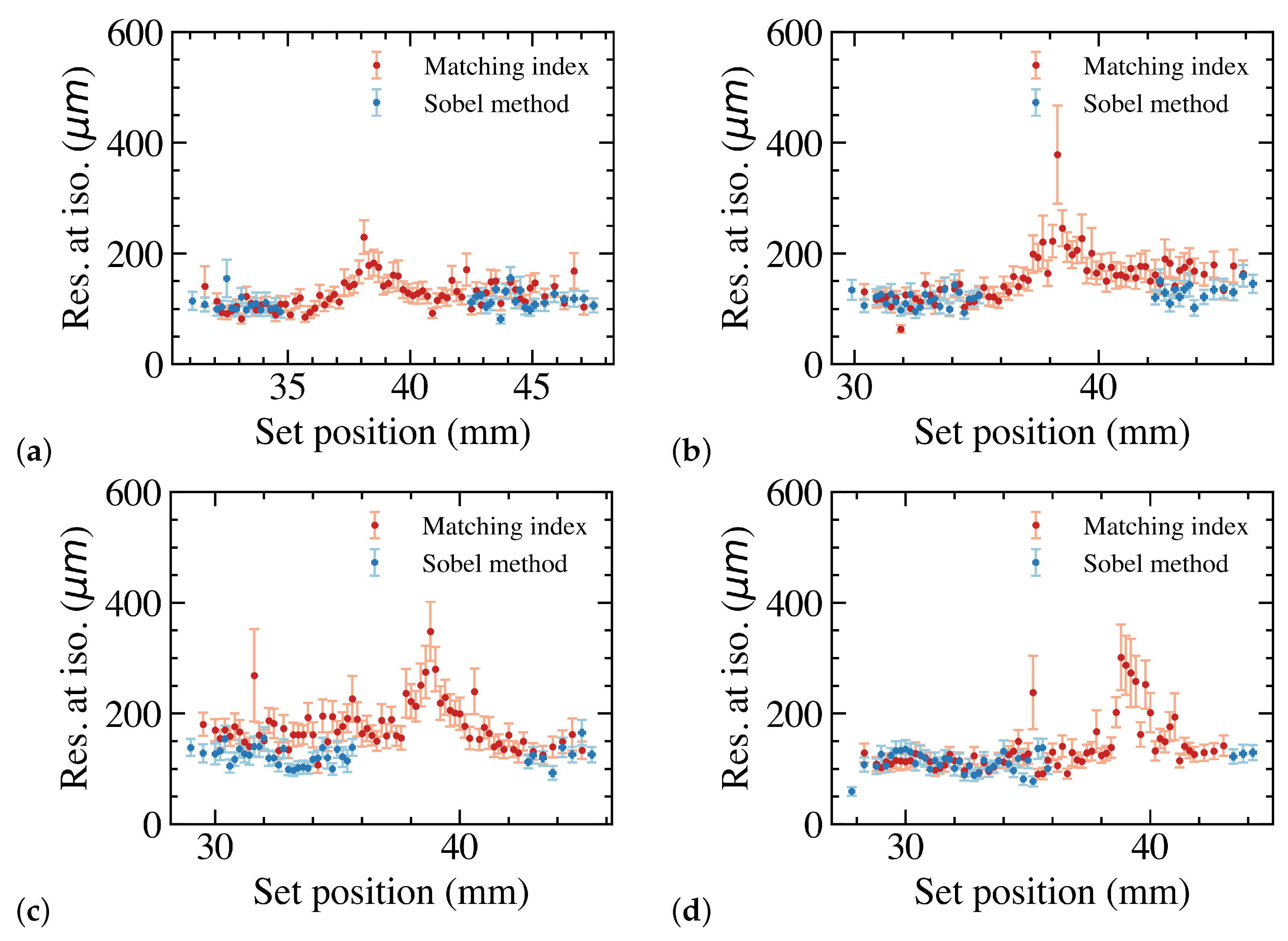
3.3. Overall Position Resolution with Leaves along the Dead Area
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EBRT | external beam radiotherapy |
| IMRT | intensity-modulated radiation therapy |
| LINAC | linear accelerator |
| MLC | multileaf collimator |
| EPID | electronic portal imaging device |
| CMOS | complementary metal-oxide-semiconductor |
| MAPS | monolithic active pixel sensors |
| PCB | printed circuit board |
| FF | flattening filter |
| FFF | flattening filter free |
References
- Staffurth, J. A Review of the Clinical Evidence for Intensity-modulated Radiotherapy. Clin. Oncol. 2010, 22, 643–657. [Google Scholar] [CrossRef]
- Pritchard, J.L.; Velthuis, J.J.; Beck, L.; De Sio, C.; Hugtenburg, R.P. High-Resolution MLC Leaf Position Measurements With a Large Area MAPS. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 5, 392–397. [Google Scholar] [CrossRef]
- Van Elmpt, W.; McDermott, L.; Nijsten, S.; Wendling, M.; Lambin, P.; Mijnheer, B. A literature review of electronic portal imaging for radiotherapy dosimetry. Radiother. Oncol. 2008, 88, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, E.; Radici, L.; Borca, V.C.; Ferrario, S.; Paolini, M.; Pasquino, M. Delta4 Discover transmission detector: A comprehensive characterization for in-vivo VMAT monitoring. Phys. Medica 2021, 85, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hoegele, W.; Zygmanski, P. Strip detector array (SDA) for beam monitoring in radiotherapy: Reconstruction of MLC parameters from multiple projections of flux. Biomed. Phys. Eng. Express 2022, 8, 055011. [Google Scholar] [CrossRef] [PubMed]
- Olaciregui-Ruiz, I.; Beddar, S.; Greer, P.; Jornet, N.; McCurdy, B.; Paiva-Fonseca, G.; Mijnheer, B.; Verhaegen, F. In vivo dosimetry in external beam photon radiotherapy: Requirements and future directions for research, development, and clinical practice. Phys. Imaging Radiat. Oncol. 2020, 15, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.W.; Moloney, S.; Bangiri, A.; Blackmore, A.; Hart, C.; Holmes-Smith, W.; Pooler, A.; Rixham, P.; Doolan, P. IPEM topical report: Results of a 2020 UK survey on the use of online treatment monitoring solutions for IMRT/VMAT. Phys. Med. Biol. 2021, 66, 22TR02. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Muir, B. Assessing the accuracy of electronic portal imaging device (EPID)-based dosimetry: I. Quantities influencing long-term stability. Med. Phys. 2022, 49, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Muir, B. Assessing the accuracy of electronic portal imaging device (EPID)-based dosimetry: II. Evaluation of a dosimetric uncertainty budget and development of a new film-in-EPID absorbed dose calibration methodology. Med. Phys. 2022, 49, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Paxton, A.B.; Sarkar, V.; Kunz, J.N.; Szegedi, M.; Zhao, H.; Huang, Y.J.; Nelson, G.; Rassiah, P.; Su, F.C.F.; Salter, B.J. Evaluation of the effects of implementing a diode transmission device into the clinical workflow. Phys. Med. 2020, 80, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Brace, O.J.; Fuduli, I.; Alnaghy, S.; Le, A.T.; Davis, J.A.; Causer, T.; Wilkinson, D.; Perevertaylo, A.; Rosenfeld, A.B.; Petasecca, M. A large area pixelated silicon array detector for independent transit in vivo dosimetry. Appl. Sci. 2022, 12, 537. [Google Scholar] [CrossRef]
- Esposito, M.; Piermattei, A.; Bresciani, S.; Orlandini, L.C.; Falco, M.D.; Giancaterino, S.; Cilla, S.; Ianiro, A.; Nigro, R.; Botez, L.; et al. Improving dose delivery accuracy with EPID in vivo dosimetry: Results from a multicenter study. Strahlenther. Und Onkol. 2021, 197, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Musoll, A.; Delgado-Tapia, P.; Gisbert, M.L.; Sala, N.J.; Sempau, J. 2022. Transit-guided radiation therapy: Proof of concept of an on-line technique for correcting position errors using transit portal images. Phys. Med. Biol. 2022, 67, 155022. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rastogi, N.; Das, K.M.; Yoganathan, S.A.; Udayakumar, D.; Naresh, R.; Kumar, S. Evaluating the dosimetric consequences of MLC leaf positioning errors in dynamic IMRT treatments. J. Radiother. Pract. 2019, 18, 225–231. [Google Scholar] [CrossRef]
- Page, R.F.; Abbott, N.L.; Davies, J.; Dyke, E.L.; Randles, H.J.; Velthuis, J.J.; Fletcher, S.; Gregory, S.D.; Hall, C.; John, A.; et al. Towards using a Monolithic Active Pixel Sensor for in vivo beam monitoring of Intensity Modulated Radiotherapy. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2013, 731, 295–298. [Google Scholar] [CrossRef]
- De Sio, C.; Velthuis, J.J.; Beck, L.; Pritchard, J.L.; Hugtenburg, R.P. r-Unet: Leaf Position Reconstruction in Upstream Radiotherapy Verification. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 272–279. [Google Scholar] [CrossRef]
- Pritchard, J.L.; Velthuis, J.J.; Beck, L.; Li, Y.; De Sio, C.; Ballisat, L.; Hugtenburg, R.P. Complex field verification using a large area CMOS MAPS upstream in radiotherapy. J. Instrum. 2022, 17, C08018. [Google Scholar] [CrossRef]
- Beck, L.; Velthuis, J.; Fletcher, S.; Haynes, J.; Page, R. Using a TRAPS upstream transmission detector to verify multileaf collimator positions during dynamic radiotherapy delivery. Appl. Rad. Isot. 2020, 156, 108951. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J. Multileaf Collimator Position Measurements Using a Pixel Sensor. Ph.D. Thesis, University of Bristol, Bristol, UK, 2023. [Google Scholar]
- Velthuis, J.; Hugtenburg, P.R.; Hall, C.; Page, R.; Stevens, P. Monitoring of a Therapeutic X-ray Beam Using an Active Pixel Sensor Detector. EU Patent EP2654895B1, 4 March 2018. [Google Scholar]
- Velthuis, J.; Hugtenburg, P.R.; Hall, C.; Page, R.; Stevens, P. Upstream Direct X-ray Detection. U.S. Patent No. 9,517,358, 13 December 2016. [Google Scholar]
- Beck, L.; Velthuis, J.J.; Page, R.F.; Hugtenburg, R.P.; De Sio, C.; Pritchard, J. A novel approach to contamination suppression in transmission detectors for radiotherapy. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 4, 637–643. [Google Scholar] [CrossRef]
- Beck, L.; Velthuis, J.; De Sio, C.; Pritchard, J.; Li, Y.; Hugtenburg, R. Towards 2D dosimetry using monolithic active pixel sensors and a copper grating. J. Instrum. 2022, 17, C12021. [Google Scholar] [CrossRef]
- Velthuis, J.; Page, R.; Hugtenburg, R.; Stevens, P. Radiation. Detector. Patent WO/2018/162904, 8 April 2021. [Google Scholar]
- Sedgwick, I.; Das, D.; Guerrini, N.; Marsh, B.; Turchetta, R. LASSENA: A 6.7 megapixel, 3-sides buttable wafer-scale CMOS sensor using a novel grid-addressing architecture. In Proceedings of the 2013 International Image Sensor Workshop, Snowbird Resort, UT, USA, 12–16 June 2013; pp. 3–6. [Google Scholar]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients (Version 1.4); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://physics.nist.gov/xaamdi (accessed on 4 October 2022).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velthuis, J.; Li, Y.; Pritchard, J.; De Sio, C.; Beck, L.; Hugtenburg, R. Performance of a Full-Scale Upstream MAPS-Based Verification Device for Radiotherapy. Sensors 2023, 23, 1799. https://doi.org/10.3390/s23041799
Velthuis J, Li Y, Pritchard J, De Sio C, Beck L, Hugtenburg R. Performance of a Full-Scale Upstream MAPS-Based Verification Device for Radiotherapy. Sensors. 2023; 23(4):1799. https://doi.org/10.3390/s23041799
Chicago/Turabian StyleVelthuis, Jaap, Yutong Li, Jordan Pritchard, Chiara De Sio, Lana Beck, and Richard Hugtenburg. 2023. "Performance of a Full-Scale Upstream MAPS-Based Verification Device for Radiotherapy" Sensors 23, no. 4: 1799. https://doi.org/10.3390/s23041799




