Evolution of Portable Sensors for In-Vivo Dose and Time-Activity Curve Monitoring as Tools for Personalized Dosimetry in Molecular Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Identified Devices
3.1.1. Thermo-Luminescence Dosimeters (TLD)
3.1.2. External Probes
3.1.3. Portable Scintillators
3.1.4. MOSFET
3.1.5. Fiber-Coupled Inorganic Scintillators (FcIS)
3.1.6. WIDMApp
3.2. Cost and Portability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, S.M.O.; Thomas, S.; Pinheiro, M.A.; Coelho, F.D.A.R.F.B.; Albernaz, M.D.S.; Dos Santos, C.L.G.; Berdeguez, M.B.T.; Zikan, F.; Medeiros, P.D.C.; Sampaio, D.D.C.P.; et al. Internal radiation dose and modeling codes in nuclear medicine: A fresh look at old problems. Int. J. Radiol. Radiat. Ther. 2017, 4, 439–443. [Google Scholar] [CrossRef]
- Della Gala, G.; Bardiès, M.; Tipping, J.; Strigari, L. Overview of commercial treatment planning systems for targeted radionuclide therapy. Phys. Med. 2021, 92, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Strigari, L.; Konijnenberg, M.; Chiesa, C.; Bardies, M.; Du, Y.; Gleisner, K.S.; Lassmann, M.; Flux, G. The evidence base for the use of internal dosimetry in the clinical practice of molecular radiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1976–1988. [Google Scholar] [CrossRef] [PubMed]
- Hardiansyah, D.; Attarwala, A.A.; Kletting, P.; Mottaghy, F.M.; Glatting, G. Prediction of time-integrated activity coefficients in PRRT using simulated dynamic PET and a pharmacokinetic model. Phys. Med. 2017, 42, 298–304. [Google Scholar] [CrossRef]
- Lassmann, M.; Chiesa Flux, G.; Bardiès, M. EANM Dosimetry Committee guidance document: Good practice of clinical dosimetry reporting. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Bolch, W.E.; Bouchet, L.G.; Robertson, J.S.; Wessels, B.W.; Siegel, J.A.; Howell, R.W.; Erdi, A.K.; Aydogan, B.; Costes, S.; Watson, E.E.; et al. MIRD Pamphlet No. 17: The Dosimetry of Nonuniform Activity Distributions-Radionuclide S Values at the Voxel Level. J. Nucl. Med. 1999, 40, 11S–36S. [Google Scholar] [PubMed]
- Rinscheid, A.; Lee, J.; Kletting, P.; Beer, A.J.; Glatting, G. A simulation-based method to determine optimal sampling schedules for dosimetry in radioligand therapy. Z. Med. Phys. 2019, 29, 314–325. [Google Scholar] [CrossRef]
- Gleisner, K.S.; Spezi, E.; Solny, P.; Gabina, P.M.; Cicone, F.; Stokke, C.; Chiesa, C.; Paphiti, M.; Brans, B.; Sandström, M.; et al. Variations in the practice of molecular radiotherapy and implementation of dosimetry: Results from a European survey. EJNMMI Phys. 2017, 4, 28. [Google Scholar] [CrossRef]
- Glatting, G.; Kletting, P.; Reske, S.N.; Hohl, K.; Ring, C. Choosing the optimal fit function: Comparison of the Akaike information criterion and the F-test. Med. Phys. 2007, 34, 4285–4292. [Google Scholar] [CrossRef]
- Sarrut, D.; Halty, A.; Badel, J.N.; Ferrer, L.; Bardies, M. Voxel-based multimodel fitting method for modeling time activity curves in SPECT images. Med. Phys. 2017, 44, 6280–6288. [Google Scholar] [CrossRef]
- Hellingman, D.; Vidal-Sicart, S. The Use of Intraoperative Small and Large Field of View Gamma Cameras for Radioguided Surgery. In Radioguided Surgery; Herrmann, K., Nieweg, O., Povoski, S., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Solfaroli Camillocci, E.; Bocci, V.; Chiodi, G.; Collamati, F.; Donnarumma, R.; Faccini, R.; Mancini Terracciano, C.; Marafini, M.; Mattei, I.; Muraro, S.; et al. Intraoperative probe detecting β- decays in brain tumour radio-guided surgery. NIMA 2017, 845, 689–692. [Google Scholar] [CrossRef]
- Council Directive 2013/59/Euratom of 5 December 2013 Laying down Basic Safety Standards for Protection against the Dangers Arising from Exposure to Ionising Radiation, and Repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/ 122/Euratom. Official Journal of the European Union L-13 of 17 January 2014. Available online: https://eurlex.europa.eu/eli/dir/2013/59/oj (accessed on 1 February 2023).
- Sgouros, G.; Bolch, W.E.; Chiti, A.; Dewaraja, Y.K.; Emfietzoglou, D.; Hobbs, R.F.; Konijnenberg, M.; Sjögreen-Gleisner, K.; Strigari, L.; Yen, C.; et al. ICRU REPORT 96, Dosimetry-Guided Radiopharmaceutical Therapy. J. ICRU 2022, 21, 1–212. [Google Scholar] [CrossRef]
- Aschan, A.C.; Toivonen, M.J.; Lampinen, J.S.; Tenhunen, M.; Kairemo, K.J.A.; Tapani Korppi-Tommola, E.; Jekunen, A.P.; Sipilä, P.; Savolainen, S.E. The Use of TL Detectors in Dosimetry of Systemic Radiation Therapy. Acta Oncol. 1999, 38, 2. [Google Scholar] [CrossRef]
- Gambarini, G.; Borroni, M.; Grisotto, S.; Maucione, A.; Cerrotta, A.; Fallai, C.; Carrara, M. Solid state TL detectors for in vivo dosimetry in brachytherapy. Appl. Radiat. Isot. 2012, 71, 48–51. [Google Scholar] [CrossRef]
- Bahamonde, S.; Díaz-Londoño, G.M.; García, M.; Albornoza, F.; Andrade, P. Design and implementation of a mobile gamma spectrometry system to in vivo measure the accumulated activity of 131I in patients with thyroid diseases. Appl. Radiat. Isot. 2017, 129, 87–95. [Google Scholar] [CrossRef]
- Brinks, P.; Van Gils k Kranenborg, E.; Lavalaye, J.; Dickerscheid, D.B.M.; Habraken, J.B.A. Measuring the actual I-131 thyroid uptake curve with a collar detector system: A feasibility study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 935–940. [Google Scholar] [CrossRef]
- Abuqbeltah, M.; Demir, M.; Yeyln, N.; Sager, S.; Gray, D. Thyroid uptake test with portable device (COTI) after 131I tracer administration: Proof of concept. Radiat. Environ. Biophys. 2020, 59, 553–558. [Google Scholar] [CrossRef]
- Williams, J.M.; Arlinghaus, L.R.; Rani, S.D.; Shone, M.D.; Abramson, V.G.; Pendyala, P.; Chakravarthy, A.B.; Gorge, W.J.; Knowland, J.G.; Lattanze, R.K.; et al. Towards real-time topical detection and characterization of FDG dose infiltration prior to PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2374–2380. [Google Scholar] [CrossRef]
- Lattanze, R.K.; Osman, M.M.; Ryan, K.A.; Frye, S.; Townsend, D.W. Usefulness of Topically Applied Sensors to Assess the Quality of 18F-FDG Injections and Validation Against Dynamic Positron Emission Tomography (PET) Images. Front. Med. 2018, 5, 303. [Google Scholar] [CrossRef]
- Haughey, A.; Coalter, G.; Mugabe, K. Evaluation of linear array MOSFET detectors for in vivo dosimetry to measure rectal dose in HDR brachytherapy. Australas Phys. Eng. Sci. Med. 2011, 34, 361–366. [Google Scholar] [CrossRef]
- Carrara, M.; Tenconi, C.; Rossi, G.; Borroni, M.; Cerrotta, A.; Grisotto, S.; Cusumano, D.; Pappalardi, B.; Cutajar, D.; Petasecca, M.; et al. In-vivo rectal wall measurements during HDR prostate brachytherapy with MOSkin dosimeters integrated on a trans-rectal US probe: Comparison with planned and reconstructed doses. Radiother. Oncol. 2016, 118, 148–153. [Google Scholar] [CrossRef]
- Carrara, M.; Romanyukha, A.; Tenconi, C.; Mazzeo, D.; Cerrotta, A.; Borroni, M.; Cutajar, D.; Petasecca, M.; Lerch, M.; Bucci, J.; et al. Clinical application of MOSkin dosimeters to rectal wall in vivo dosimetry in gynecological HDR brachytherapy. Phys. Med. 2017, 41, 5–12. [Google Scholar] [CrossRef]
- Jamalludin, Z.; Jong, W.L.; Malik, R.A.; Rosenfeld, A.B.; Ung, U.M. Evaluation of rectal dose discrepancies between planned and in vivo T dosimetry using MOSkin detector and PTW 9112 semiconductor probe during 60Co HDR CT-based intracavitary cervix brachytherapy. Phys. Med. 2020, 69, 52–60. [Google Scholar] [CrossRef]
- Jamalludin, Z.; Malik, R.A.; Ung, N.M. Correlation analysis of CT-based rectal planning dosimetric parameters with in vivo dosimetry of MOSkin and PTW 9112 detectors in Co-60 source HDR intracavitary cervix brachytherapy. Phys. Eng. Sci. Med. 2021, 44, 773–783. [Google Scholar] [CrossRef]
- Jørgensen, E.B.; Buus, S.; Bentzen, L.; Hokland, S.B.; Rylander, S.; Kertzscher, G.; Beddar, S.; Tanderup, K.; Johansen, J.G. 3D dose reconstruction based on in vivo dosimetry for determining the dosimetric impact of geometric variations in high-dose-rate prostate brachytherapy. Radiother. Oncol. 2022, 171, 62–68. [Google Scholar] [CrossRef]
- Jørgensen, E.B.; Johansen, J.G.; Overgaard, J.; Piché-Meunier, D.; Tho, D.; Rosales, H.M.L.; Tanderup, K.; Beaulieu, L.; Kertzscher, G.; Beddar, S. A high-Z inorganic scintillator–based detector for time-resolved in vivo dosimetry during brachytherapy. Med. Phys. 2021, 48, 7382–7398. [Google Scholar] [CrossRef]
- Debnath, S.B.C.; Ferre, M.; Tonneau, D.; Fauquet, C.; Tallet, A.; Goncalves, A.; Darreon, J. High resolution small-scale inorganic scintillator detector: HDR brachytherapy application. Med. Phys. 2021, 48, 1485–1496. [Google Scholar] [CrossRef]
- Morganti, S.; Collamati, F.; Faccini, R.; Iaccarino, G.; Mancini-Terracciano, C.; Mirabelli, R.; Nicolanti, F.; Pacilio, M.; Soriani, A.; Solfaroli-Camillocci, E.; et al. Technical note: A wearable radiation measurement system for collection of patient-specific time-activity data in radiopharmaceutical therapy: System design and Monte Carlo simulation results. Med. Phys. 2021, 48, 8117–8126. [Google Scholar] [CrossRef]
- Knowland, J.G.; Scarantino, C.W.; Lattanze, R.K. System for the Detection of Gamma Radiation from a Radioactive Analyte. U.S. Patents 20130324844 A1, 5 December 2013. [Google Scholar]
- Qi, Z.-Y.; Deng, X.-W.; Huang, S.-M.; Lu, J.; Lerch, M.; Cutajar, D.; Rosenfeld, A. Verification of the plan dosimetry for high dose rate brachytherapy using metal–oxide–semiconductor field effect transistor detectors. Med. Phys. 2007, 34, 2007–2013. [Google Scholar] [CrossRef]
- Kertzscher, G.; Beddar, S. Inorganic scintillation detectors for 192Ir brachytherapy. Phys. Med. Biol. 2019, 64, 225018. [Google Scholar] [CrossRef]
- Kaveckyte, V.; Jørgensen, E.B.; Kertzscher, G.; Johansen, J.G.; Carlsson Tedgren, Å. Monte Carlo characterization of high atomic number inorganic scintillators for in vivo dosimetry in 192 Ir brachytherapy. Med. Phys. 2022, 49, 4715–4730. [Google Scholar] [CrossRef]
- Bunjes, D.; Buchmann, I.; Duncker, C.; Seitz, U.; Kotzerke, J.; Wiesneth, M.; Dohr, D.; Stefanic, M.; Buck, A.; Harsdorf, S.V.; et al. Rhenium 188-labeled anti-CD66 (a, b, c, e) monoclonal antibody to intensify the conditioning regimen prior to stem cell transplantation for patients with high-risk acute myeloid leukemia or myelodysplastic syndrome: Results of a phase I-II study. Blood 2001, 98, 565–572. [Google Scholar] [CrossRef]
- Glatting, G.; Kull, T.; Blumstein, N.M.; Bunjes, D.; Neumaier, B.; Buck, A.; Reske, S.N. Dosimetry with 188Re-labelled monoclonal anti-CD66 antibodies: A simplified approach based on a single measurement 3 h.p.i. Nuklearmedizin 2006, 45, 134–138. [Google Scholar]
- De Sadeleer, C.; Van Laere, K.; Georges, B.; Piepsz, A.; Ham, H.R. Influence of time interval and number of blood samples on the error in renal clearance determination using a mono-exponential model: A Monte Carlo simulation. Nucl. Med. Commun. 2000, 21, 741–745. [Google Scholar] [CrossRef]
- De Sadeleer, C.; Piepsz, A.; Ham, H.R. Influence of errors in sampling time and in activity measurement on the single sample clearance determination. Nucl. Med. Commun. 2001, 22, 429–432. [Google Scholar] [CrossRef]
- Kuruc, A.; Treves, S.T.; Rosen, P.R.; Greenberg, D. Estimating the plasma time-activity curve during radionuclide renography. J. Nucl. Med. 1987, 28, 1338–1340. [Google Scholar]
- D’Alessio, D.; Giliberti, C.; Benassi, M.; Strigari, L. Potential third-party radiation exposure from patients undergoing therapy with 131I for thyroid cancer or metastases. Health Phys. 2015, 108, 319–325. [Google Scholar] [CrossRef]
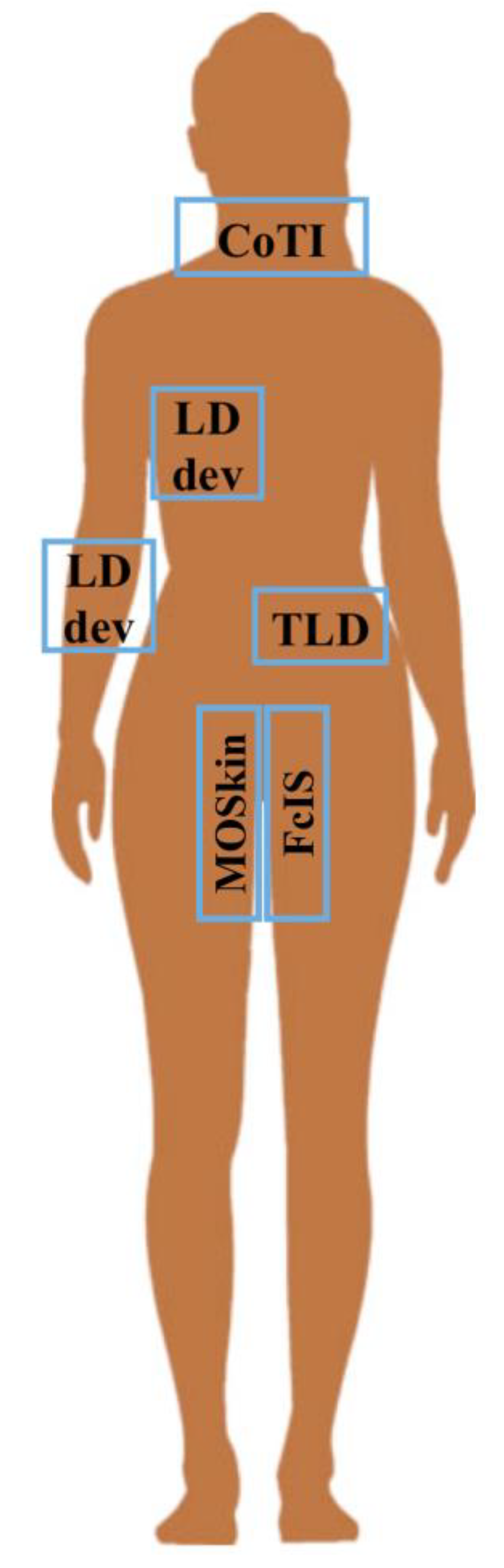

| Device | Description and Adopted Technology | Operation Modality and Read Out System | Tested in MRT | Tested in HDR-BT | Investigated Body Site | N. Involved Patients | Monitored Time Range | Ref. |
|---|---|---|---|---|---|---|---|---|
| TLD | Cheap, easy, portable, wearable and comfortable devices. Thermo-luminescence detectors. BT: 3.2 × 3.2 × 0.9 mm3 | Passive. Offline elaboration by dedicated external read out system. | (a) 131I thyroid treatment: TIA determination | (b) Prostate cancer HDR-BT: point dose measurement | (a) Kidney (b) Prostate | (a) 6 pts. (b) 4 pts | (a) 8 data points in 6 days | (a) [15] (b) [16] |
| External probe | Mobile gamma spectrometry system based on a 7.6 × 7.6 cm2 NaI(Tl) scintillation crystal | Active. Online registration of the average counts | TIA determination in whole body | Thyroid or Whole body | 5 pts | Up to 8 data points in 4 days | [17] | |
| CoTI | Wearable, lightweight collar with up to 4 gamma detectors: 3 × 3 mm2 CsI(Tl) scintillation crystal + SiPM | Active. Electronics read out on board transmits to external control unit for data logging | 131I thyroid uptake measurement | Thyroid | (a) 3 pts (b) 89 pts | (a) Data points for 48 h (b) Data points at 2 and 24 h (81 pts) and up to 96 h (8 pts) | (a) [18] (b) [19] | |
| LDdev | Scintillation crystal and photodetector | Active. Three/four sensors read out by a single box | (a) Routine 18F-FDG PET/CT imaging: TIA determination (b) Locally advanced breast carcinoma: tumour uptake | (a) Injection site, arms and liver (b) Tumour | (a) 21 pts. (b) 8 pts. | 45/60 min | (a) [20] (b) [21] | |
| LAM | Commercial linear array MOSFET detectors inserted in a flatus tube | Active. Integrated read-out electronics | Point dose determination for QA in (a) gynaecological or (b) prostate HDR-BT | Rectum | (a) 4 gynaecological pts (7 sessions in total) (b) 22 prostate pts (2 sessions) | Treatment time range | [22] | |
| microMOSFET/ MOSkin | Trans-rectal probe with 2/4 customized MOSFET Dosimeter (sensitive volume: 4.8 × 10−6 mm3) | Active. Battery operated read-out system or directly connected to laptop | Point dose determination for QA in (a/c) gynaecological or (b) prostate HDR-BT | (a/b) Rectal wall (c) Rectum | (a) 9 pts (26 sessions) (b) 12 pts (18 sessions) | Treatment time range | (a) [23] (b) [24] (c) [25,26] | |
| FcIS | The detector probe consisted of inorganic scintillator crystal glued onto a 15 m-long optical fibre with a 0.5-mm core diameter. The detector probe was placed in the prostate inside a standard BT catheter | Active. The optical reader consisted of a photodiode unit, a data acquisition device, and a single-board computer | Point dose for determining the dosimetric impact of geometric variations during HDR-BT | Prostate | (a) 18 pts (b) phantom | Treatment time range | (a) [27,28] (b) [29] | |
| WIDMApp | Wearable multichannel detector and data processing system | Under development | 131I thyroid: simulation study—not yet tested | Thyroid, Kidneys, Liver, Spleen, Bladder | NO | Treatment time range | [30] |
| Device | Advantages | Limitation Factors | Ref. |
|---|---|---|---|
| TLD | Low dependence on photon energy, dose rate and angular incidence of radiation. Allow several point mapping. | Only integral measurement. Uncertainty due to TLD substitution for TIA measurement. Relative expensive read out system and time-consuming process. | [15,16] |
| External probe | Patient can do measurement her/himself. | Cumbersome. No wearability and low portability. Require relative calibration strategy. | [17] |
| CoTI |
Wearable and portable system. No presence of staff is required. | Long data taking pause at night due to feeling of discomfort experienced by patients wearing collar. | [18,19] |
| LDdev | Data are recorded on a on-board reader and exported afterwards. | Low comfort. Staff assistance is required. | [20,21] |
| LAM | Very small size. Dose rate independence. | Require frequent detector recalibration. Need sensor position. Angular and source energy spectrum dependent. | [22] |
| microMOSFET/ MOSkin | Optimized for high steep dose gradient. Use of markers to identify the actual dosimeter position in CT images. A correction of the angular dependence is necessary. | Need frequent single element calibration. Require very accurate detector element localization and orientation within patient. | [23,24,25,26] |
| FcIS | Optimized for high steep dose gradient. | The detector calibration is directly related to the accuracy of the radial catheter shift. A calibration uncertainty of less than 4.5% results in source-tracking errors smaller than 1 mm for source-to-detector probe distances of less than 45 mm. Moreover, some scintillators used in this research work have shown unstable scintillating intensity, significant afterglow, and require significant energy, temperature, and fibre signal attenuation corrections. | [27,28,29] |
| WIDMApp | Wearable and portable system. Simultaneous monitoring of all the emitting volumes involved. Very accurate TIA assessment (uptake and long-term retain). No constant presence of staff is required. | Under development/no-commercially available. Not yet tested on patients. No voxel-based. | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strigari, L.; Marconi, R.; Solfaroli-Camillocci, E. Evolution of Portable Sensors for In-Vivo Dose and Time-Activity Curve Monitoring as Tools for Personalized Dosimetry in Molecular Radiotherapy. Sensors 2023, 23, 2599. https://doi.org/10.3390/s23052599
Strigari L, Marconi R, Solfaroli-Camillocci E. Evolution of Portable Sensors for In-Vivo Dose and Time-Activity Curve Monitoring as Tools for Personalized Dosimetry in Molecular Radiotherapy. Sensors. 2023; 23(5):2599. https://doi.org/10.3390/s23052599
Chicago/Turabian StyleStrigari, Lidia, Raffaella Marconi, and Elena Solfaroli-Camillocci. 2023. "Evolution of Portable Sensors for In-Vivo Dose and Time-Activity Curve Monitoring as Tools for Personalized Dosimetry in Molecular Radiotherapy" Sensors 23, no. 5: 2599. https://doi.org/10.3390/s23052599
APA StyleStrigari, L., Marconi, R., & Solfaroli-Camillocci, E. (2023). Evolution of Portable Sensors for In-Vivo Dose and Time-Activity Curve Monitoring as Tools for Personalized Dosimetry in Molecular Radiotherapy. Sensors, 23(5), 2599. https://doi.org/10.3390/s23052599






