Study on the Psychological States of Olfactory Stimuli Using Electroencephalography and Heart Rate Variability
Abstract
:1. Introduction
2. Related Studies
2.1. Methods for Estimating Psychological States
2.2. Relationship between Olfactory Stimuli and Psychological States
2.3. Relationship between Olfactory Stimuli and Biological Signals
3. Experimental Method
3.1. Olfactory Stimuli
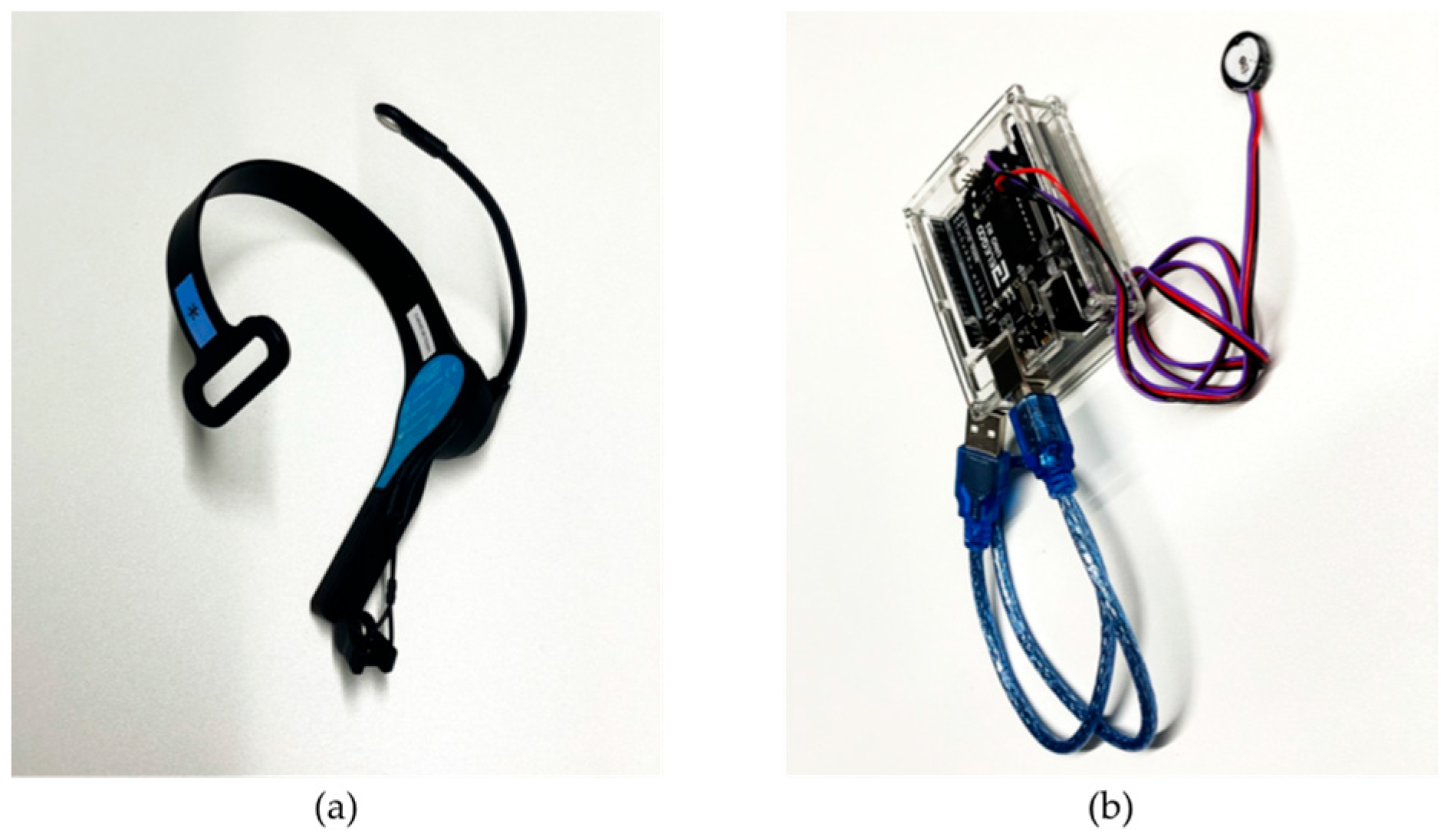
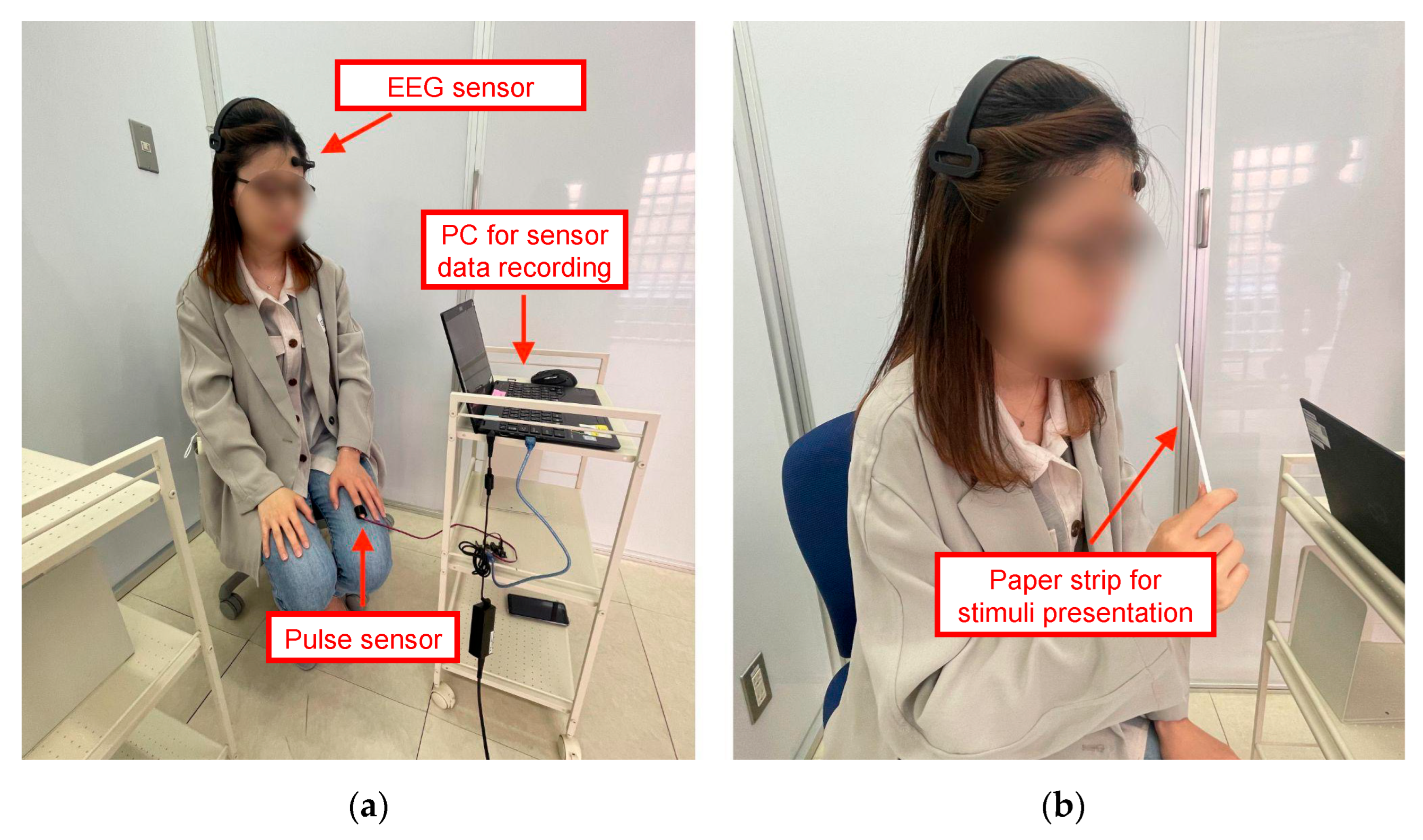
3.2. Measuring Instruments
- EEG sensor (Figure 1a): We placed an electrode near the left frontal lobe, namely, the AF3 channel as defined by the International 10–20 EEG system, using Mindwave Mobile 2 manufactured by NeuroSky. It is a simple, low-invasive single-channel EEG sensor with a sampling rate of 512 Hz. The output from this EEG sensor is acquired approximately once per second.
- Pulse sensor (Figure 1b): We attached the pulse sensor to an index finger using a reflection-type PPG pulse sensor manufactured by SparkFun Electronics that works in combination with an Arduino kit for data transmission to the computer. It is a low-cost, fast-response, and simple sensor with a sampling rate of 500 Hz. The output from this pulse sensor is acquired approximately once per second, along with the EEG output.
3.3. Experimental Setup and Procedure
- Explain the experiment and obtain written informed consent for experiment cooperation;
- Attach pulse sensor and EEG sensor and start sensor recording;
- Perform pre-resting condition by sitting still for 1 min;
- Perform stimulus presentation condition for 1 min;
- Answer the questionnaire about the stimulus;
- Perform post-resting condition by sitting still for 1 min;
- Repeat steps 3 to 6 until all stimuli are presented;
- Stop sensor recording and finish the experiment.
3.4. Participants
4. Data Analysis Method
4.1. EEG and HRV Indexes
4.2. Data Normalization Method
4.3. Data Grouping
- Stimulus exposure time
- ○
- The entire exposure time (60 s for all aromas, 30 s for IVA)
- ○
- Half of the exposure time (first half, latter half)
- Participant gender and generation
- ○
- Male, 20s–30s (age range 20–39 years old) (N = 15)
- ○
- Male, 40s–50s (age range 40–59 years old) (N = 15)
- ○
- Female, 20s–30s (age range 20–39 years old) (N = 17)
- ○
- Female, 40s–50s (age range 40–59 years old) (N = 15)
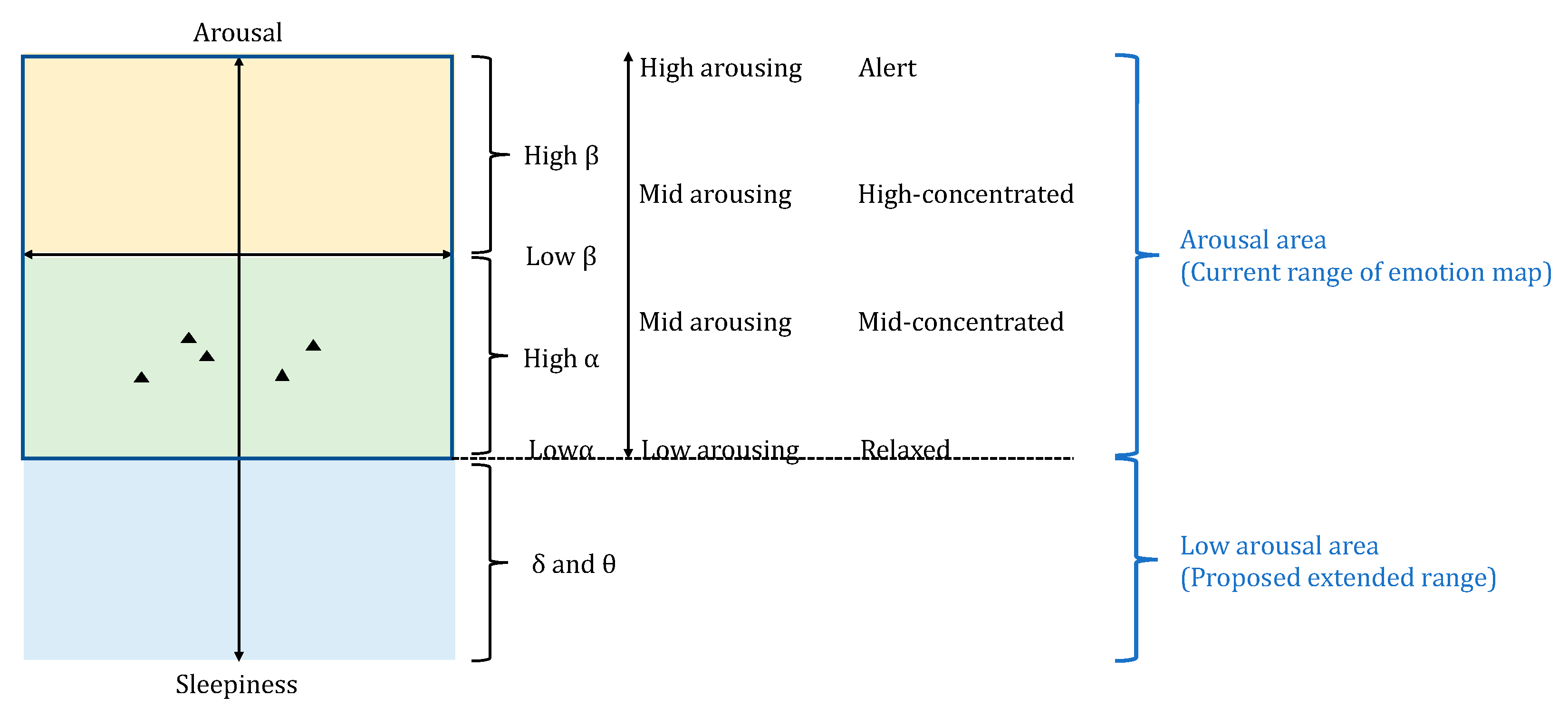
4.4. Data Visualization by Emotion Map
- Attention−Meditation: According to NeuroSky’s definition, Attention and Meditation have values ranging from 0 to 100 [50]. Since we calculated them by subtraction, the range becomes −100 to 100 with the origin point of 0.
- MA Hβ/Hα: Since the MA Hβ/Hα is a ratio value, the minimum value is 0. The maximum value is scaled by the maximum value of all data to include all data on the emotion map. In this paper, we set the maximum value as 2, which covers all data with balanced scaling between negative and positive ranges on the axis.
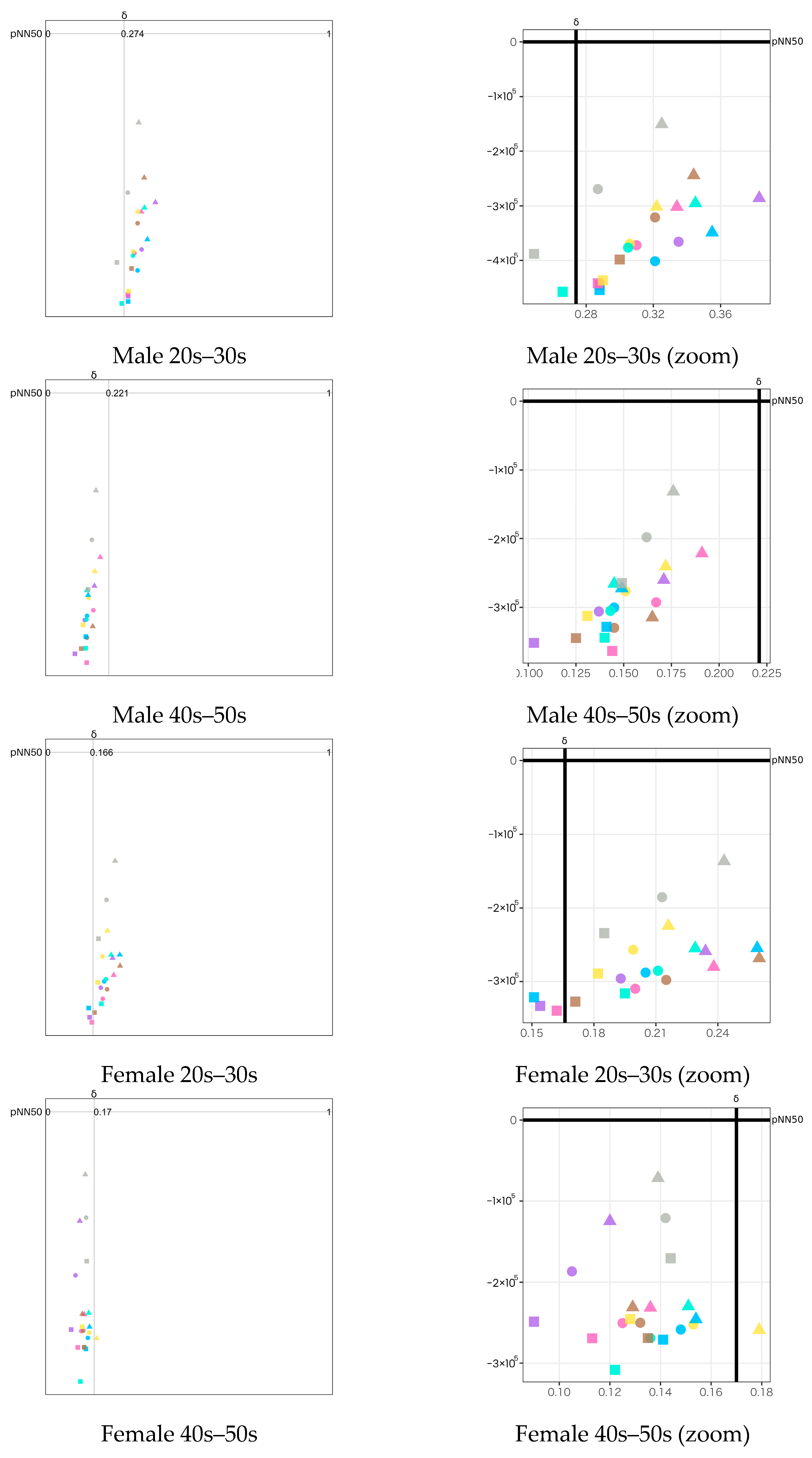
- δ wave: Since we used its raw data, its values represent the magnitude of EEG wave and have no units. Thus, it is meaningful when being considered as relative quantity and temporal fluctuations. We used this index as supporting information to interpret the Attention−Meditation. Therefore, we did not limit the range of this index like other indexes.
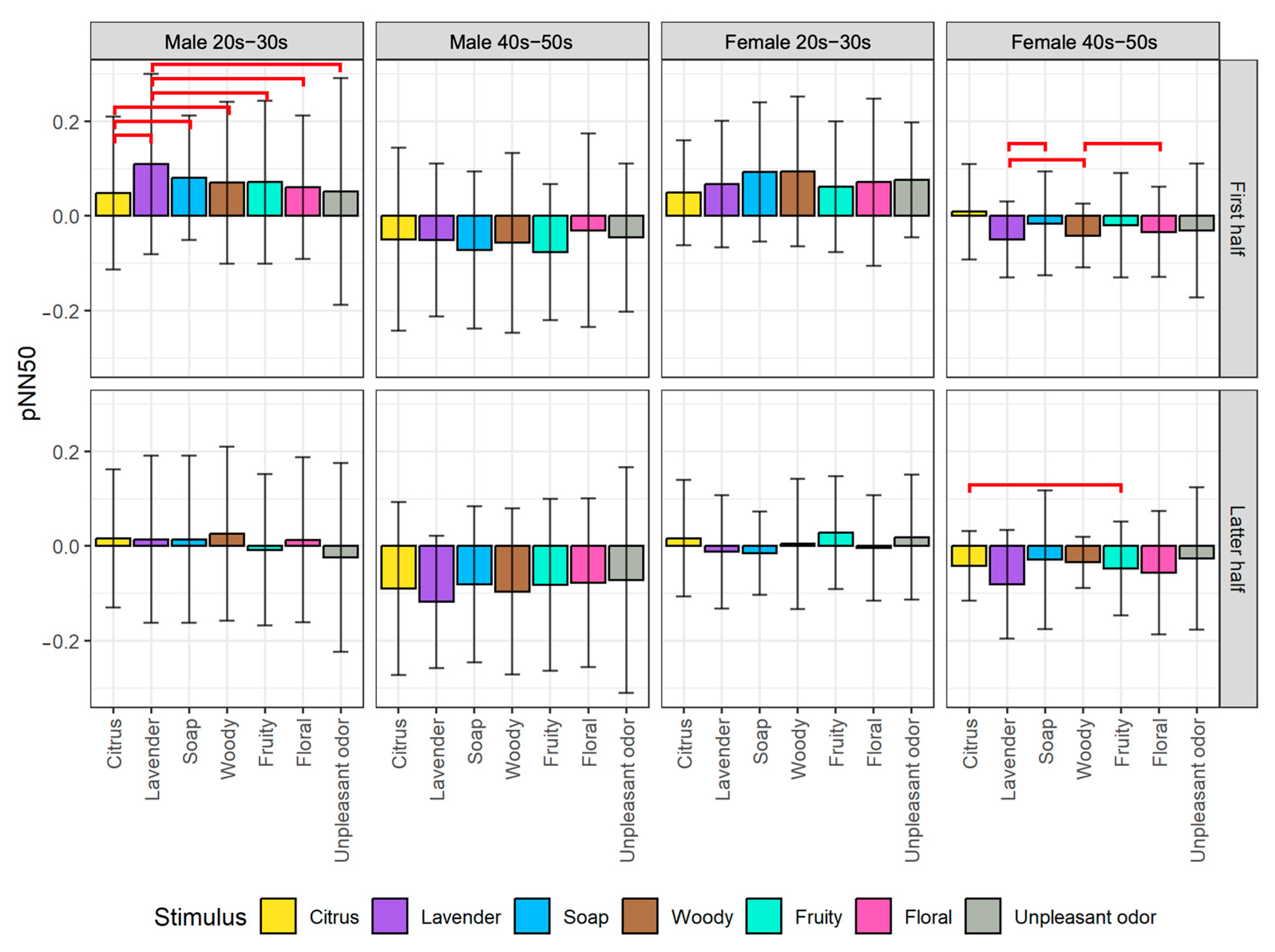
- pNN50: The range is 0 to 1, with the origin calculated from the average resting condition at the start of the experiment. Thus, the origin is different for each emotion map, making the scale between the left and right sides of the origin point unbalanced. Note that we did not use RMSSD to plot the emotion map because it is typically used for the stress-relax index [58], which is slightly different from the pNN50 proposed as the valence index. Thus, we only used it in the statistical analysis to support the interpretation of pNN50.
5. Experimental Results and Discussion
5.1. Statistical Comparison among Olfactory Stimuli
5.1.1. Analysis of EEG Indexes
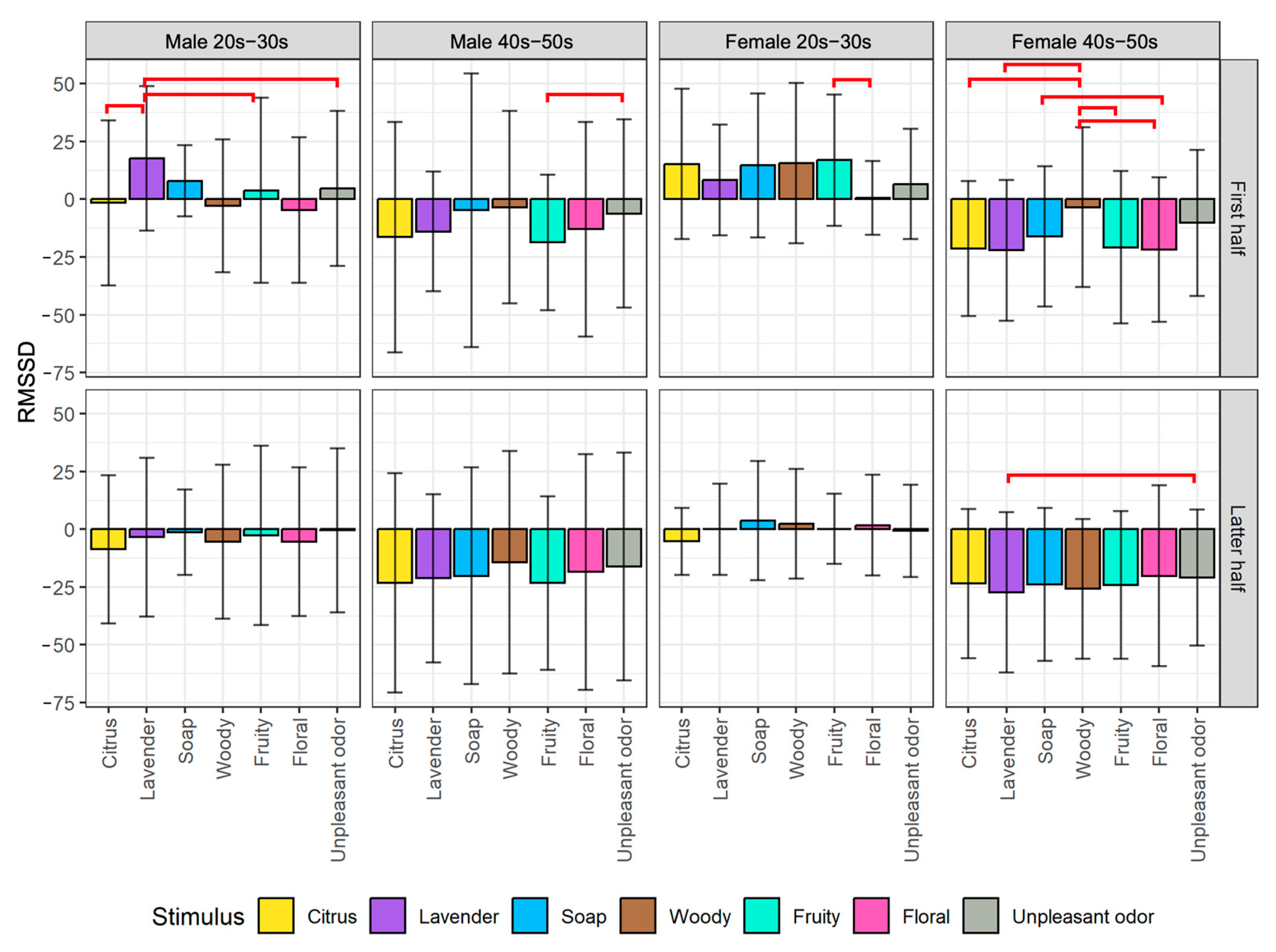
5.1.2. Analysis of HRV Indexes
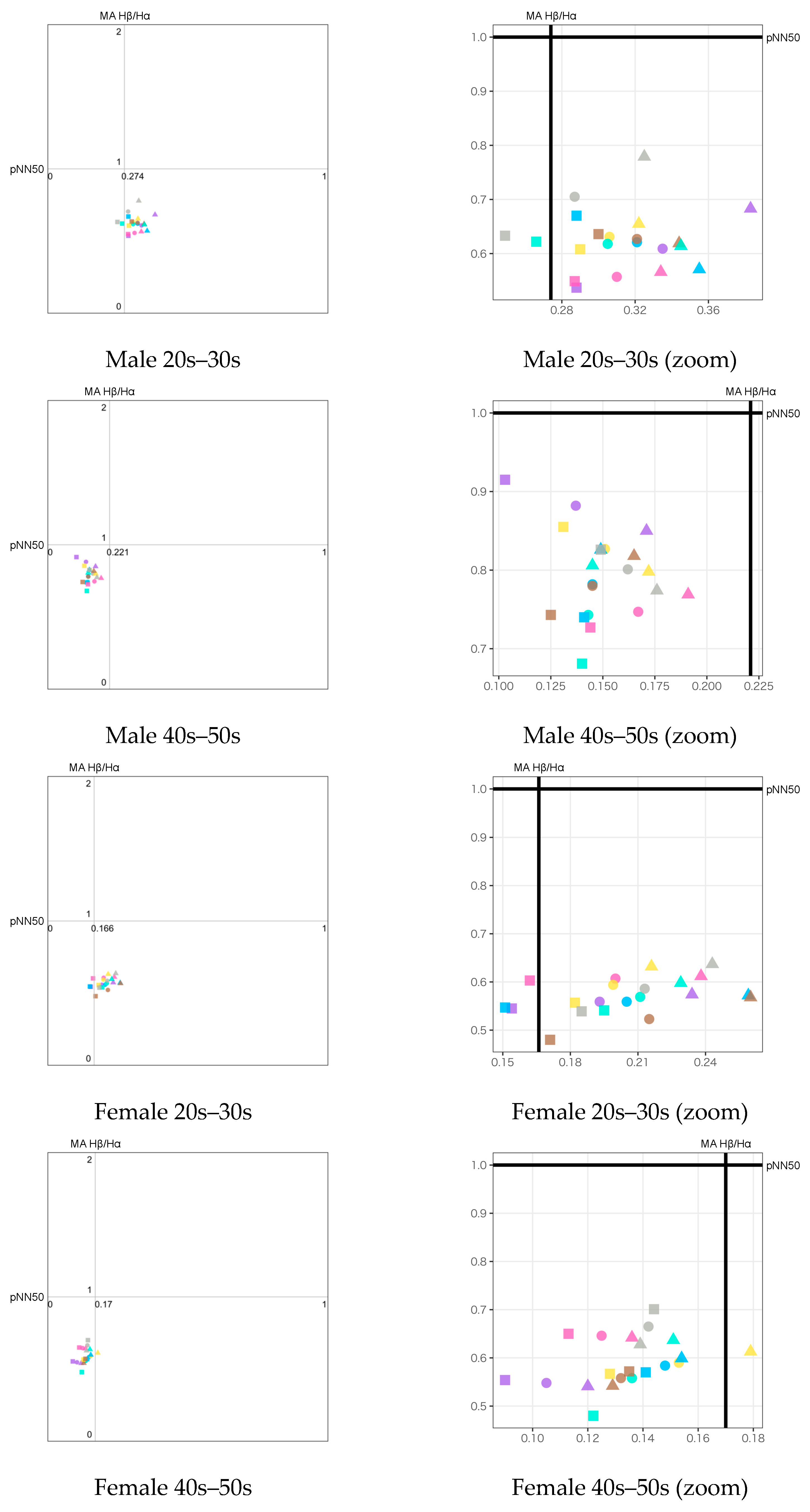
5.2. Emotion Maps
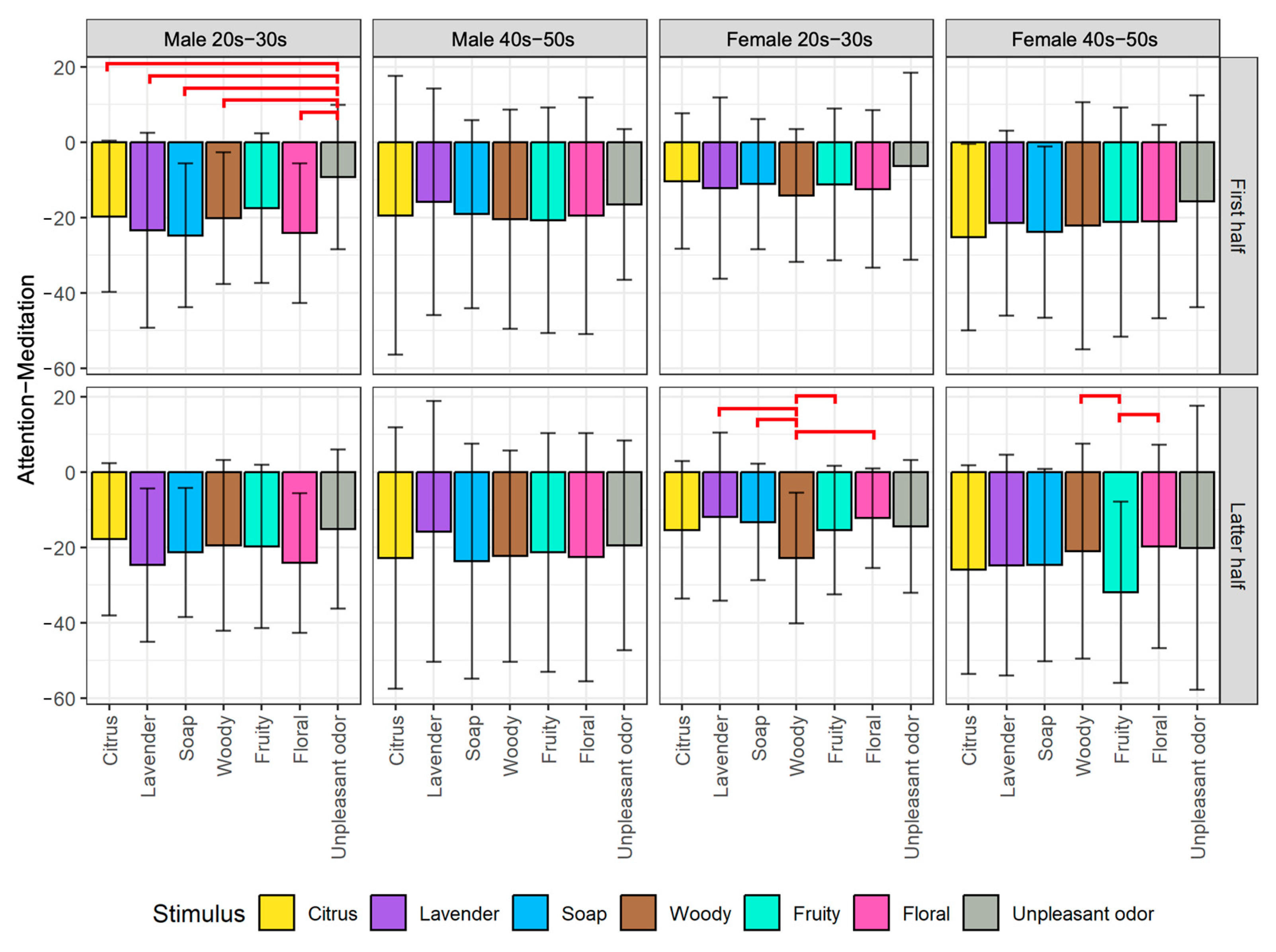
- The emotion maps for both young male and female groups show low arousal and high valence levels (LAHV) near the lower right quadrant.
- The emotion maps of both older male and female groups show low arousal and low valence levels (LALV) near the lower left quadrant.
6. Discussion
- For valence level (pNN50 on X-axis), the emotion maps of the younger generation show a tendency toward parasympathetic nerve dominance or relaxation. On the other hand, the emotion maps of the older generation show a tendency toward sympathetic nerve dominance. These results suggest that the younger generation tends to be more relaxed, while the older generation tends to be more nervous when they smell aromas. For both younger and older generations, genders tend to have less influence on psychological states.
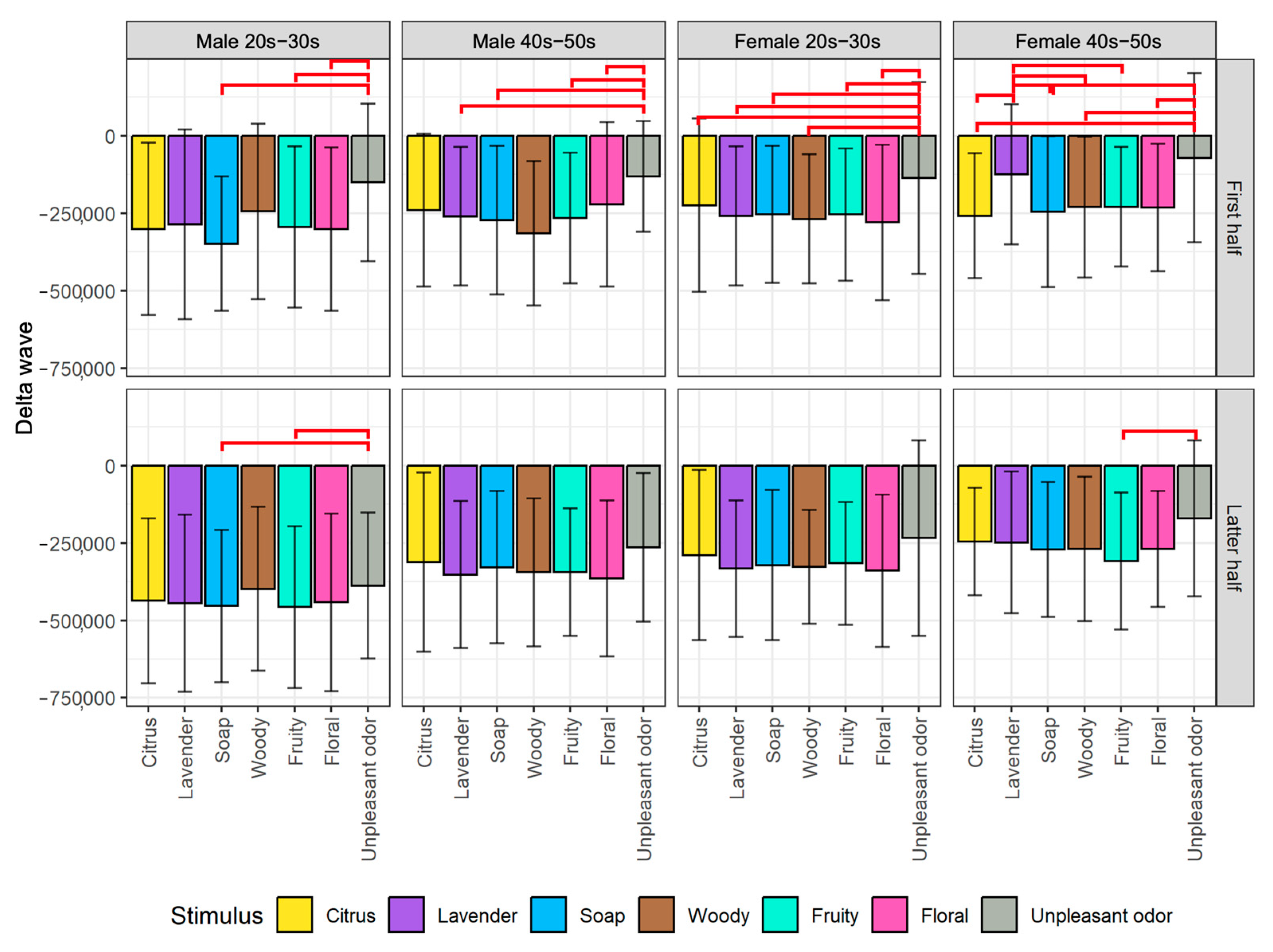
- For arousal level (Attention−Meditation or MA Hβ/Hα on Y-axis), all of the emotion maps show that the values are low. This indicates that the values of Meditation and Hα tend to be larger regardless of any aromas or grouping. According to the literature survey, higher Hα is associated with a more relaxed mental state, which governs overall mental coordination, calmness, alertness, and mind–body integration. These results suggest that all participants may be relaxed and well-focused while smelling the aroma. For the δ wave, the values show certain differences among the olfactory stimuli. Thus, this index might be effective in classifying aromas and unpleasant odor.
- Results between Attention−Meditation and MA Hβ/Hα are consistent. However, further analysis is difficult because Attention and Meditation can only be obtained from NeuroSky’s original algorithm. Instead, MA Hβ/Hα is considered a helpful index because it allows detailed analysis based on different frequency bands of Hβ and Hα, and yet yielded the same result as Attention−Meditation. However, some results of δ wave show a different trend, which indicates we need to further investigate how to appropriately employ it on the emotion map.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A. Time Series Plot of Delta Waves
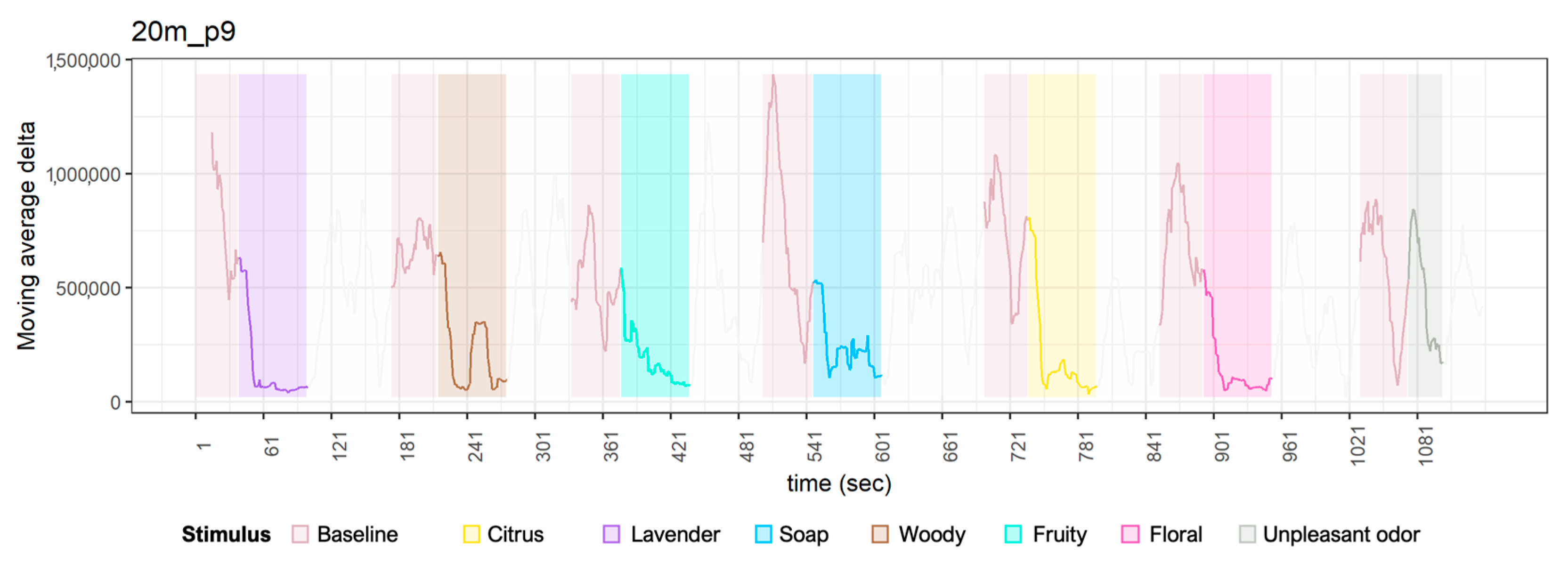

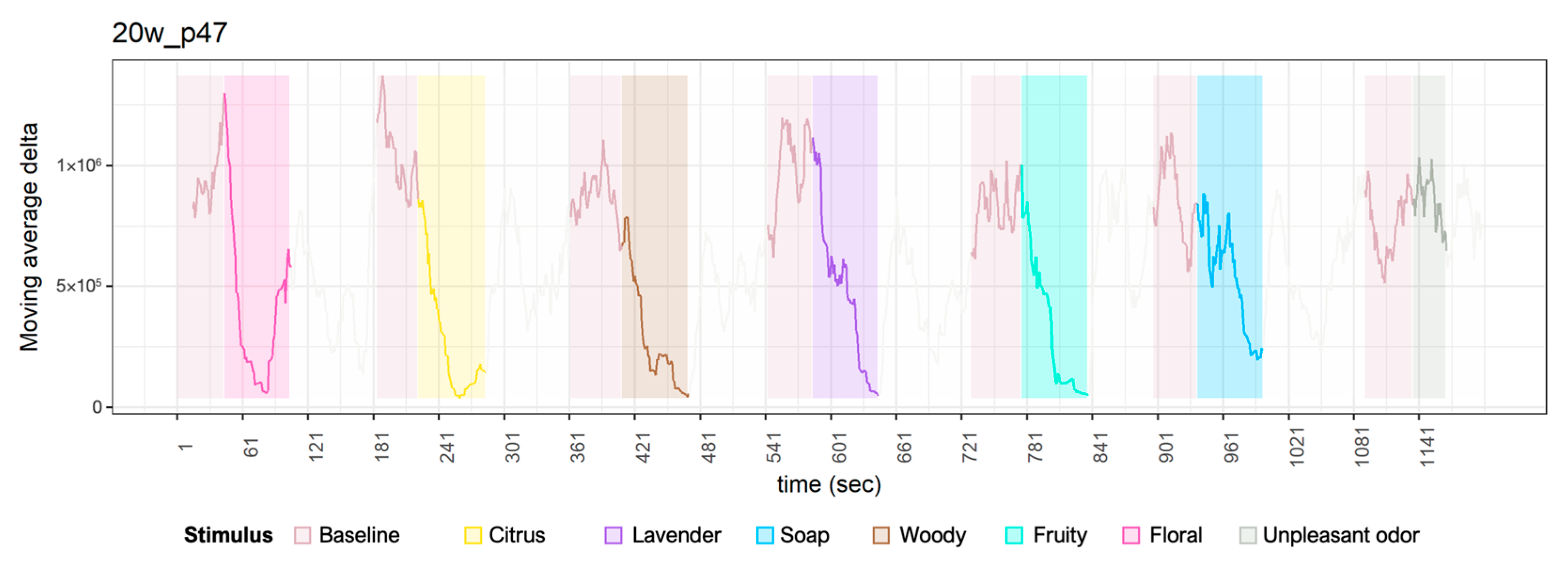
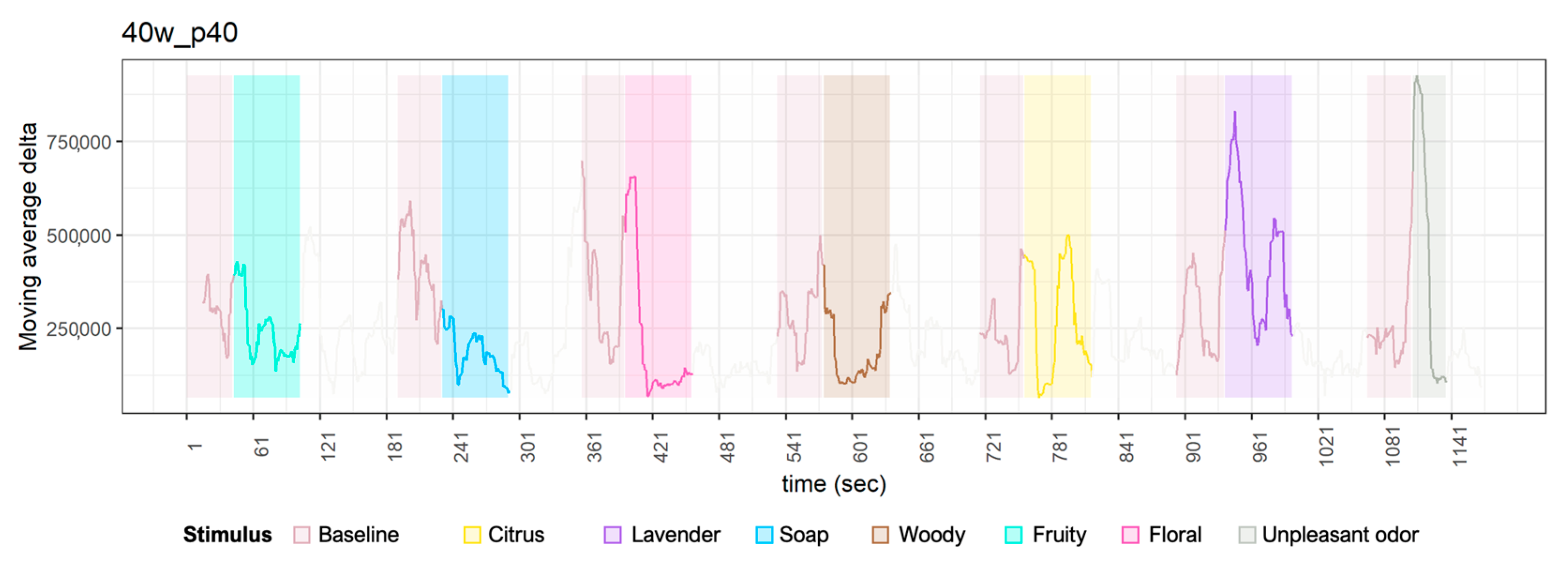
References
- Overview of the 2018 Occupational Safety and Health Survey (Fact-Finding Survey). Available online: www.mhlw.go.jp/toukei/list/dl/h30-46-50_gaikyo.pdf (accessed on 31 January 2023). (In Japanese).
- Awareness and Fact-Finding Survey Regarding Aromatherapy. Available online: www.aromakankyo.or.jp/basics/literature/result/vol3.php (accessed on 31 January 2023).
- Suzuki, A.; Okubo, N. Aromatherapy Research in Nursing and Its Present State. St. Luke’s Coll. Nurs. Mag. 2009, 24, 17–27. (In Japanese) [Google Scholar]
- Moontaha, S.; Schumann, F.E.F.; Arnrich, B. Online Learning for Wearable EEG-Based Emotion Classification. Sensors 2023, 23, 2387. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Tashiro, M.; Wu, D.; Yambe, T. Heart rate variability in autonomic function and localization of cerebral activity during inhalation of perfumed fragrances. J. Int. Soc. Life Inf. Sci. 2006, 24, 383–395. [Google Scholar]
- Ikeda, Y.; Horie, R.; Sugaya, M. Estimating Emotion with Biological Information for Robot Interaction. Procedia Comput. Sci. 2017, 112, 1589–1600. [Google Scholar] [CrossRef]
- Russell, J. A circumplex model of affect. J. Personal. Soc. Psychol. 1980, 39, 1161–1172. [Google Scholar] [CrossRef]
- Advantages and Disadvantages of Questionnaires. Available online: www.surveyanyplace.com/questionnaire-pros-and-cons (accessed on 31 January 2023).
- Lokman, A.M. Design & Emotion: The Kansei Engineering Methodology. Malays. J. Comput. 2010, 1, 1–14. [Google Scholar]
- Lévy, P.; Yamanaka, T.; Tomico, O. Psychophysiological Applications in Kansei Engineering. In Kansei Engineering and Soft Computing: Theory and Practice, 1st ed.; Dai, Y., Chakraborty, B., Shi, M., Eds.; IGI Global: Hershey, PA, USA, 2011; pp. 266–286. [Google Scholar]
- Pei, G.; Li, T. A Literature Review of EEG-Based Affective Computing in Marketing. Front. Psychol. 2021, 12, 602843. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Tiwari, P.; Song, D.; Hu, B.; Yang, M.; Zhao, Z.; Kumar, N.; Marttinen, P. EEG Based Emotion Recognition: A Tutorial and Review. ACM Comput. Surv. 2022, 55, 1–57. [Google Scholar] [CrossRef]
- Dadebayev, D.; Goh, W.W.; Tan, E.X. EEG-based emotion recognition: Review of commercial EEG devices and machine learning techniques. J. King Saud Univ.-Comput. Inf. Sci. 2022, 34, 4385–4401. [Google Scholar] [CrossRef]
- Hussin, S.S.; Sudirman, R. Sensory Response through EEG Interpretation on Alpha Wave and Power Spectrum. Procedia Eng. 2013, 53, 288–293. [Google Scholar] [CrossRef]
- Sahar, N.S.; Safri, N.M.; Zakaria, N.A. Use of EEG Technique in a Cognitive Process Study—A Review. Elektr. J. Electr. Eng. 2022, 21, 24–32. [Google Scholar] [CrossRef]
- Takahashi, N.; Inoue, S.; Seki, H.; Ushio, S.; Saito, Y.; Hasegawa, K.; Ohkura, M. Proposal for Driver Distraction Indexes Using Biological Signals Including Eye Tracking. In Proceedings of the 15th International Conference on Human-Computer Inter-action (HCI2013), Las Vegas, NV, USA, 21–26 July 2013. [Google Scholar]
- Nabaraj, D.; Nanda, N.; Andrew, N.; Zorica, N. Modeling of Cognition Using EEG: A Review and A New Approach. In Proceedings of the IEEE Region 10 Conference (TENCON2011), Bali, Indonesia, 21–24 November 2011. [Google Scholar]
- Gao, Y.; Lee, H.J.; Mehmood, R.M. Deep Learning of EEG Signals for Emotion Recognition. In Proceedings of the IEEE International Conference on Multimedia & Expo Workshops (ICMEW2015), Turin, Italy, 29 June–3 July 2015. [Google Scholar]
- Xiao-Wei, W.; Dan, N.; Bao-Liang, L. Emotional state classification from EEG data using machine learning approach. Neurocomputing 2014, 129, 94–106. [Google Scholar]
- Alarcao, S.M.; Fonseca, M.J. Emotions Recognition Using EEG Signals: A Survey. IEEE Trans. Affect. Comput. 2019, 10, 374–393. [Google Scholar] [CrossRef]
- Fraiwan, M.; Alafeef, M.; Almomani, F. Gauging human visual interest using multiscale entropy analysis of EEG signals. J. Ambient. Intell. Humaniz. Comput. 2021, 12, 2435–2447. [Google Scholar] [CrossRef]
- Stangl, F.J.; Riedl, R. Measurement of Heart Rate and Heart Rate Variability with Wearable Devices: A Systematic Review. In Proceedings of the 17th International Conference on Wirtschaftsinformatik, Nürnberg, Germany, 21–23 February 2022. [Google Scholar]
- Pham, T.; Lau, Z.; Chen, S.; Makowski, D. Heart Rate Variability in Psychology: A Review of HRV Indices and an Analysis Tutorial. Sensors 2021, 21, 3998. [Google Scholar] [CrossRef]
- Nardelli, M.; Lanata, A.; Francesco, F.D.; Scilingo, E.P. Investigating complex cardiovascular dynamics during hedonic olfactory elicitation. In Proceedings of the 11th Conference of the European Study Group on Cardiovascular Oscillations (ESGCO2020), Pisa, Italy, 15 July 2020. [Google Scholar]
- Tonacci, A.; Billeci, L.; Di Mambro, I.; Marangoni, R.; Sanmartin, C.; Venturi, F. Wearable Sensors for Assessing the Role of Olfactory Training on the Autonomic Response to Olfactory Stimulation. Sensors 2021, 21, 770. [Google Scholar] [CrossRef]
- Grol, M.; De Raedt, R. The link between resting heart rate variability and affective flexibility. Cogn. Affect. Behav. Neurosci. 2020, 20, 746–756. [Google Scholar] [CrossRef]
- Chang, C.Y.M.; Vries, M.; Groom, M.; Michelini, G.; Bellato, A. The Association Between Autonomic Arousal, Emotion Regulation, Anxiety & Quality of Life in Young Adults. Authorea 2023, 1–8. [Google Scholar] [CrossRef]
- Tivatansakul, S.; Ohkura, M. Emotion Recognition using ECG Signals with Local Pattern Description Methods. Int. J. Affect. Eng. 2016, 15, 51–61. [Google Scholar] [CrossRef]
- Ito, K.; Usuda, S.; Yasunaga, K.; Ohkura, M. “Feelings of Excitement” Caused by a VR Interactive System with Unknown Experience Using ECG. In Proceedings of the International Conference on Affective and Pleasurable Design (AHFE2017), Los Angeles, NV, USA, 17–21 July 2017. [Google Scholar]
- Kido, S.; Hashizume, A.; Baba, T.; Matsui, T. Development and Evaluation of a Smartphone Application for Self-estimation of Daily Mental Stress Level. Int. J. Affect. Eng. 2016, 15, 183–187. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F. An Emotional Analysis Method Based on Heart Rate Variability. In Proceedings of the IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI2012), Hong Kong/Shenzhen, China, 2–7 January 2012. [Google Scholar]
- Nagasawa, T.; Masui, K.; Doi, H.; Ogawa-Ochiai, K.; Tsumura, N. Continuous estimation of emotional change using multi-modal responses from remotely measured biological information. Artif. Life Robot. 2022, 27, 19–28. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Z.; Chen, P.; Nichele, S. Emotion recognition using multi-modal data and machine learning techniques: A tutorial and review. Inf. Fusion 2020, 59, 103–126. [Google Scholar] [CrossRef]
- He, Z.; Li, Z.; Yang, F.; Wang, L.; Li, J.; Zhou, C.; Pan, J. Advances in Multimodal Emotion Recognition Based on Brain–Computer Interfaces. Brain Sci. 2020, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Seipäjärvi, S.M.; Tuomola, A.; Juurakko, J.; Rottensteiner, M.; Rissanen, A.-P.; Kurkela, J.L.; Kujala, U.M.; Laukkanen, J.; Wikgren, J. Measuring psychosocial stress with heart rate variability-based methods in different health and age groups. Physiol. Meas. 2022, 43, 055002. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Laohakangvalvit, T.; Matsubara, R.; Sugaya, M. Constructing an Emotion Estimation Model Based on EEG/HRV Indexes Using Feature Extraction and Feature Selection Algorithms. Sensors 2021, 21, 2910. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A. Autonomic Modulation of Olfactory Signaling. Sci. Signal. 2011, 4, pe1. [Google Scholar] [CrossRef]
- Imanishi, J. Medical Aromatherapy. Jpn. J. Complement. Altern. Med. 2004, 1, 53–61. (In Japanese) [Google Scholar] [CrossRef]
- Kanzaki, S. Time course of autonomic nerve function affected by olfactory (aroma) stimulation. Annu. Rep. Cosmetol. 2015, 23, 148–154. (In Japanese) [Google Scholar]
- Tanida, M.; Niijima, A.; Shen, J.; Nakamura, T.; Nagai, K. Olfactory stimulation with scent of essential oil of grapefruit affects autonomic neurotransmission and blood pressure. Brain Res. 2005, 1058, 44–55. [Google Scholar] [CrossRef]
- Masago, R.; Matsuda, T.; Kikuchi, Y.; Miyazaki, Y.; Iwanaga, K.; Harada, H.; Katsuura, T. Effects of Inhalation of Essential Oils on EEG Activity and Sensory Evaluation. J. Physiol. Anthr. Appl. Hum. Sci. 2000, 19, 35–42. [Google Scholar] [CrossRef]
- Plutchik, R. Emotions and Life: Perspectives from Psychology, Biology, and Evolution, 1st ed.; American Psychological Association: Washington, DC, USA, 2003. [Google Scholar]
- Duan, X.; Tashiro, M.; Di Wu, D.; Yambe, T.; Wang, Q.; Sasaki, T.; Kumagai, K.; Luo, Y.; Nitta, S.-I.; Itoh, M. Autonomic nervous function and localization of cerebral activity during lavender aromatic immersion. Technol. Health Care 2007, 15, 69–78. [Google Scholar] [CrossRef]
- Babini, M.H.; Kulish, V.V.; Krejcar, O.; Namazi, H. Decoding of heart–brain relation by complexity-based analysis of heart rate variability (hrv) and electroencephalogram (eeg) signals. Fractals 2022, 30, 2250190. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, L.; Luo, H.; Li, X.; Guo, B.; Jiang, M.; Jia, Y.; Ma, J.; Sun, L.; Huang, Z. Effect of Fragrant Primula Flowers on Physiology and Psychology in Female College Students: An Empirical Study. Front. Psychol. 2021, 12, 607876. [Google Scholar] [CrossRef]
- Lekamge, S.; Nakachi, M.; Sato, S.; Ito, K.; Nomura, S. Psychophysiological Effects of Aroma Inhalation during a Short-Term Cognitive Stressor: A Preliminary Study Using Eight Different Aromas. Int. J. Affect. Eng. 2017, 16, 213–220. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, G.; Guo, L.; Zhang, B.; Niu, Y. The Effects of Olfactory Stimulation by Tree Peony Flowers on Autonomic Nervous System, Emotional States, and Brain Electrical Activity. Res. Sq. 2019, 1–17. [Google Scholar] [CrossRef]
- Zhang, R.; Feng, C.; Sripian, P.; Sugaya, M. A Preliminary Experiment on the Evaluation of Aroma Effects Using Biological Signals. In Proceedings of the 22nd HCI International Conference (HCII2020), Copenhagen, Denmark, 19–24 July 2020. [Google Scholar]
- Olfactory Measurement Method in Japan. Available online: https://www.env.go.jp/content/900450153.pdf (accessed on 17 March 2023).
- NeuroSky Inc. MindSet Communications Protocol. Available online: http://developer.neurosky.com/docs/lib/exe/fetch.php?media=mindset_communications_protocol.pdf (accessed on 27 March 2023).
- Nafea, M.; Hisham, A.B.; Abdul-Kadir, N.A.; Che Harun, F.K. Brainwave-Controlled System for Smart Home Applications. In Proceedings of the 2nd International Conference on BioSignal Analysis, Processing and Systems (ICBAPS2018), Kuching, Malaysia, 24–26 July 2018. [Google Scholar]
- Constatnt, I.; Sabourdin, N. The EEG signal: A window on the cortical brain activity. Paediatr. Anaesth. 2012, 22, 539–552. [Google Scholar] [CrossRef]
- Nedvedova, M.; Marek, J. Comparing EEG signals and emotions provoked by images with different aesthetic variables using emotive insight and neurosky mindwave. In Proceedings of the 17th Conference on Applied Mathematics (APLIMAT2018), Bratislava, Slovak Republic, 6–8 February 2018. [Google Scholar]
- Girase, P.D.; Deshmukh, M.P. Mindwave Device Wheelchair Control. Int. J. Sci. Res. 2013, 5, 2172–2176. [Google Scholar]
- Serrano-Barroso, A.; Siugzdaite, R.; Guerrero-Cubero, J.; Molina-Cantero, A.J.; Gomez-Gonzalez, I.M.; Lopez, J.C.; Vargas, J.P. Detecting Attention Levels in ADHD Children with a Video Game and the Measurement of Brain Activity with a Single-Channel BCI Headset. Sensors 2021, 21, 3221. [Google Scholar] [CrossRef]
- Fontanella, F.; Pinelli, S.; Babiloni, C.; Lizio, R.; Percio, C.D.; Lopez, S.; Noce, G.; Giubilei, F.; Stocchi, F.; Frisoni, G.B.; et al. Machine Learning to Predict Cognitive Decline of Patients with Alzheimer’s Disease Using EEG Markers: A Preliminary Study. In Proceedings of the 21st International Conference on Image Analysis and Processing (ICIAP2022), Lecce, Italy, 23–27 May 2022. [Google Scholar]
- Kotte, S.; Dabbakuti, J.R.K.K. Methods for removal of artifacts from EEG signal: A review. In Proceedings of the 1st International Conference on Advances in Physical Sciences and Materials (ICAPSM2020), Coimbatore, India, 13–14 August 2020. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Cunningham, D.W.; Wallraven, C. Experimental Design: From User Studies to Psychophysics, 1st ed.; Routledge: Oxfordshire, UK, 2012; pp. 285–288. [Google Scholar]
- Kawala-Sterniuk, A.; Podpora, M.; Pelc, M.; Blaszczyszyn, M.; Gorzelanczyk, E.J.; Martinek, R.; Ozana, S. Comparison of Smoothing Filters in Analysis of EEG Data for the Medical Diagnostics Purposes. Sensors 2020, 20, 807. [Google Scholar] [CrossRef]




| Stimulus Name | Stimulus Type |
|---|---|
| Citrus | Aroma |
| Lavender | Aroma |
| Soap | Aroma |
| Woody | Aroma |
| Fruity | Aroma |
| Floral | Aroma |
| Unpleasant odor | Isovaleric acid (IVA) |
| Gender | Generation | Number of Participants |
|---|---|---|
| Male | 20s | 9 |
| Female | 20s | 10 |
| Male | 30s | 6 |
| Female | 30s | 7 |
| Male | 40s | 3 |
| Female | 40s | 9 |
| Male | 50s | 12 |
| Female | 50s | 6 |
| EEG Index | Frequency Band (Hz) | Related |
|---|---|---|
| Delta (δ) | 1–3 | Deep, dreamless sleep, unconscious |
| Theta (θ) | 4–7 | Intuitive, recall, relaxed meditative |
| Low alpha (α) | 8–9 | Relaxed but not drowsy, calm, eyes closed |
| High alpha (α) | 10–12 | Relaxed but not drowsy, conscious |
| Low beta (β) | 13–17 | Relaxed yet focused, integrated |
| High beta (β) | 18–30 | Thinking, aware of self and surroundings, alert |
| Gamma (γ) | 31–50 | Cognition, information processing, awareness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laohakangvalvit, T.; Sripian, P.; Nakagawa, Y.; Feng, C.; Tazawa, T.; Sakai, S.; Sugaya, M. Study on the Psychological States of Olfactory Stimuli Using Electroencephalography and Heart Rate Variability. Sensors 2023, 23, 4026. https://doi.org/10.3390/s23084026
Laohakangvalvit T, Sripian P, Nakagawa Y, Feng C, Tazawa T, Sakai S, Sugaya M. Study on the Psychological States of Olfactory Stimuli Using Electroencephalography and Heart Rate Variability. Sensors. 2023; 23(8):4026. https://doi.org/10.3390/s23084026
Chicago/Turabian StyleLaohakangvalvit, Tipporn, Peeraya Sripian, Yuri Nakagawa, Chen Feng, Toshiaki Tazawa, Saaya Sakai, and Midori Sugaya. 2023. "Study on the Psychological States of Olfactory Stimuli Using Electroencephalography and Heart Rate Variability" Sensors 23, no. 8: 4026. https://doi.org/10.3390/s23084026





