Efficacy of the Cardiac Implantable Electronic Device Multisensory Triage-HF Algorithm in Heart Failure Care: A Real-World Clinical Experience
Abstract
:1. Introduction
2. Materials and Methods
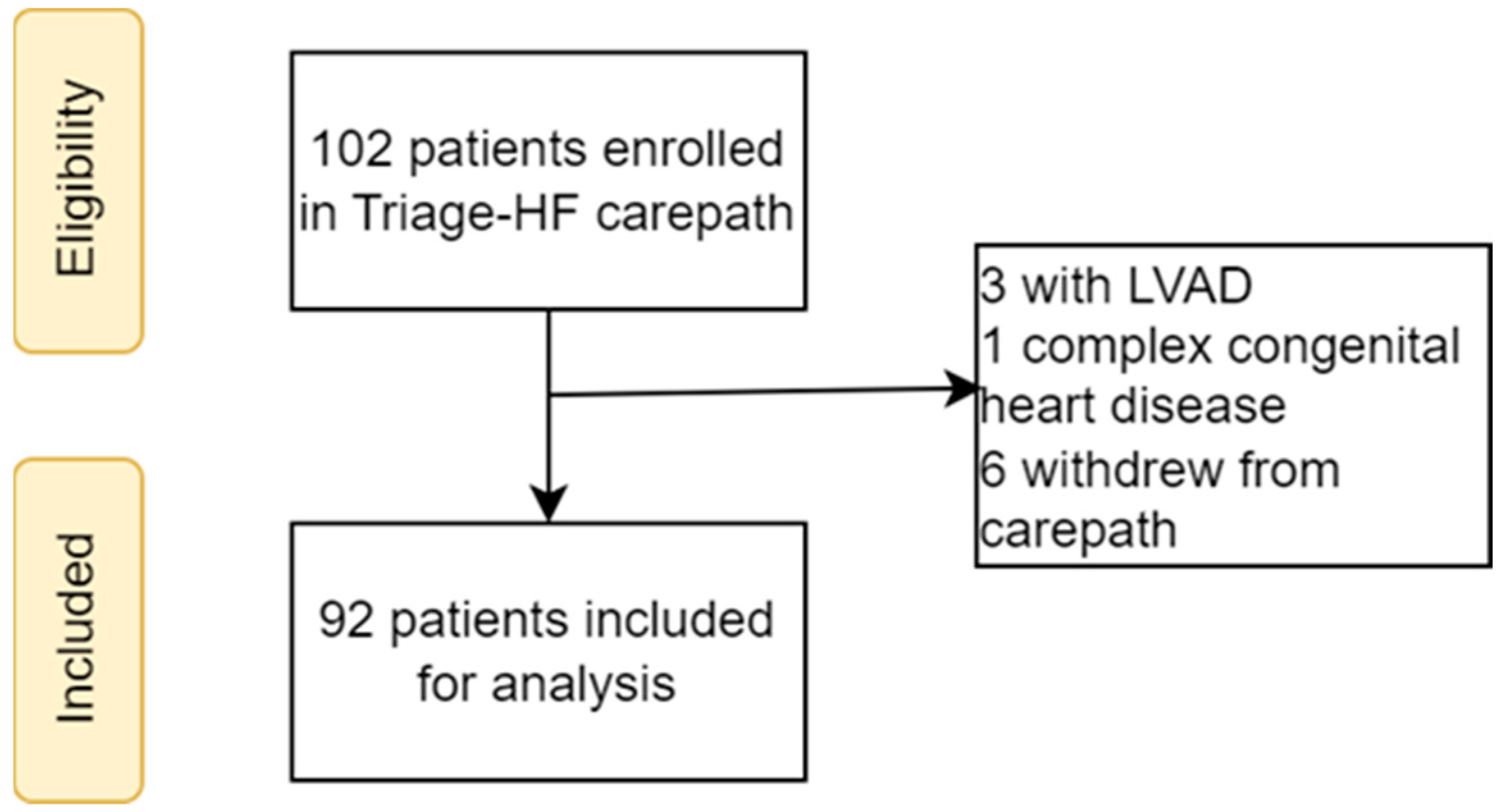
2.1. Study Population
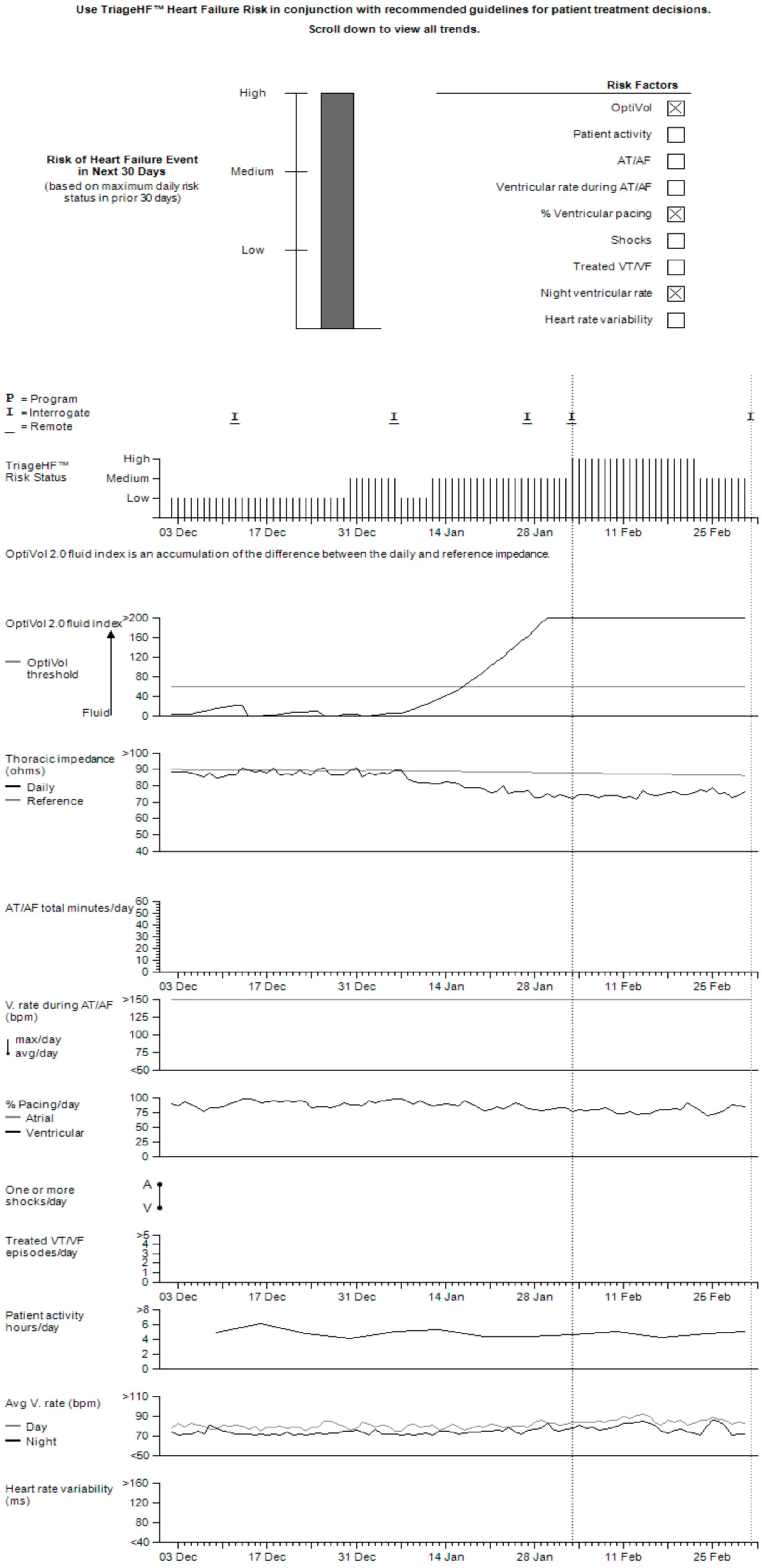
2.2. Triage-HF Risk Score
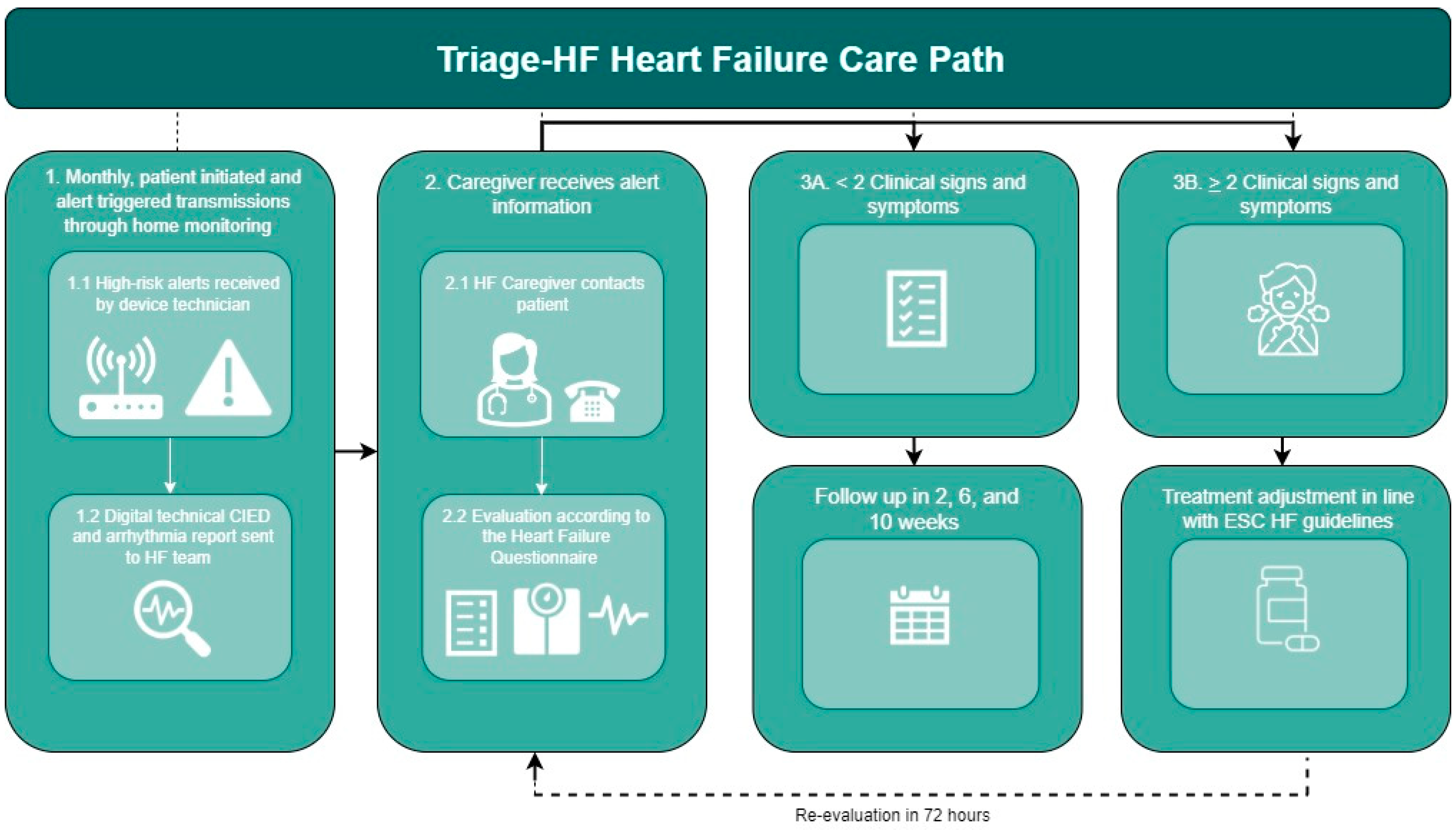
2.3. Triage-HF Alert-Guided Carepath
2.4. Data Sources and Collection
2.5. Alert Definition
2.6. Outcome Measures
2.7. Statistical Analysis
2.8. Ethics Statement
3. Results
3.1. Study Population
3.2. Triage-HF Alerts
3.3. Clinical Performance of the Triage-HF Alerts
3.4. Alert Handling and Impact on Patient Management
3.5. Timing of True Positive Alert Adjudication
3.6. Device-Specific Diagnostic Parameters Contributing to a Triage-HF High-Risk Status
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, A.; Allman, R.M.; Fonarow, G.C.; Love, T.E.; Zannad, F.; Dell’Italia, L.J.; White, M.; Gheorghiade, M. Incident Heart Failure Hospitalization and Subsequent Mortality in Chronic Heart Failure: A Propensity-Matched Study. J. Card. Fail. 2008, 14, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, S.; Stevenson, L.W.; Schneeweiss, S. Repeated Hospitalizations Predict Mortality in the Community Population with Heart Failure. Am. Heart J. 2007, 154, 260–266. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Čelutkiene, J.; Chioncel, O.; Cleland, J.G.F.; Coats, A.J.S.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.F.; Li, Y.; Pfeffer, M.A.; Solomon, S.D.; Weinfurt, K.P.; Velazquez, E.J.; Califf, R.M.; Rouleau, J.L.; Kober, L.; White, H.D.; et al. Impact of Cardiovascular Events on Change in Quality of Life and Utilities in Patients after Myocardial Infarction: A VALIANT Study (Valsartan in Acute Myocardial Infarction). JACC Heart Fail. 2014, 2, 159–165. [Google Scholar] [CrossRef]
- Gupta, A.; Fonarow, G.C. The Hospital Readmissions Reduction Program: Evidence for Harm. JACC Heart Fail. 2018, 6, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Nichols, M.; Scarborough, P.; Rayner, M. Cardiovascular Disease in Europe—Epidemiological Update 2015. Eur. Heart J. 2015, 36, 2696–2705. [Google Scholar] [CrossRef]
- Adamson, P.B. Pathophysiology of the Transition from Chronic Compensated and Acute Decompensated Heart Failure: New Insights from Continuous Monitoring Devices. Curr. Heart Fail. Rep. 2009, 6, 287–292. [Google Scholar] [CrossRef]
- Abraham, W.T.; Perl, L. Implantable Hemodynamic Monitoring for Heart Failure Patients. J. Am. Coll. Cardiol. 2017, 70, 389–398. [Google Scholar] [CrossRef]
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B. Sustained Efficacy of Pulmonary Artery Pressure to Guide Adjustment of Chronic Heart Failure Therapy: Complete Follow-up Results from the CHAMPION Randomised Trial. Lancet 2016, 387, 453–461. [Google Scholar] [CrossRef]
- Van Veldhuisen, D.J.; Braunschweig, F.; Conraads, V.; Ford, I.; Cowie, M.R.; Jondeau, G.; Kautzner, J.; Muñoz Aguilera, R.; Lunati, M.; Yu, C.M.; et al. Intrathoracic Impedance Monitoring, Audible Patient Alerts, and Outcome in Patients with Heart Failure. Circulation 2011, 124, 1719–1726. [Google Scholar] [CrossRef]
- Böhm, M.; Drexler, H.; Oswald, H.; Rybak, K.; Bosch, R.; Butter, C.; Klein, G.; Gerritse, B.; Monteiro, J.; Israel, C.; et al. Fluid Status Telemedicine Alerts for Heart Failure: A Randomized Controlled Trial. Eur. Heart J. 2016, 37, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Sarkar, S.; Koehler, J.; Whellan, D.J.; Crossley, G.H.; Tang, W.H.W.; Abraham, W.T.; Sharma, V.; Santini, M. Development and Validation of an Integrated Diagnostic Algorithm Derived from Parameters Monitored in Implantable Devices for Identifying Patients at Risk for Heart Failure Hospitalization in an Ambulatory Setting. Eur. Heart J. 2013, 34, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Whellan, D.J.; Ousdigian, K.T.; Al-Khatib, S.M.; Pu, W.; Sarkar, S.; Porter, C.B.; Pavri, B.B.; O’Connor, C.M. Combined Heart Failure Device Diagnostics Identify Patients at Higher Risk of Subsequent Heart Failure Hospitalizations: Results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) Study. J. Am. Coll. Cardiol. 2010, 55, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Gula, L.J.; Wells, G.A.; Yee, R.; Koehler, J.; Sarkar, S.; Sharma, V.; Skanes, A.C.; Sapp, J.L.; Redfearn, D.P.; Manlucu, J.; et al. A Novel Algorithm to Assess Risk of Heart Failure Exacerbation Using ICD Diagnostics: Validation from RAFT. Heart Rhythm. 2014, 11, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Taborsky, M.; Glikson, M.; Heinrich, U.; Schumacher, B.; Katz, A.; Brachmann, J.; Lewalter, T.; Goette, A.; Block, M.; et al. Implant-Based Multiparameter Telemonitoring of Patients with Heart Failure (IN-TIME): A Randomised Controlled Trial. Lancet 2014, 384, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.M.; Kitt, S.; Gill, J.; McComb, J.M.; Andre Ng, G.; Raftery, J.; Roderick, P.; Seed, A.; Williams, S.G.; Witte, K.K.; et al. Remote Management of Heart Failure Using Implantable Electronic Devices. Eur. Heart J. 2017, 38, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Da Costa, A.; Quesada, A.; Ricci, R.P.; Favale, S.; Boscolo, G.; Clementy, N.; Amori, V.; Mangoni di S. Stefano, L.; Burri, H. Effects of Remote Monitoring on Clinical Outcomes and Use of Healthcare Resources in Heart Failure Patients with Biventricular Defibrillators: Results of the MORE-CARE Multicentre Randomized Controlled Trial. Eur. J. Heart Fail. 2017, 19, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Palfy, J.A.; Benezet-Mazuecos, J.; Martinez Milla, J.; Iglesias, J.A.; de la Vieja, J.J.; Sanchez-Borque, P.; Miracle, A.; Rubio, J.M. CorVue Algorithm Efficacy to Predict Heart Failure in Real Life: Unnecessary and Potentially Misleading Information? Pacing Clin. Electrophysiol. 2018, 41, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients with Implanted Devices: Results from the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef] [PubMed]
- D’onofrio, A.; Solimene, F.; Calò, L.; Calvi, V.; Viscusi, M.; Melissano, D.; Russo, V.; Rapacciuolo, A.; Campana, A.; Caravati, F.; et al. Combining Home Monitoring Temporal Trends from Implanted Defibrillators and Baseline Patient Risk Profile to Predict Heart Failure Hospitalizations: Results from the SELENE HF Study. Europace 2022, 24, 234–244. [Google Scholar] [CrossRef]
- Virani, S.A.; Sharma, V.; McCann, M.; Koehler, J.; Tsang, B.; Zieroth, S. Prospective Evaluation of Integrated Device Diagnostics for Heart Failure Management: Results of the TRIAGE-HF Study. ESC Heart Fail. 2018, 5, 809–817. [Google Scholar] [CrossRef]
- Burri, H.; Da Costa, A.; Quesada, A.; Ricci, R.P.; Favale, S.; Clementy, N.; Boscolo, G.; Villalobos, F.S.; Di Mangoni Stefano, L.; Sharma, V.; et al. Risk Stratification of Cardiovascular and Heart Failure Hospitalizations Using Integrated Device Diagnostics in Patients with a Cardiac Resynchronization Therapy Defibrillator. Europace 2018, 20, e69–e77. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.Z.; Taylor, J.K.; Green, C.; Moore, L.; Goode, A.; Black, P.; Howard, L.; Fullwood, C.; Zaidi, A.; Seed, A.; et al. Triage-HF Plus: A Novel Device-Based Remote Monitoring Pathway to Identify Worsening Heart Failure. ESC Heart Fail. 2020, 7, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Sasaki, S.; Kusano, K.; Mine, T.; Fujii, K.; Iwasa, A.; Sunagawa, O.; Yamabe, H.; Takahashi, N.; Ishii, S.; et al. Evaluation of an Integrated Device Diagnostics Algorithm to Risk Stratify Heart Failure Patients—Results from the SCAN-HF Study. Circ. J. 2020, 84, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Koehler, J.; Sarkar, S.; Butler, J. Prediction of Worsening Heart Failure Events and All-Cause Mortality Using an Individualized Risk Stratification Strategy. ESC Heart Fail. 2020, 7, 4277–4289. [Google Scholar] [CrossRef] [PubMed]
- Sammut-Powell, C.; Taylor, J.K.; Motwani, M.; Leonard, C.M.; Martin, G.P.; Ahmed, F.Z. Remotely Monitored Cardiac Implantable Electronic Device Data Predict All-Cause and Cardiovascular Unplanned Hospitalization. J. Am. Heart Assoc. 2022, 11, e024526. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Costanzo, M.R.R.; Ippolito, E.M.; Zhang, Y.; Stapleton, R.; Sadhu, A.; Jimenez, J.; Hobbs, J.; Sharma, V.; Warman, E.N.; et al. INTERVENE-HF: Feasibility Study of Individualized, Risk Stratification-Based, Medication Intervention in Patients with Heart Failure with Reduced Ejection Fraction. ESC Heart Fail. 2021, 8, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Feijen, M.; Egorova, A.D.; Beeres, S.L.M.A.; Treskes, R.W. Early Detection of Fluid Retention in Patients with Advanced Heart Failure: A Review of a Novel Multisensory Algorithm, HeartLogicTM. Sensors 2021, 21, 1361. [Google Scholar] [CrossRef] [PubMed]
- Feijen, M.; Egorova, A.D.; Treskes, R.W.; Mertens, B.J.A.; Jukema, J.W.; Schalij, M.J.; Beeres, S.L.M.A. Performance of a HeartLogicTM Based Care Path in the Management of a Real-World Chronic Heart Failure Population. Front. Cardiovasc. Med. 2022, 9, 883873. [Google Scholar] [CrossRef] [PubMed]
- Treskes, R.W.; Beles, M.; Caputo, M.L.; Cordon, A.; Biundo, E.; Maes, E.; Egorova, A.D.; Schalij, M.J.; Van Bockstal, K.; Grazioli-Gauthier, L.; et al. Clinical and Economic Impact of HeartLogicTM Compared with Standard Care in Heart Failure Patients. ESC Heart Fail. 2021, 8, 1541–1551. [Google Scholar] [CrossRef]
- Feijen, M.; Beles, M.; Tan, Y.Z.; Cordon, A.; Dupont, M.; Treskes, R.W.; Caputo, M.L.; Van Bockstal, K.; Auricchio, A.; Egorova, A.D.; et al. Fewer Worsening Heart Failure Events with HeartLogic on Top of Standard Care: A Propensity-Matched Cohort Analysis. J. Card. Fail. 2023, 29, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Mairesse, G.H.; Braunschweig, F.; Klersy, K.; Cowie, M.R.; Leyva, F. Implementation and Reimbursement of Remote Monitoring for Cardiac Implantable Electronic Devices in Europe: A Survey from the Health Economics Committee of the European Heart Rhythm Association. Europace 2015, 17, 814–818. [Google Scholar] [CrossRef] [PubMed]

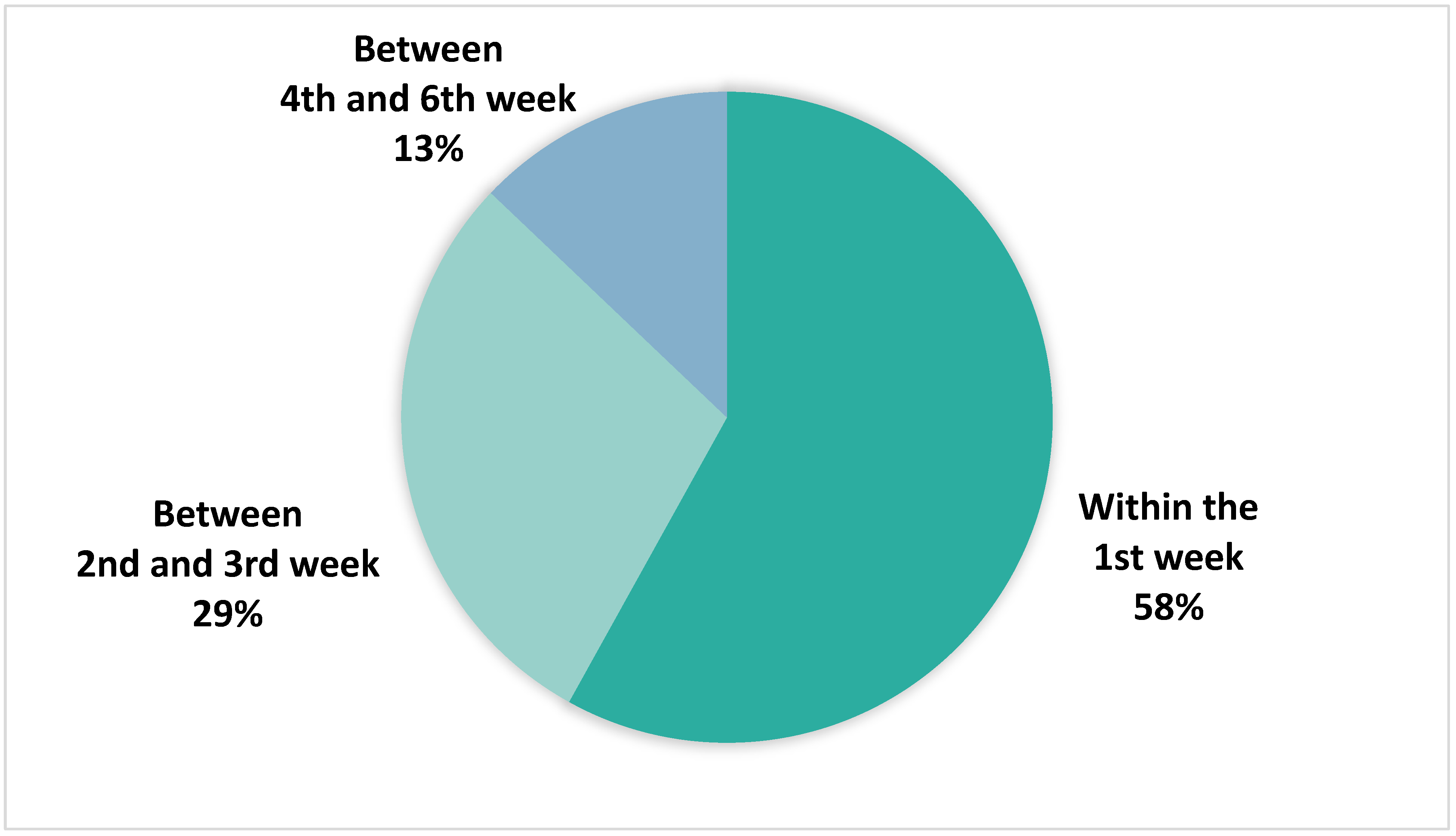

| n = 92 | |
|---|---|
| Age in years, median [IQR] | 69 [59–75] |
| Males, n (%) | 72 (78%) |
| Years since HF diagnosis, median [IQR] | 10 [6–17] |
| BMI (kg/m2), median [IQR] | 26 [24–30] |
| LVEF in % (SD) | 38 ± 10 |
| NYHA class, n (%) | |
| I | 18 (20%) |
| II | 60 (65%) |
| III–IV | 14 (15%) |
| Etiology | |
| Ischemic | 37 (40%) |
| Non-ischemic | 55 (60%) |
| Device | |
| CRT, n (%) | 62 (67%) |
| Percentage of biv-pacing (%), median [IQR] | 99 [95–100] |
| DDD/VVI ICD | 30 (33%) |
| Cardiac history | |
| CABG, n (%) | 16 (17%) |
| Valve surgery, n (%) | 18 (20%) |
| Co-morbidities | |
| Atrial fibrillation, n (%) | 37 (40%) |
| Hypertension, n (%) | 24 (26%) |
| Diabetes mellitus, n (%) | 23 (25%) |
| Ischemic CVA/TIA, n (%) | 16 (17%) |
| Laboratory findings | |
| NT-proBNP in ng/L, median [IQR] | 512 [165–1668] |
| Hb in mmol/L, median [IQR] | 8.7 [8.1–9.4] |
| eGFR in mL/min/1.73 m2, median [IQR] | 66 [46–83] |
| Pharmacotherapy | |
| Beta-blockers, n (%) | 88 (92%) |
| ACE/ARB/ARNI, n (%) | 84 (91%) |
| MRA, n (%) | 59 (64%) |
| SGLT2i, n (%) | 29 (32%) |
| Diuretics, n (%) | 63 (69%) |
| Population Mean | 95% Confidence Interval | |
|---|---|---|
| Sensitivity | 83% | 65–92% |
| Specificity | 97% | 92–99% |
| Positive predictive value | 89% | 73–96% |
| Negative predictive value | 94% | 89–97% |
| Unexplained alert rate | 0.05 alerts per patient year |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslan, U.; Beeres, S.L.M.A.; Feijen, M.; Mulder, G.M.; Jukema, J.W.; Egorova, A.D. Efficacy of the Cardiac Implantable Electronic Device Multisensory Triage-HF Algorithm in Heart Failure Care: A Real-World Clinical Experience. Sensors 2024, 24, 3664. https://doi.org/10.3390/s24113664
Aslan U, Beeres SLMA, Feijen M, Mulder GM, Jukema JW, Egorova AD. Efficacy of the Cardiac Implantable Electronic Device Multisensory Triage-HF Algorithm in Heart Failure Care: A Real-World Clinical Experience. Sensors. 2024; 24(11):3664. https://doi.org/10.3390/s24113664
Chicago/Turabian StyleAslan, Ugur, Saskia L. M. A. Beeres, Michelle Feijen, Gerlinde M. Mulder, J. Wouter Jukema, and Anastasia D. Egorova. 2024. "Efficacy of the Cardiac Implantable Electronic Device Multisensory Triage-HF Algorithm in Heart Failure Care: A Real-World Clinical Experience" Sensors 24, no. 11: 3664. https://doi.org/10.3390/s24113664





