Role of Graphene in Surface Plasmon Resonance-Based Biosensors
Abstract
1. Introduction
2. Methodology
2.1. The Review Goal
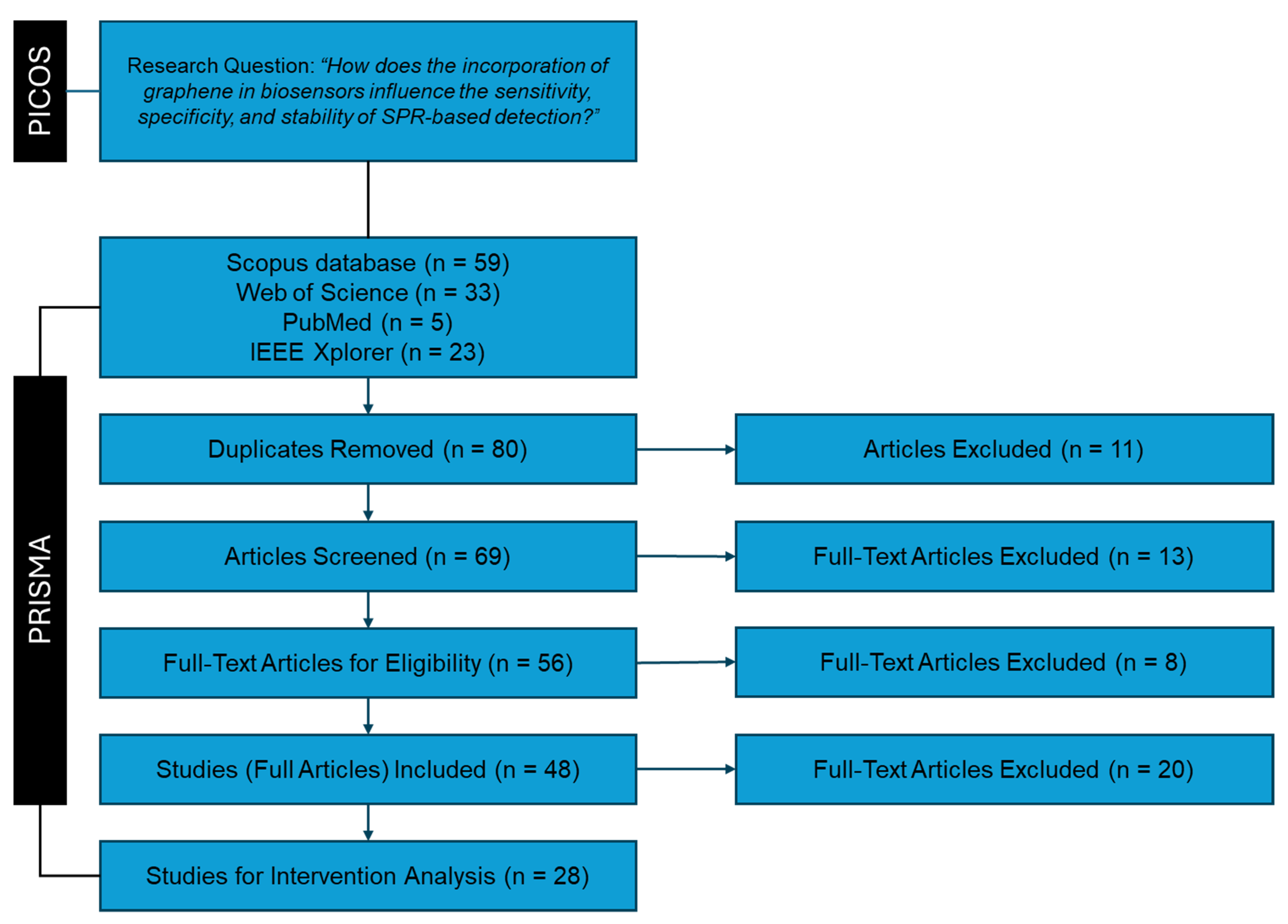
2.2. Identification Stage
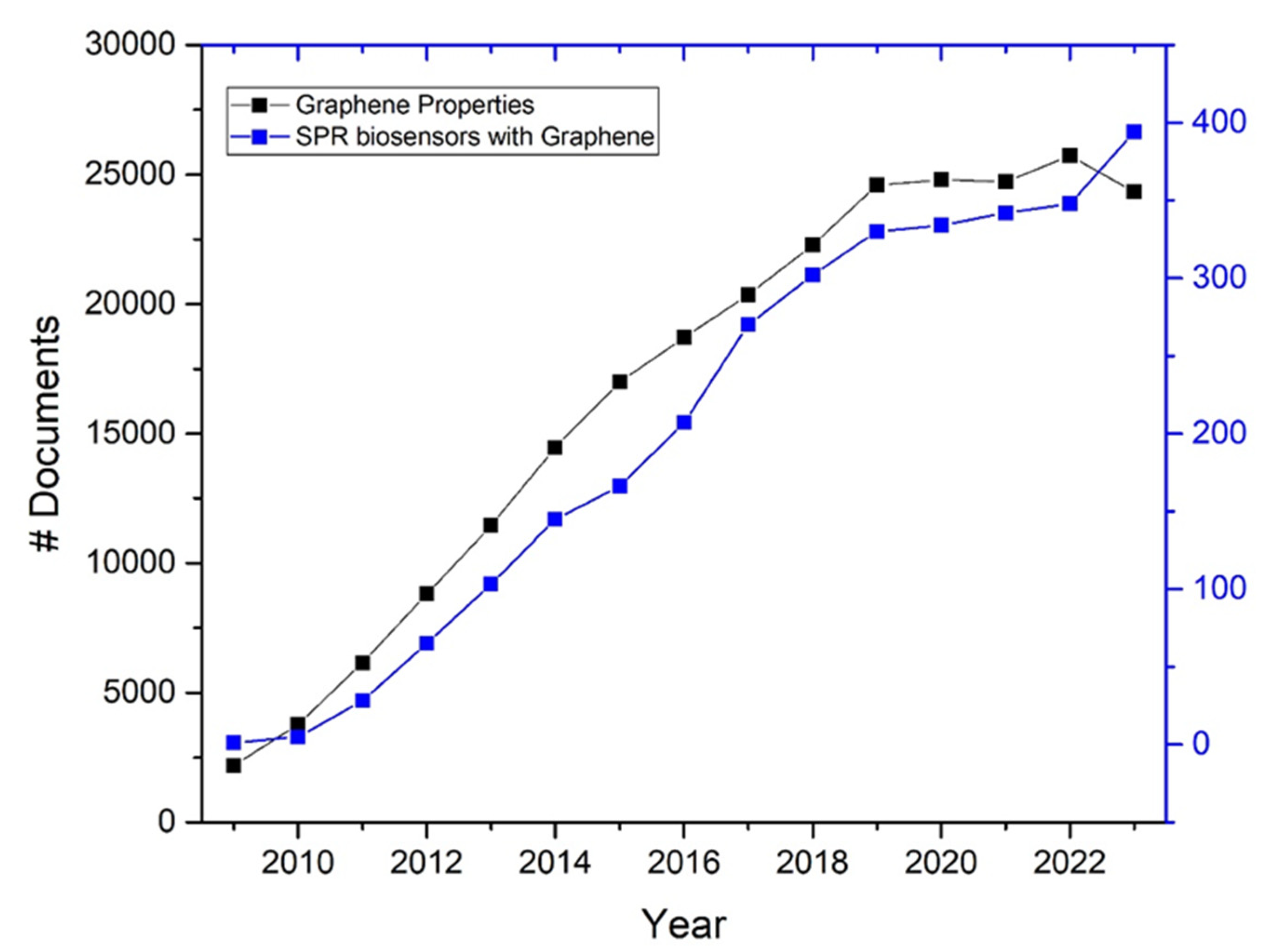
- The field of graphene research, particularly its application in biosensors, has seen significant advancements and a surge in scientific publications over the past decade. The unique properties of graphene were more thoroughly understood and explored in this period, leading to numerous innovative applications in biosensing technologies (see Figure 3, black line, data obtained from the Scopus database).
- Starting in 2010, there was a noticeable increase in the number of studies focusing on the integration of graphene in SPR sensors. This period captures the maturation of graphene technology from basic research to more applied studies, including experimental and comparative studies that directly assess the performance improvements brought by graphene (see Figure 3, blue line, data obtained from the Scopus database).
2.3. Screening Stage
- Inclusion of review articles, full research articles, and proceedings papers.
- Focus on articles specifically addressing graphene.
- Relevance to SPR-based biosensors.
- Inclusion of articles irrespective of language.
- Eight articles did not focus on or include graphene-related materials.
- One article did not pertain to biosensor technologies.
- Two articles were not available.
2.4. Eligibility Stage
- The full text of the article is available in any language.
- The article focuses on the use of graphene in biosensors.
- The article centers on SPR or related technologies.
- The article specifically discusses the application of graphene in SPR-based biosensors.
- Nine articles did not focus on biosensors utilizing graphene.
- Four articles were narrative review papers discussing the general topic of sensors using low-dimensional materials.
2.5. Included Stage
- Sensitivity.
- Specificity.
- Stability.
- Eight articles did not report conclusive metrics. For example, some articles were excluded due to the lack of rigorous experimental methodology to calculate the refractive indices. From the theoretical part, in some studies, models assumed at the same time ideal conditions such as perfect immobilization of biomolecules, absence of non-specific binding, and uniform distribution of the analyte in the sample.
3. Results
3.1. Summary of Search Results
- Type of Intervention describes the specific approach or modification applied.
- Type of Biosensor specifies the biosensor types used.
- The Graphene Integration Method details the methods used for incorporating graphene into the biosensors.
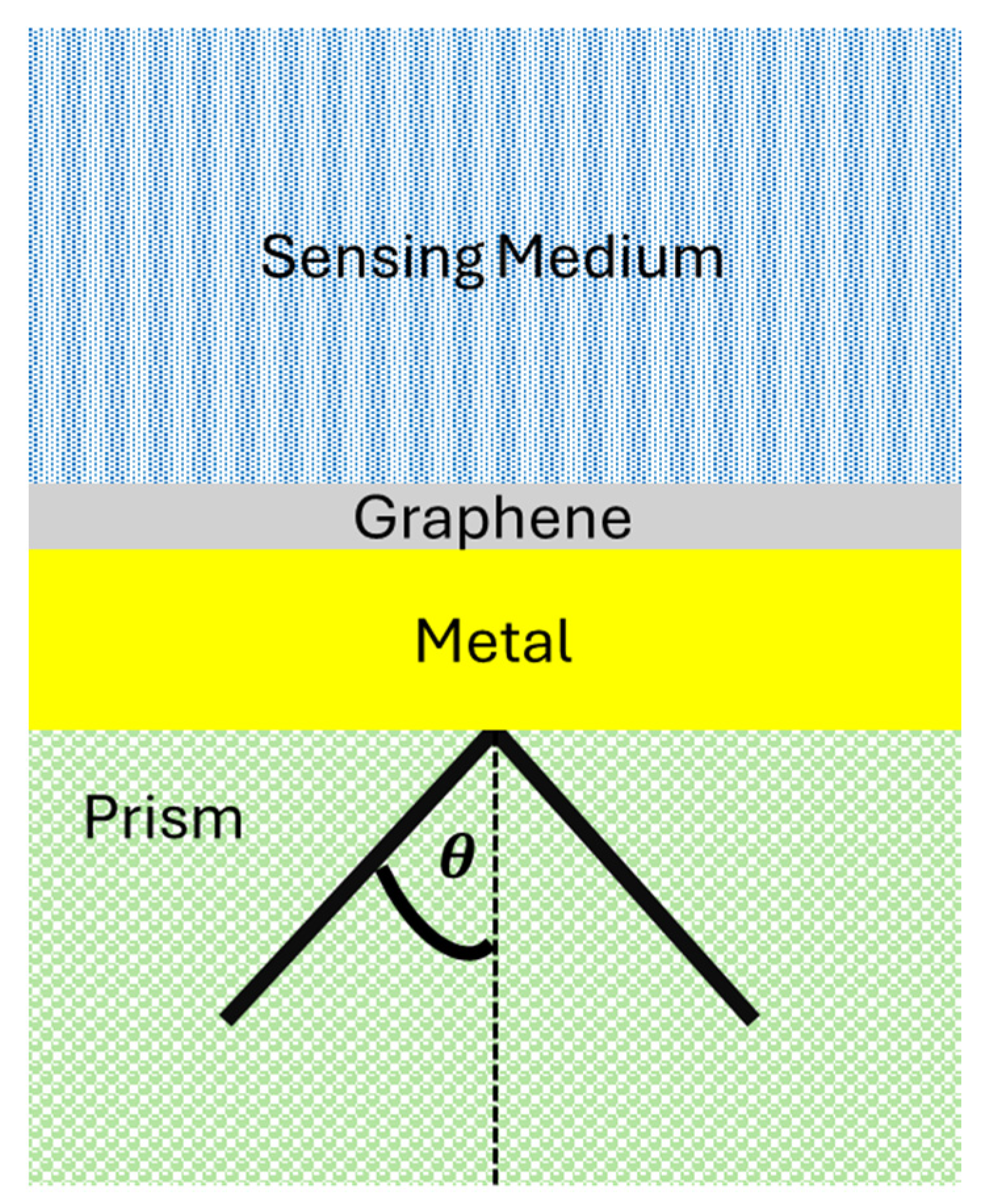
- Surface Plasmon Resonance Configuration describes the SPR configurations employed, such as angular, wavelength, or intensity modulation.
- Comparison Material provides a list of the conventional materials used for comparisons, such as gold or silver.
- Changes in the sensitivity of the biosensors due to graphene integration. Sensitivity refers to the ability to detect small changes in analyte concentration by the variation of refractive indices after biomolecule adsorption. Factors influencing sensitivity include the sensor surface material, surface functionalization quality, and optical setup design.
- Modifications in the specificity of the biosensors when graphene is used. Specificity is the ability to selectively detect the target analyte amidst other non-target molecules. High specificity minimizes false positives and ensures accurate detection of the desired analyte. This is achieved by functionalizing the sensor surface with specific biomolecules like antibodies, aptamers, or DNA that have a high affinity for the target molecule.
- Observed improvements in the stability of the biosensors. Stability refers to maintaining consistent performance over time and under various conditions. High stability ensures the reliability of the sensor over extended periods and different environments. Factors influencing stability include the robustness of materials, surface chemistry, and the ability to withstand environmental changes such as temperature and pH variations.
3.2. Summary of Interventions
3.3. Summary of Specific Interventions
3.4. Summary of Performance Metrics
3.5. Data Analysis
3.6. Generated Data
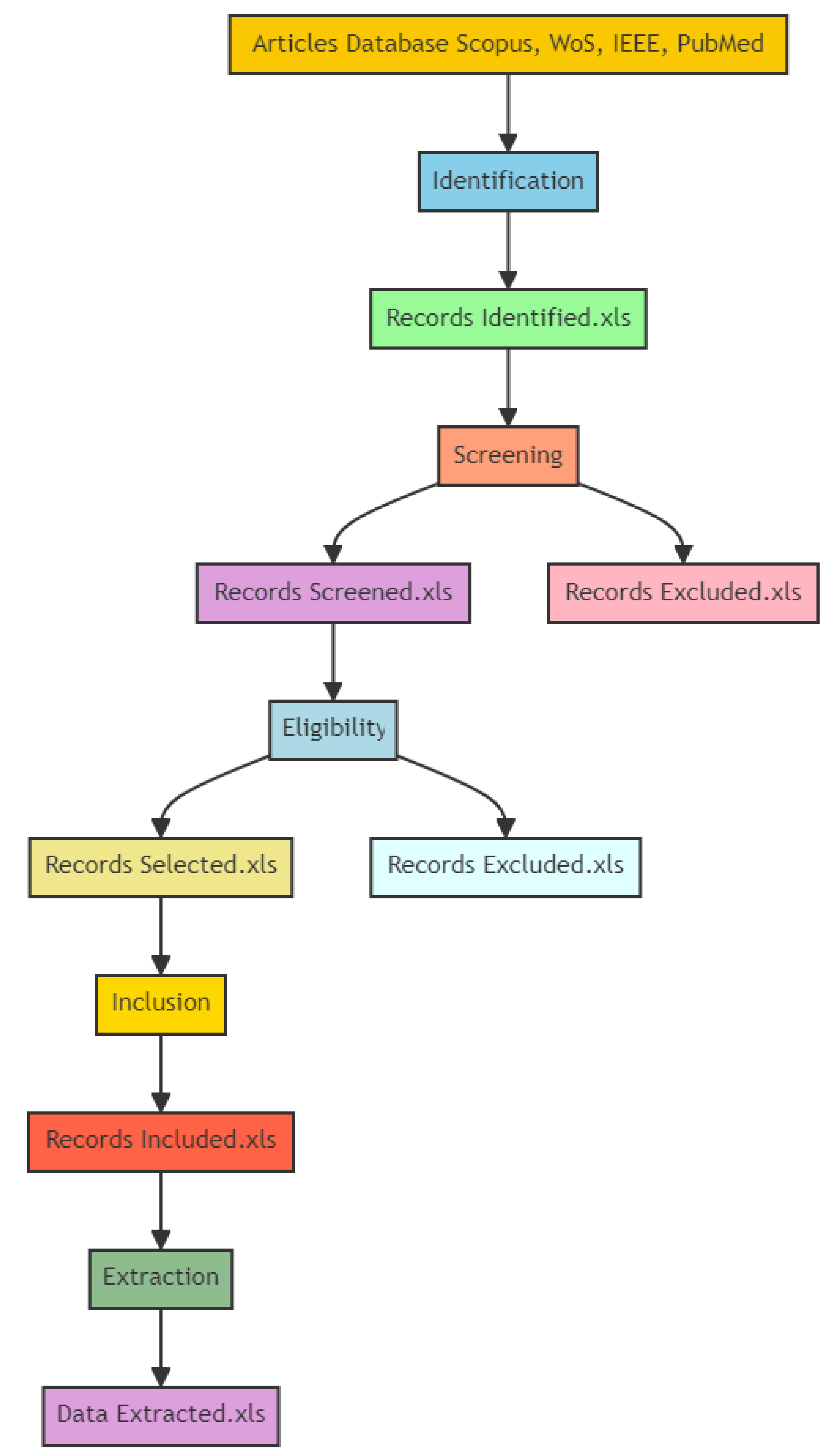
- Identification;
- Screening;
- Eligibility;
- Inclusion;
- Extraction.
4. Discussion
4.1. Enhancements in SPR Biosensors through Graphene Integration
4.2. Target Analytes and Comparative Materials
4.3. Sensitivity
4.4. Specificity
4.5. Stability
5. Limitations of the Current Systematic Review
- One of the primary limitations of this review is the relatively limited number of studies focused on the specificity and response time of graphene-enhanced SPR biosensors. While sensitivity and stability metrics were well-represented and thoroughly analyzed, the specificity metrics were based on fewer studies. This limited scope could impact the generalizability of the findings related to these specific performance aspects.
- The studies included in this review employed a wide range of experimental conditions, including different types of graphene, biosensor configurations, target analytes, and detection methods. This variability can make it challenging to directly compare results across studies and may introduce inconsistencies in the reported performance metrics. Although efforts were made to categorize and analyze the studies systematically, the inherent differences in experimental setups should be considered when interpreting the results.
- There is a possibility of publication bias, where studies reporting significant improvements in biosensor performance with graphene integration are more likely to be published than those reporting minimal or no improvements. This bias could skew the overall conclusions of the review, presenting an overly optimistic view of the impact of graphene on SPR biosensors.
- Many of the studies included in this review were conducted under controlled laboratory conditions. There is limited information on the performance and reliability of graphene-enhanced SPR biosensors in realistic applications. Future research should emphasize the testing and validation of these biosensors in practical settings to better understand their applicability and robustness in real-life scenarios.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference | Type of Biosensor | Graphene Integration Method | SPR Configuration |
|---|---|---|---|
| Maharana et al. [50] | SPR | Deposition of a monolayer of graphene on top of the silver layer | The setup consists of a helio–neon laser (632.8 nm), a polarizer, a BK7 prism on a high-precision rotary stage, and a photodetector. The optimized thickness of silver and graphene is deposited on the prism surface. |
| Islam et al. [51] | SPR | Deposition of a 2 nm thick graphene layer on top of a 50 nm gold thin film, followed by placing a periodic dielectric subwavelength grating on top of the graphene layer | Kretschmann configuration with a fused silica glass prism, gold thin film (50 nm), and a graphene layer (2 nm), over which a periodic dielectric subwavelength grating made of polymethyl methacrylate (PMMA) is placed. The setup uses a TM-polarized plane wave light of fixed wavelength 632.8 nm. |
| Nayak et al. [52] | Fiber optic sensor based on SPR | Use of 2D materials (graphene, graphene oxide, molybdenum disulfide) on silicon over a silver layer. | High-index chalcogenide core fiber with a silicon overlayer and different 2D materials on top of silver. |
| Meshingqalam et al. [53] | SPR | The study models the interaction of sensing molecules with a graphene layer to observe changes in its electronic band structure and refractive index, influencing SPR response. | The study involves modeling the refractive index variations and SPR response using a transverse magnetic wave model in a graphene-based sensor configuration. |
| Sharma et al. [54] | SPR | Graphene-based electro-optical SPR biosensor | Kretschmann–Raether configuration-based SPR sensor with a ZBLAN–silver–graphene-analyte structure. |
| Conceicao et al. [55] | SPCE | Graphene layers are modeled as surface impedances with conductivity described by the Kubo formula. | Multilayer structure: air/graphene/silicon dioxide/graphene/prism, excited by a THz source. |
| Liang et al. [56] | SPR | Periodically arranged graphene elliptic-circular nanodisk resonators on a dielectric substrate. | Graphene elliptical and circular nanodisks are periodically arranged on a dielectric substrate, utilizing the finite difference time domain (FDTD) method for simulations. |
| Rouf et al. [57] | SPR | The graphene layer is integrated using a layered structure where it is sandwiched between the metal silver and the sensing layers, serving as a protective layer and biomolecular recognition element. | The configuration includes a BK7 prism, Ti adhesion layer, silver layer, molybdenum diselenide layer, and graphene layer. The sensor operates with p-polarized monochromatic light, optimized at a 750 nm excitation wavelength. |
| Wu et al. [58] | SPR | Coating of graphene on gold surface using a transfer printing technique | Kretschmann configuration with a prism coupler |
| Islam et al. [59] | LSPR | Coating of a graphene layer on top of the gold thin film | Kretschmann configuration with a prism coupler |
| Islam et al. [60] | LSPR | The graphene sheet is deposited on top of the gold layer. | Kretschmann configuration with a triangular prism and variable incidence angles, using a gold thin film with a graphene layer on top, operating with a fixed wavelength of 632.8 nm. |
| Islam et al. [61] | LSPR | The graphene sheet is deposited on top of the gold layer using electron beam lithography and nano-sphere lithography. | Kretschmann configuration with a triangular prism, various incidence angles, and a 632.8 nm laser beam. |
| Islam et al. [62] | LSPR | The graphene layer is incorporated on top of a thin gold film, with periodic dielectric grating placed on top. | Kretschmann configuration with a thin gold film, graphene layer, and periodic dielectric grating on top. The setup includes a fused silica glass prism and a TM-polarized plane wave light of 632 nm. |
| Wu et al. [63] | SPR | Graphene ribbons were fabricated and integrated into the sensor design through a periodic array on a quartz substrate. | The sensor configuration includes a graphene ribbon array on top of a quartz (silicon dioxide) substrate. The SPR effect is measured by detecting spectral shifts of the resonant transmission dip due to changes in the refractive index of the medium above the sensor. |
| Maharana et al. [64] | SPR | Graphene layers were incorporated on top of a gold film, forming part of a multilayer structure. | The sensor configuration includes a chalcogenide glass prism, a teflon layer, gold film, and a graphene layer. The SPR setup utilizes the Kretschmann configuration, where TM polarized light is launched at the prism and reflectivity is measured. |
| Islam et al. [65] | SPR | A graphene layer is deposited on top of a gold thin film, followed by a periodic subwavelength dielectric grating. | Kretschmann configuration with a gold thin film, graphene layer, and periodic dielectric grating. The setup includes a fused silica glass prism, and the excitation is achieved using TM-polarized plane wave light at 632.8 nm. |
| Toloue et al. [66] | SPR | Graphene layers are added on top of the copper thin film as a binding recognition element (BRE). | The setup includes a 50 nm copper film with multiple layers of graphene on one side and SF10 glass on the other. The thickness of each graphene layer is 0.34 nm, and the sensor operates at a fixed wavelength of 633 nm using a helio-neon laser. |
| Verma et al. [67] | SPR | The graphene layer is coated on a silver layer, which is deposited on a chromium substrate | Kretschmann configuration with a prism (SF10), air gap, chromium layer, silver layer, graphene layer, affinity layer, and sensing medium |
| Wu et al. [68] | LRSPR | Graphene layer coated on a metal thin film (such as copper, aluminum, or silver) deposited on a chalcogenide glass (2S2G) prism with a cytop thin film. | The setup involves a 2S2G glass prism, cytop thin film, metal thin film, and the graphene layer. The excitation light wavelength used is 633 nm. |
| Verma et al. [69] | SPR | Graphene is integrated into the gold film, showing stable adsorption of carbon-based biomolecules (e.g., ssDNA). | Kretschmann configuration, where a high refractive index prism is coated with a metal film touching the sensing medium. |
| An et al. [70] | Quasi-D-Shaped Optical Fiber Plasmonic Biosensor | Graphene is deposited over ITO, which is then coated on the polished plane of the quasi-D-shaped photonic crystal fiber (PCF). | The configuration involves a quasi-D-shaped PCF with a short polishing depth. ITO coated with graphene is deposited on the polished plane. The sensing mechanism is based on the wavelength interrogation sensitivity of the sensor. |
| Huang et al. [71] | THz sensor. This sensor operates using the SPR phenomenon at terahertz frequencies | Continuous suspended monolayer graphene (MLG) and graphene/insulator stacks (GIS). | Otto configuration with a prism, analyte, substrate, and MLG or GIS layers. The GIS is separated by a buffer layer with a thickness of 20 nm and a refractive index of 1.535, and deposited on a substrate with a refractive index of 1.6. In the suspended MLG configuration, the substrate refractive index was set to 1 |
| Lin et al. [72] | Intensity-sensitive Bloch Surface Wave (BSW) biosensor. | Graphene layers are used as defect layers on the surface of 1DPC, with the number of layers optimized for performance. | Kretschmann configuration with a ZF10 half-cylinder coupling prism, 1DPC composed of titanium dioxide and silicon dioxide layers. |
| Farmani et al. [73] | SPR | Monolayer CVD graphene was transferred onto silicon dioxide/silicene substrate using poly(methyl methacrylate) (PMMA) as a supporting layer, followed by etching of PMMA with dilute hydrochloric acid. | Otto configuration, an attenuated total reflection (ATR)-SPR setup with incident light system, Otto configuration system, and reflected light detection system. |
| Hossain et al. [74] | Optical biosensor for glucose detection | Modification of mono-layer graphene Fermi energy (Ef) by a gate voltage (Vg) | Transfer matrix method (TMM)-based angular interrogation technique, using incident light with a 633-nm wavelength and TM polarization |
| Behboudi et al. [75] | THz sensor | Perforated stripe with periodic grooves made of graphene, laid over a polyimide substrate | The sensor operates in reflection mode using a photoconductive antenna (PCA) as the THz source in its pulse mode, operating over a frequency range of 0.2–6 THz |
| Mostufa et al. [76] | SPR | Graphene is integrated as a monolayer in a multilayered structure with other materials including tungsten disulfide and barium titanate. | Kretschmann configuration with a p-polarized light source (helio-neon laser) at a wavelength of 633 nm incident on a BK7 prism, detectors (CCD or CMOS) measure output reflection intensity and angle of incidence. |
| Ishtiak et al. [77] | SPR | Graphene integrated through deposition methods (details on deposition methods for other layers are provided). | Calcium fluoride prism as substrate, zinc oxide layer for adhesion, silver layer, thin Si layer, and graphene layer. Multiple light sources are used for detection. |
| Reference | Specific Intervention | Target | Comparison Material |
|---|---|---|---|
| Maharana et al. [50] | Incorporation of a monolayer of graphene on silver to enhance field and performance | Detection and identification of different biomolecules of carbon-based ring structure | Bimetallic silver–gold configuration |
| Islam et al. [51] | Incorporation of a periodic array of subwavelength grating on top of a layer of graphene sheet in the multilayer SPR biosensor | Biomolecular interactions of cDNA–ssDNA | The traditional gold thin film without graphene, a conventional SPR biosensor, and a graphene-based SPR biosensor (G-SPRB) |
| Nayak et al. [52] | Incorporation of graphene, graphene oxide, and molybdenum disulfide as sensing layers in an SPR-based biosensor. | Not applicable | Silver without any 2D materials. |
| Meshingqalam et al. [53] | Modeling the effect of molecular adsorption on the electro-optical properties of graphene-based sensors for SPR detection. | Molecules like graphene–beryllium, graphene–hydrogen, and graphene–calcium, which impact the electro-optical properties of the sensor. | Not explicitly mentioned, but the study focuses on the changes in graphene properties due to molecular adsorption. |
| Sharma et al. [54] | Incorporation of a graphene monolayer as a protective and enhancing layer in the SPR sensor. | H2O and D2O samples. | Silver layer used alone, and potential use of silver–gold bimetallic layers. |
| Conceicao et al. [55] | Numerical analysis of a graphene-based SPR sensor using the Finite Element Method. | Fluorescent nanoparticles and other compounds in a microfluidic channel | Traditional SPCE sensor models use gold and operate in the optical spectrum. |
| Liang et al. [56] | Design of a tunable triple-band graphene refractive index sensor with good angle-polarization tolerance | Refractive index changes in the surrounding medium, useful for detecting gases, liquids, or mixed solutions | Traditional SPR sensors typically use metals like gold and silver. |
| Rouf et al. [57] | The study presents a graphene–molybdenum-enhanced SPR biosensor incorporating a silver metallic layer and a titanium adhesion layer to improve performance and chemical stability. | Biological analytes including glucose, the stroma of the cornea, blood plasma, and DNA templates of the hepatitis B virus | Traditional materials such as gold and conventional SPR sensors without molybdenum diselenide and graphene. |
| Wu et al. [58] | Use of graphene sheet coated above a gold thin film | Biomolecules with carbon-based ring structures, such as single-stranded DNA | Gold thin film |
| Islam et al. [59] | Incorporation of a graphene sheet on top of a gold thin film in an LSPR biosensor | Biomolecular interactions of biotin–streptavidin | Gold, silver, copper, and aluminum thin films |
| Islam et al. [60] | Introduction of an additional graphene sheet layer on top of a gold thin film in a variable incidence angle LSPR biosensor. | Biotin–streptavidin interaction | Traditional materials such as gold and configurations without the graphene layer. |
| Islam et al. [61] | Introduction of an additional graphene sheet layer on top of a gold layer in a multilayer LSPR biosensor for enhanced sensitivity and detection accuracy. | Streptavidin (biotin–streptavidin interactions) | Traditional materials such as gold, silver, copper, and aluminum thin films. |
| Islam et al. [62] | Introduction of a periodic array of dielectric grating on top of a graphene layer to improve the sensitivity of an LSPR biosensor for monitoring biomolecular interactions of biotin–streptavidin. | Biotin–streptavidin interactions | Traditional materials include gold, copper, silver, and aluminum thin films. |
| Wu et al. [63] | Development of a SPR biosensor using a graphene ribbon array on a quartz substrate for infrared wavelength detection. | Detection of refractive index changes in gases and low-refractive-index materials in aqueous environments. | Traditional materials such as metallic nanostructures and metal–dielectric configurations are used for SPR sensing in visible and near-infrared wavelengths. |
| Maharana et al. [64] | Development of a low-index dielectric (Teflon)-mediated SPR sensor based on graphene in a dielectric-metal–dielectric configuration for near-infrared measurements. | Specific biomolecules detectable in the near-infrared spectrum | Traditional materials used for comparison include gold and silver |
| Islam et al. [65] | Integration of a periodic array of subwavelength grating on top of a layer of graphene sheet to improve sensitivity for DNA hybridization detection. | Biomolecular interactions, specifically focusing on the binding of biotin–streptavidin | Gold thin films and other metal–dielectric configurations are used in traditional SPR sensors. |
| Toloue et al. [66] | The study incorporates graphene layers on a conventional copper SPR biosensor to enhance sensitivity. This is based on the high adsorption efficiency of graphene due to π-stacking interaction with carbon-based ring biomolecules like single-stranded DNA. | DNA molecules | Traditional materials compared in the study include copper alone without the addition of graphene. |
| Vermaet al. [67] | Use of graphene and an air gap as dielectric layers in the SPR biosensor | Detection of Pseudomonas and Pseudomonas-like bacteria | Silver and gold |
| Wu et al. [68] | Incorporation of a graphene layer on the metal surface of a LRSPR biosensor. | Enhancing the sensitivity and detection accuracy of biosensors for chemical examination, medical diagnosis, and biological detection. | Traditional materials used for comparison include gold, copper, aluminum, and silver. |
| Verma et al. [69] | The study proposes to use a graphene/metamaterial film to enhance the adsorption of biomolecules. The film of graphene/metamaterial is coated on a gold film in the conventional SPR biosensor. | Biomolecule detection | The study compares the proposed biosensor with existing two-dimensional nanomaterials such as graphene-based biosensors and conventional SPR biosensors using gold or silver. |
| An et al. [70] | The study involves the use of chemically stable graphene and indium tin oxide (ITO) layers outside the fiber structure to realize a simple detection mechanism. | Refractive index sensing for biomolecules, water quality analysis, and other analytes. | The study compares the proposed biosensor with traditional D-shaped optical fibers and other conventional SPR-based sensors. |
| Huang et al. [71] | Use of continuous suspended monolayer graphene (MLG) and graphene/insulator stacks (GIS) for SPR-based THz plasmonic sensing | Enhancing detection accuracy and sensitivity for gas sensing applications | Traditional materials are not explicitly listed, but the study focuses on the advantages of graphene over noble metal plasmons. |
| Lin et al. [72] | Use of graphene as a defect layer attached to the surface of a one-dimensional photonic crystal (1DPC) to enhance biosensor performance. SPR biosensors rely on the excitation of surface plasmons on a metal layer, and BSW biosensors use a 1DPC to excite Bloch surface waves | The target for the biosensor is not explicitly mentioned, but it is designed to enhance sensitivity and detection accuracy, likely for various biomolecules. | Traditional BSW sensors without graphene, and conventional SPR biosensors. |
| Farmani et al. [73] | Use of a monolayer of chemical vapor deposition (CVD) graphene as the sensing layer. | High-resolution detection of refractive index changes in environmental monitoring applications, including temperature sensing and photodetectors for atomic force microscopy | Traditional materials such as noble metals (gold and silver) are used in conventional SPR configurations. |
| Hossain et al. [74] | Gate-controlled graphene SPR glucose sensor | Detection of glucose in blood samples | Gold-coated dielectric materials, Au–chromium nano-laminated SPR sensors |
| Behboudi et al. [75] | Use of a graphene-based metasurface for THz sensing | Wide range of biological tissues and chemical compounds. | Noble metals such as gold, copper, and platinum |
| Mostufa et al. [76] | Incorporation of a graphene-based multilayered structure (BK7/tungsten disulfide/gold/barium titanate/graphene) for SPR biosensor designed for rapid detection of the novel coronavirus (COVID-19). | Virus spike receptor-binding domain (RBD) and interactions with monoclonal antibodies (mAbs). | Traditional materials such as gold, platinum diselenide, and transition metal dichalcogenides (TMDCs) like molybdenum disulfide and tungsten disulfide. |
| Ishtiak et al. [77] | Incorporation of graphene for enhanced sensitivity in water salinity detection using SPR. | The concentration of salinity in water | Silver layer, compared with existing works. |
| Reference | Sensitivity Improvement | Specificity Improvement | Stability | Key Results |
|---|---|---|---|---|
| Maharana et al. [50] | More than 22% higher sensitivity compared to bimetallic silver–gold configuration | The study does not explicitly mention specificity improvement percentages but indicates enhanced biomolecule adhesion due to π-stacking interaction with graphene. | Graphene prevents oxidation of silver, resulting in an ultra-stable biosensor | Incorporation of a graphene monolayer on silver enhances the electric field by over 30% compared to the silver–gold bimetallic configuration. The proposed graphene-based sensor has more than 38% narrower full-width at half-maximum (FWHM) than the bimetallic configuration, indicating better detection accuracy. The graphene monolayer improves biomolecule adhesion, providing better interaction with the sensing layer and leading to higher performance in real-time biomolecular interactions. |
| Islam et al. [51] | Improved (specific percentage not provided, but sensitivity increases compared to both conventional SPRB and G-SPRB) | Enhanced (qualitative improvement indicated, the specific percentage not provided) | Graphene prevents oxidation of the underlying metal layer, resulting in more stable performance over time | The incorporation of a periodic array of subwavelength grating on a graphene layer significantly enhances the sensitivity of the SPR biosensor. The proposed GG-SPRB (graphene grating-based SPR biosensor) shows a larger shift in the resonance peak angle compared to conventional SPRB and G-SPRB, indicating better sensitivity. The study demonstrates improved field amplification and coupling of surface plasmon polaritons (SPPs) in the dielectric grating, leading to enhanced detection performance. The tunable operation of the GG-SPRB allows for optimized performance by varying design parameters, such as the number of graphene layers and the thickness of the biomolecular layer. |
| Nayak et al. [52] | Sensitivity increases from 190.47 nm/RIU for a single layer to 196.63 nm/RIU for five layers of graphene. The GO-based sensor showed a sensitivity of 202.2 nm/RIU compared to 189.4 nm/RIU for a graphene-based sensor. | Specificity improvement is not explicitly mentioned, but the study indicates better performance in terms of FOM (Figure of Merit) for graphene oxide and molybdenum disulfide compared to graphene. | Graphene and its derivatives, such as graphene oxide, enhance stability by preventing the oxidation of the silicon layer. | The incorporation of graphene and its derivatives significantly enhances the sensitivity and electric field intensity of SPR-based biosensors. Graphene oxide shows the highest sensitivity (202.2 nm/RIU) and lowest FWHM, resulting in the highest Figure of Merit (FOM). The electric field enhancement for the silver–silicon–graphene oxide system is 4.65 × 104%. |
| Meshingqalam et al. [53] | Sensitivity is improved due to the high specific surface area and attractive properties of graphene, such as high electron mobility and low electrical noise. Quantitative improvements are not provided but the improvement is noted as significant due to these factors. | Specificity improvement is not explicitly quantified, but the study notes the use of graphene enhances detection capabilities due to its unique electronic properties and high adsorption efficiency for sensing molecules. | The stability of graphene-incorporated biosensors is enhanced due to graphene’s high chemical and thermal stability, as well as its functionalization capability which makes it suitable for high-performance label-free chemical sensing. | The incorporation of graphene significantly enhances the sensitivity and electrical properties of SPR-based biosensors. The adsorption of sensing molecules on graphene’s surface leads to changes in its electrical conductivity, which can be attributed to local carrier concentration changes. Refractive index deviations based on band gap variations are modeled, showing that sensing molecule adsorption results in a non-zero band gap and changes in the refractive index, ultimately affecting SPR responses. |
| Sharma et al. [54] | Qualitative improvement: Enhanced sensitivity observed in the near-IR region for graphene monolayers. The combined sensitivity factor (CSF) can be maximized for a chemical potential range of 0.7 < µ < 1 eV. | Enhanced detection accuracy compared to traditional metal-only sensors due to the narrow spectral width of silver combined with graphene. | Graphene provides a protective coating atop the silver layer, preventing oxidation and improving chemical stability. | The study demonstrates that the incorporation of graphene in the “ZBLAN fluoride glass–silver–graphene” plasmonic structure significantly enhances the sensing performance. Optimal performance is achieved with a graphene monolayer and a chemical potential between 0.7 and 1 eV. Using multilayer graphene can slightly improve sensitivity, but the combined performance parameter (CSF) decreases with increased layers due to the damping effect. |
| Conceicao et al. [55] | The study indicates enhanced sensitivity due to the dynamic doping of graphene, although a specific percentage is not provided. | The graphene-based sensor shows improved specificity in the terahertz range compared to traditional SPCE sensors operating in the optical spectrum. | The study discusses the stability of the graphene-incorporated sensor, noting the enhanced stability provided by the dynamic control of graphene’s surface conductivity. | The study demonstrates that the graphene-based SPCE sensor has better performance in terms of sensitivity and specificity in the terahertz range compared to traditional gold-based SPCE sensors in the optical spectrum. The dynamic doping of graphene layers significantly influences the sensor’s response. |
| Liang et al. [56] | The article does not provide a specific percentage improvement in sensitivity but indicates a sensitivity of up to 11.56 μm/RIU | Enhanced | Good chemical stability, and high biocompatibility. | The study demonstrates that the graphene-based sensor has good angle-polarization tolerance and maintains polarization insensitivity over a wide angular range (0–60°). The sensor shows a large sensing range and high sensitivity, with a sensitivity of up to 11.56 μm/RIU. The sensing characteristics can be actively adjusted by changing the doping level of graphene, which is not possible with conventional SPR sensors. The graphene sensor can monitor tiny variations in the refractive index, making it suitable for detecting gases, liquids, or mixed solutions. |
| Rouf et al. [57] | Results are 2.42 times higher than the conventional SPR sensor and 2.35 times higher than the graphene-based sensor. | The improved sensitivity indicates better specificity as well. | Improved stability due to the inclusion of a Ti adhesion layer that protects the silver layer from oxidation, and graphene providing a protective layer. | The proposed sensor shows significant sensitivity enhancement with a sensitivity of 215.5°/RIU. The quality factor is slightly compromised, being 86% and 88% of conventional and graphene-based sensors, respectively. The sensor effectively detects biological analytes such as glucose, the stroma of the cornea, blood plasma, and DNA templates of the hepatitis B virus (HBV). The sensitivity improvement is due to the enhanced evanescent fields and increased interaction volume. |
| Wu et al. [58] | Up to 25% improvement in sensitivity with 10 layers of graphene compared to conventional gold SPR biosensors | The study does not explicitly mention specificity improvement percentages but indicates enhanced adsorption efficiency due to graphene’s interaction with biomolecules. | Improved stability due to stronger and more stable adsorption of biomolecules on graphene | The incorporation of graphene on gold thin films in SPR biosensors leads to a substantial increase in sensitivity, which can be attributed to two main factors: (a) the strong and stable adsorption of biomolecules on graphene, and (b) the modification of SPR curves by the optical properties of graphene. The overall sensitivity is increased by a factor of (1 + 0.025 L) × γ, where γ > 1. |
| Islam et al. [59] | Improved (specific percentage not provided, but sensitivity increases almost linearly with the number of graphene layers) | Enhanced (qualitative improvement mentioned, but no specific percentage provided) | Improved stability due to the stronger adsorption of biomolecules on graphene compared to gold, resulting in consistent performance | The study demonstrated that the incorporation of a graphene layer on top of a gold thin film in an LSPR biosensor significantly enhanced both sensitivity and adsorption efficiency compared to conventional LSPR biosensors. Sensitivity improvements were influenced by the number of graphene layers and the operating wavelength. The graphene-on-gold LSPR biosensor exhibited better sensitivity at lower operating wavelengths and with a larger number of graphene layers. |
| Islam et al. [60] | The sensitivity increases with the addition of graphene and is linearly related to the number of graphene layers. | The improved sensitivity indicates better specificity. | Improved stability due to the introduction of the graphene layer, which enhances the adsorption of biomolecules. | The study investigates the enhancement of the sensitivity of a variable incidence angle LSPR biosensor by introducing an additional graphene sheet layer on top of the gold thin film. The sensitivity, indicated by the shift of the plasmon resonance angle, increases with graphene deposited onto the gold layers and is linearly related to the number of graphene layers. The investigation was carried out for different analyte interfaces (air and water), and it was found that the graphene biosensor has better sensitivity for triangular prisms, higher prism angles, and water interfaces. |
| Islam et al. [61] | Enhanced sensitivity was observed with the introduction of graphene layers, with an overall improvement indicated by a higher shift in the plasmon dip compared to traditional materials. | Enhanced specificity due to the increased adsorption efficiency of graphene. | Improved stability with the inclusion of a graphene layer and an additional layer of silica-doped boron trioxide (sdB2O3), which compensates for the reduced signal-to-noise ratio. | The study investigates the improvement in sensitivity, adsorption efficiency, and detection accuracy of a multilayer LSPR graphene biosensor. The sensitivity of the conventional LSPR biosensor is enhanced by using the graphene sheet as a biomolecular recognition element on top of the gold thin film. The sensitivity can be further improved by selecting the appropriate operating wavelength, prism configuration, interface with prism, and thickness of target biomolecules. Introducing a layer of sdB2O3 under the graphene layer significantly improves the signal-to-noise ratio (SNR), though sensitivity is slightly reduced. The proposed design achieves a balance between high sensitivity and high detection accuracy. |
| Islam et al. [62] | The study shows a significant enhancement in sensitivity with the introduction of a graphene layer, resulting in improved sensitivity compared to traditional metal-only LSPR sensors. | Enhanced specificity due to the stronger adsorption of biomolecules on the graphene layer. | Improved stability due to the incorporation of graphene, which enhances the adsorption efficiency and stability of the biosensor. | The study presents a multilayer design for an LSPR biosensor incorporating a graphene layer and a periodic array of dielectric grating on top. The proposed design significantly improves the sensitivity of the LSPR biosensor for monitoring biomolecular interactions. The sensitivity enhancement is attributed to the creation of local hot spots and an enlarged reaction area due to near-field interactions around the nanostructured metal surfaces. The numerical simulations using the FDTD method show that the proposed design provides optimal functioning conditions with improved sensitivity. |
| Wu et al. [63] | Sensitivity improvement is substantial, with a sensitivity of 4720 nm/RIU for gas detection and 5520 nm/RIU for low-refractive-index materials in an aqueous environment. | Enhanced specificity due to the increased adsorption of biomolecules on the graphene surface. | Improved stability, with the graphene ribbon array providing dynamic tunability and consistent performance over a wide range of wavelengths from infrared to THz. | The study presents a novel transmission-type SPR sensor based on a graphene ribbon array for infrared wavelengths. Significant improvement in sensitivity and figure of merit (FOM) compared to traditional metal-based SPR sensors. The sensitivity of the sensor is 4720 nm/RIU for detecting gas (refractive index change from 1.0 to 1.05) and 5520 nm/RIU for low-refractive-index materials in an aqueous environment (refractive index change from 1.30 to 1.35). The dynamic tunability of graphene enables detectable refractive index changes covering a broad wavelength range from infrared to THz. The sensor’s performance is influenced by the Fermi level and the number of graphene layers, which provide guidelines for optimizing sensor design. |
| Maharana et al. [64] | The imaging sensitivity of the proposed sensor is approximately 50% greater than that of the conventional 2S2G–gold–graphene-based affinity sensor. | Enhanced specificity due to the improved adsorption of biomolecules on the graphene layer. | The graphene layer acts as a passivating layer, preserving the plasmonic properties of silver and enhancing stability by preventing oxidation and corrosion. | The study proposes a low-index dielectric (Teflon)-mediated SPR sensor based on graphene in a dielectric–metal–dielectric (D–M–D) configuration for near-infrared measurements. At a wavelength of 850 nm, the field intensity enhancement factor at the graphene-sensing layer interface for the proposed chalcogenide Ge20Ga5Sb10S65 (2S2G)–teflon–gold–graphene-based sensor is 20% greater than for the 2S2G–gold–graphene-based sensor. The penetration depth of the field into the sensing region for the proposed sensor is 340% greater than for the conventional 2S2G–gold–graphene SPR sensor. The FWHM (full width at half maximum) of the SPR curve for the low loss SP (LLSP) mode is 90% smaller than that of the conventional SP (CSP) resonance sensor, enhancing detection accuracy. The proposed sensor demonstrates high imaging sensitivity and stability, making it suitable for high-throughput assessment of multiple simultaneous molecular interactions. |
| Islam et al. [65] | Approximately 18% sensitivity improvement compared to conventional SPRB. | Enhanced specificity due to improved adsorption efficiency and field amplification. | Improved stability by integrating graphene, which acts as a passivating layer, preventing oxidation and corrosion of the gold layer. | The incorporation of a periodic array of subwavelength grating on top of a graphene layer significantly enhances the sensitivity of the SPR biosensor. The proposed multilayer grating-graphene SPRB demonstrates improved performance in sensitivity, with approximately 18% enhancement compared to traditional SPRB structures. The sensor’s performance is optimized by adjusting the grating configurations (rectangular, sinusoidal, triangular), grating depth, and volume factor. The sensor shows a larger shift of resonance peak, indicating improved sensitivity. Numerical simulations validate the performance enhancements, showing strong plasmonic resonances at optimized configurations. |
| Toloue et al. [66] | Not explicitly specified in terms of percentage, but the study indicates a significant enhancement. | Not explicitly specified in terms of percentage. | The text mentions improved stability with the prevention of oxidation by the graphene layer. | The study demonstrates that the incorporation of graphene significantly enhances the sensitivity of SPR-based biosensors compared to conventional sensors. This is primarily due to the higher adsorption efficiency of graphene and a greater change in refractive index near the copper surface. |
| Vermaet al. [67] | The study indicates that the sensitivity of the proposed SPR biosensor with graphene and an air gap is 2.35 times greater than that of a graphene-based SPR sensor without an air gap. | Not explicitly specified in terms of percentage. | The graphene layer provided protection against the oxidation of the silver layer, improving the stability and performance of the biosensor. | The incorporation of both graphene and an air gap significantly enhances the sensitivity of the SPR biosensor. The optimized structure, with a 23 nm air gap and 53 nm silver layer, shows improved performance due to strong excitation of surface plasmons, resulting in a large change in the refractive index at the sensing medium interface. |
| Wu et al. [68] | Nearly tenfold improvement in sensitivity compared to the conventional SPR biosensor. | Significant increase in detection accuracy (DA). | The graphene layer enhances the absorption of biomolecules and prevents the oxidation of metals, improving stability. | The incorporation of graphene in the LRSPR biosensor leads to nearly tenfold improvement in sensitivity and a significant increase in detection accuracy. The graphene layer enhances surface plasmons and the absorption of biomolecules, leading to better performance. |
| Verma et al. [69] | The study indicates that the sensitivity of the proposed SPR biosensor with metamaterial and graphene is significantly higher compared to traditional materials. Specifically, the sensitivity improvement is quantified as follows: At 750 nm: 54.75 degrees/RIU for metamaterial, 41.47 degrees/RIU for graphene, and 40.42 degrees/RIU for conventional SPR. At 850 nm: 52.05 degrees/RIU for metamaterial, 42.71 degrees/RIU for graphene, and 41.66 degrees/RIU for conventional SPR. At 1000 nm: 49.10 degrees/RIU for metamaterial, 43.85 degrees/RIU for graphene, and 43.80 degrees/RIU for conventional SPR. | The detection accuracy (DA) is used as a measure of specificity. The study shows the following: At 750 nm: 5.63/degree for metamaterial, 0.88/degree for graphene, and 0.98/degree for conventional SPR. At 850 nm: 7.34/degree for metamaterial, 1.13/degree for graphene, and 1.22/degree for conventional SPR. At 1000 nm: 17.67/degree for metamaterial, 1.56/degree for graphene, and 1.65/degree for conventional SPR. | The study describes that the incorporation of graphene enhances the stability of the biosensor due to the stable adsorption of carbon-based biomolecules (e.g., ssDNA). Additionally, the metamaterial film further improves the adsorption efficiency, leading to enhanced stability. | The study demonstrates that the incorporation of graphene and metamaterial significantly enhances the performance of the SPR biosensor. The metamaterial-based SPR biosensor exhibits superior sensitivity, detection accuracy, and quality factor compared to graphene-based and conventional SPR biosensors. The enhancement is attributed to the high adsorption efficiency of the metamaterial film and the stable adsorption properties of graphene. |
| An et al. [70] | The sensitivity of the proposed quasi-D-shaped optical fiber plasmonic biosensor shows a wavelength interrogation sensitivity of 3908–10,693 nm/RIU in the dynamic index range from 1.33 to 1.38. This indicates a significant improvement in sensitivity compared to traditional materials. | The specificity improvement is not explicitly mentioned in terms of percentage, but the study demonstrates a maximum amplitude sensitivity of 95 RIU−1 at the wavelength of 2040 nm with the analyte refractive index of 1.37, suggesting an enhanced specificity. | The incorporation of graphene enhances the stability of the biosensor due to the stable adsorption properties of graphene and the ITO layer. This stability is further improved by the design of the quasi-D-shaped fiber, which reduces the likelihood of damage during manufacturing. | The study demonstrates that the incorporation of graphene and ITO layers in the quasi-D-shaped optical fiber plasmonic biosensor significantly enhances the performance. The sensor exhibits a high wavelength interrogation sensitivity of 10,693 nm/RIU and a maximum amplitude sensitivity of 95 RIU−1. The quasi-D-shaped design also simplifies the manufacturing process and enhances the impact toughness of the sensor. |
| Huang et al. [71] | Improved sensitivity by using multiple layers of graphene. The sensitivity values were calculated as 51.0, 16.8, 8.4, 4.43, and 2.12°/RIU for different layers of graphene. | Specificity was enhanced due to the higher detection accuracy of the graphene-based sensor. The study noted that more layers of graphene result in higher detection accuracy. | The stability of the graphene-incorporated biosensor is indicated to be high, with the potential for consistent performance over time due to the strong mechanical and electronic properties of graphene. | The study demonstrated that the incorporation of graphene significantly enhances the sensitivity, specificity, and detection accuracy of SPR-based biosensors compared to traditional materials like gold and silver. The optimal performance was achieved with monolayer graphene, which offered a preferable balance between sensitivity and detection accuracy. |
| Lin et al. [72] | The maximum intensity sensitivity of the proposed biosensor with graphene is 3.5 × 104/RIU, which is significantly greater than that in the conventional SPR or BSW sensors. For conventional SPR structures, the intensity sensitivity is about 100/RIU. | The specificity improvement is not quantitatively specified, but the study indicates that the proposed graphene-incorporated biosensor shows superior performance in terms of full width at half maximum (FWHM) and detection accuracy compared to conventional SPR sensors. | The study does not explicitly mention stability over time. However, it suggests that the energy losses are within an acceptable range with the graphene layer number varying from 2 to 5, implying stable performance within these parameters. | The novel biosensor configuration consisting of a one-dimensional photonic crystal (1DPC) and graphene as a defect layer significantly enhances the intensity sensitivity. The optimized structure achieves a maximum sensitivity of 3.5 × 104/RIU, outperforming conventional SPR and BSW sensors. The findings emphasize the superior performance in terms of FWHM and detection accuracy with the inclusion of graphene. |
| Farmani et al. [73] | The study demonstrated that the highest sensitivity is achieved with a monolayer CVD graphene thickness of 0.335 nm and silicon dioxide/silicon thickness of 300 nm at an incident angle of 62.5°. | The use of graphene led to significant improvements in specificity due to the strong coupling condition between the incident light and the graphene–silicon dioxide/silicon structure. | The study indicates that the graphene-incorporated biosensor showed stable performance with low linear and surface defects, verified through atomic force microscopy (AFM) and scanning electron microscopy (SEM). | The study presented a simple, low-cost, compact-footprint, label-free, and high-resolution graphene plasmonic sensor. The optimal performance was achieved with a monolayer CVD graphene thickness of 0.335 nm, silicon dioxide/silicon thickness of 300 nm, and an incident angle of 62.5°. Both theoretical and experimental results verified that the strong coupling condition significantly enhances the resolution and sensitivity of the SPR-based biosensor compared to traditional materials. |
| Hossain et al. [74] | Total of 21.48% improvement in sensitivity when a 20-V gate voltage is applied to the graphene monolayer compared to no gate voltage. | Enhanced specificity compared to state-of-the-art glucose sensors. The sensor is highly selective to blood sugar levels (BSL). | The sensor shows consistent performance and is less sensitive to environmental variations. The detection error remains within 4.75% on average and within 7.40% in the worst-case scenario when temperature varies by ±10 °C from a reference 25 °C. | The gate-controlled graphene SPR glucose sensor offers significant improvement in detection sensitivity and figure of merit compared to state-of-the-art SPR biosensors. The proposed sensor does not require labels or extensive sample preparation and is highly sensitive and selective to blood sugar levels while being less affected by environmental changes. |
| Behboudi et al. [75] | The study demonstrated a sensitivity of approximately 1.5 THz/Permittivity unit, which is adequate for sensing purposes at the given frequency range and thickness. | Enhanced specificity is achieved through unique spectral features such as Accumulated Spectral Power (ASP) and Averaged Group Delay (AGD), which are independent of the resonance frequencies and operate over a broad range of the spectrum. | The graphene-based sensor exhibits high stability and consistent performance, able to detect relative permittivity variations up to 4 with a resolution of 0.1 and thickness variations from 5 µm to 600 µm with a resolution of 0.5 µm. These capabilities are considered significantly higher than previously reported works. | The novel THz spectroscopy technique proposed in the study utilizes a graphene-based metasurface that operates in reflection mode. The sensor can detect variations in relative permittivity and thickness with high precision, supported by spectral features like ASP and AGD. The study highlights the superior field confinement and surface plasmonic resonance capabilities of the graphene-based metasurface compared to traditional noble metals such as gold, copper, and platinum. |
| Mostufa et al. [76] | Achieved a sensitivity of 230.77 deg/RIU, which is higher compared to other configurations (e.g., 140.35 deg/RIU for traditional materials). | Enhanced specificity indicated by high detection accuracy (DA) of 0.161 deg−1 and a figure of merits (FOM) of 37.22 RIU−1. | Demonstrated consistent and reproducible performance in detecting the SARS-CoV-2 virus, showing reliable results across different concentrations of analytes. | The study showed that the graphene-based multilayered SPR biosensor has significantly higher angular sensitivity (230.77 deg/RIU) and improved performance metrics compared to traditional SPR biosensors. The sensor can effectively detect the presence of the SARS-CoV-2 virus rapidly without false-positive results. The proposed configuration outperformed other existing models, demonstrating superior sensitivity and specificity. |
| Ishtiak et al. [77] | The proposed sensor achieved a significantly higher value of sensitivity at 397.71 deg./RIU. | Enhanced specificity, achieving a wide range of detection accuracy from 0.199 to 0.498 (1/deg.) with a high-quality factor of 99.50 (1/RIU). | The sensor shows high stability with a considerably low minimum reflectance of 0.05 (normalized) for the entire salinity detection range. | The study presents a graphene-based SPR sensor for rapid water salinity concentration detection. The sensor demonstrated significantly higher sensitivity and specificity, improved stability, and high efficiency in detecting salinity levels from 1% to 30% using a multiple-light source technique. |
References
- Camarcha, A.; Varriale, A.; Capo, A.; Pennacchio, A.; Calabrese, A.; Giannattasio, C.; Staiano, M. Emergent Biosensing Technologies Based on Fluorescence Spectroscopy and Surface Plasmon Resonance. Sensors 2021, 21, 906. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Z.H.; Zhao, W.M.; Wang, L.; Yan, X.; Zhu, A.S.; Zhu, X.F.; Tian, W.; Zhang, K.K. Research Advances on Surface Plasmon Resonance Biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef]
- Soler, M.; Lechuga, L.M. Principles, Technologies, and Applications of Plasmonic Biosensors. J. Appl. Phys. 2021, 129, 111102. [Google Scholar] [CrossRef]
- Janith, G.I.; Herath, H.S.; Hendeniya, N.; Attygalle, D.; Amarasinghe, D.A.S.; Logeeshan, V.; Weerakoon, B.S.; Mendis, H.; Samarasinghe, R.; Manathunga, D.G.; et al. Advances in Surface Plasmon Resonance Biosensors for Medical Diagnostics: An Overview of Recent Developments and Techniques. J. Pharm. Biomed. Anal. Open 2023, 2, 100019. [Google Scholar] [CrossRef]
- Mohseni-Dargah, M.; Falahati, Z.; Dabirmanesh, B.; Nasrollahi, P.; Khajeh, K. Machine Learning in Surface Plasmon Resonance for Environmental Monitoring. In Artificial Intelligence and Data Science in Environmental Sensing; Academic Press: Cambridge, MA, USA, 2022; pp. 269–298. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar, S.; CA, M.; Thirunavookarasu, S.N.; CK, S. Recent Advances in Surface Plasmon Resonance (SPR) Biosensors for Food Analysis: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1055–1077. [Google Scholar] [CrossRef]
- Polshettiwar, S.A.; Deshmukh, C.D.; Baheti, A.M.; Wani, M.S.; Bompilwar, E.; Jambhekar, D.; Kolhe, A.; Manwar, J.V.; Parwe, S.; Bhogade, R.; et al. Recent Trends on Biosensors in Healthcare and Pharmaceuticals: An Overview. Int. J. Pharm. Investig. 2021, 11, 131–136. [Google Scholar] [CrossRef]
- Topor, C.V.; Puiu, M.; Bala, C. Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing. Biosensors 2023, 13, 465. [Google Scholar] [CrossRef]
- Lu, M.; Peng, W.; Lin, M.; Wang, F.; Zhang, Y. Gold Nanoparticle-Enhanced Detection of DNA Hybridization by a Block Copolymer-Templating Fiber-Optic Localized Surface Plasmon Resonance Biosensor. Nanomaterials 2021, 11, 616. [Google Scholar] [CrossRef]
- Falkowski, P.; Lukaszewski, Z.; Gorodkiewicz, E. Potential of Surface Plasmon Resonance Biosensors in Cancer Detection. J. Pharm. Biomed. Anal. 2021, 194, 113802. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cao, S.; Gao, X.; Chen, X.; Zhang, D. Improving the Detection Accuracy of an Ag/Au Bimetallic Surface Plasmon Resonance Biosensor Based on Graphene. Chemosensors 2021, 10, 10. [Google Scholar] [CrossRef]
- Lambert, A.S.; Valiulis, S.N.; Malinick, A.S.; Tanabe, I.; Cheng, Q. Plasmonic Biosensing with Aluminum Thin Films under the Kretschmann Configuration. Anal. Chem. 2020, 92, 8654–8659. [Google Scholar] [CrossRef]
- Kumar, D.; Khurana, M.; Sharma, M.; Singh, V. Analogy of Gold, Silver, Copper and Aluminium Based Ultra-Sensitive Surface Plasmon Resonance Photonic Crystal Fiber Biosensors. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Nath, N.; Chilkoti, A. Label-Free Biosensing by Surface Plasmon Resonance of Nanoparticles on Glass: Optimization of Nanoparticle Size. Anal. Chem. 2004, 76, 5370–5378. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Alharthi, M.D.; Demchenko, A.V.; Stine, K.J. Adhesion Layer-Free Attachment of Gold on Silicon Wafer and Its Application in Localized Surface Plasmon Resonance-Based Biosensing. Sens. Actuators A Phys. 2020, 312, 112155. [Google Scholar] [CrossRef]
- Scarano, S.; Manera, M.G.; Colombelli, A.; Minunni, M.; Rella, R. Nanostructures and Polymers: Emerging Nanocomposites for Plasmonic Resonance Transducers. Thin Solid Films 2020, 698, 137859. [Google Scholar] [CrossRef]
- Šípová-Jungová, H.; Jurgová, L.; Hemmerová, E.; Homola, J. Interaction of Tris with DNA Molecules and Carboxylic Groups on Self-Assembled Monolayers of Alkanethiols Measured with Surface Plasmon Resonance. Appl. Surf. Sci. 2021, 546, 148984. [Google Scholar] [CrossRef]
- Anthi, J.; Vaněčková, E.; Spasovová, M.; Houska, M.; Vrabcová, M.; Vogelová, E.; Dolanský, J.; Kolivoška, V. Probing Charge Transfer through Antifouling Polymer Brushes by Electrochemical Methods: The Impact of Supporting Self-Assembled Monolayer Chain Length. Anal. Chim. Acta 2023, 1276, 341640. [Google Scholar] [CrossRef]
- Jiang, B.; Hou, Y.; Wu, J.; Ma, Y.; Gan, X.; Zhao, J. In-fiber photoelectric device based on graphene-coated tilted fiber grating. Opto-Electron. Sci. 2023, 2, 230012. [Google Scholar] [CrossRef]
- Jamil, N.A.; Menon, P.S.; Said, F.A.; Tarumaraja, K.A.; Mei, G.S.; Majlis, B.Y. Graphene-Based Surface Plasmon Resonance Urea Biosensor Using Kretschmann Configuration. In Proceedings of the 2017 IEEE Regional Symposium on Micro and Nanoelectronics (RSM), Penang, Malaysia, 23–25 August 2017; IEEE: New York, NY, USA, 2017; pp. 112–115. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Cheng, S.; Zhang, H.; Yi, Z.; Sun, T.; Wu, P.; Zeng, Q.; Raza, R. Tunable metamaterial absorption device based on Fabry–Perot resonance as temperature and refractive index sensing. Opt. Lasers Eng. 2024, 181, 108368. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Fang, F.; Li, L.; Yan, Z.; Zhang, L.; Sun, Q. Highly sensitive and miniature microfiber-based ultrasound sensor for photoacoustic tomography. Opto-Electron. Adv. 2022, 5, 200076. [Google Scholar] [CrossRef]
- Liu, J.; Bao, S.; Wang, X. Applications of Graphene-Based Materials in Sensors: A Review. Micromachines 2022, 13, 184. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Song, W. Physical and Chemical Sensors on the Basis of Laser-Induced Graphene: Mechanisms, Applications, and Perspectives. ACS Nano 2021, 15, 18708–18741. [Google Scholar] [CrossRef]
- Ma, Z.; Tian, Z.; Li, X.; You, C.; Wang, Y.; Mei, Y.; Di, Z. Self-Rolling of Monolayer Graphene for Ultrasensitive Molecular Sensing. ACS Appl. Mater. Interfaces 2021, 13, 49146–49152. [Google Scholar] [CrossRef]
- Szunerits, S.; Maalouli, N.; Wijaya, E.; Vilcot, J.P.; Boukherroub, R. Recent Advances in the Development of Graphene-Based Surface Plasmon Resonance (SPR) Interfaces. Anal. Bioanal. Chem. 2013, 405, 1435–1443. [Google Scholar] [CrossRef]
- Akib, T.B.A.; Mou, S.F.; Rahman, M.M.; Rana, M.M.; Islam, M.R.; Mehedi, I.M.; Rahman, M.T.; Bappy, M.S.I.; Shimu, S.A.; Kouzani, A.Z. Design and Numerical Analysis of a Graphene-Coated SPR Biosensor for Rapid Detection of the Novel Coronavirus. Sensors 2021, 21, 3491. [Google Scholar] [CrossRef]
- Zahra, Q.U.A.; Fang, X.; Luo, Z.; Ullah, S.; Fatima, S.; Batool, S.; Ahmad, M.; Rahman, Z.U.; Shahzad, F. Graphene Based Nanohybrid Aptasensors in Environmental Monitoring: Concepts, Design and Future Outlook. Crit. Rev. Anal. Chem. 2023, 53, 1433–1454. [Google Scholar] [CrossRef]
- Wang, C.F.; Sun, X.Y.; Su, M.; Wang, Y.P.; Lv, Y.K. Electrochemical Biosensors Based on Antibody, Nucleic Acid and Enzyme Functionalized Graphene for the Detection of Disease-Related Biomolecules. Analyst 2020, 145, 1550–1562. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, B.; Xu, J.; Qing, T.; Zhang, P.; Qing, Z. Graphene Biosensors for Bacterial and Viral Pathogens. Biosensors 2020, 166, 112471. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, R.; Wang, X.; Xue, T.; Liu, Y.; Nel, A.; Huang, Y.; Duan, X. Real-Time Electrical Detection of Nitric Oxide in Biological Systems with Sub-Nanomolar Sensitivity. Nat. Commun. 2013, 4, 2225. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Song, D.; Zhang, D.; Zhang, H.; Ding, Y.; Yu, Y.; Sun, Y. A Highly Sensitive SPR Biosensor Based on a Graphene Oxide Sheet Modified with Gold Bipyramids, and Its Application to an Immunoassay for Rabbit IgG. Microchim. Acta 2015, 182, 1739–1746. [Google Scholar] [CrossRef]
- Shushama, K.N.; Rana, M.M.; Inum, R.; Hossain, M.B. Graphene Coated Fiber Optic Surface Plasmon Resonance Biosensor for the DNA Hybridization Detection: Simulation Analysis. Opt. Commun. 2017, 383, 186–190. [Google Scholar] [CrossRef]
- Taya, S.A.; Daher, M.G.; Almawgani, A.H.; Hindi, A.T.; Zyoud, S.H.; Colak, I. Detection of Virus SARS-CoV-2 Using a Surface Plasmon Resonance Device Based on BiFeO3-Graphene Layers. Plasmonics 2023, 18, 1441–1448. [Google Scholar] [CrossRef]
- Park, C.S.; Yoon, H.; Kwon, O.S. Graphene-Based Nanoelectronic Biosensors. J. Ind. Eng. Chem. 2016, 38, 13–22. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-Based Biosensors for Detection of Biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef]
- Tran, P.T.; Choi, Y.S.; Jeong, H.Y.; Ku, B.C.; Kim, I.T. Emerging Graphene-Based Sensors for the Detection of Food Adulterants and Toxicants—A Review. Food Chem. 2021, 355, 129547. [Google Scholar] [CrossRef]
- Báez, D.F.; Brito, T.P.; Espinoza, L.C.; Méndez-Torres, A.M.; Sierpe, R.; Sierra-Rosales, P.; Venegas, C.J.; Yáñez, C.; Bollo, S. Graphene-Based Sensors for Small Molecule Determination in Real Samples. Microchem. J. 2021, 167, 106303. [Google Scholar] [CrossRef]
- Badillo-Ramírez, I.; Carreón, Y.J.P.; Rodríguez-Almazán, C.; Medina-Durán, C.M.; Islas, S.R.; Saniger, J.M. Graphene-Based Biosensors for Molecular Chronic Inflammatory Disease Biomarker Detection. Biosensors 2022, 12, 244. [Google Scholar] [CrossRef]
- Chandrasekar, N.; Balaji, R.; Perala, R.S.; Nik Humaidi, N.Z.; Shanmugam, K.; Liao, Y.-C.; Hwang, M.T.; Govindaraju, S. A Brief Review of Graphene-Based Biosensors Developed for Rapid Detection of COVID-19 Biomarkers. Biosensors 2023, 13, 307. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 386. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and Weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Mongeon, P.; Paul-Hus, A. The Journal Coverage of Web of Science and Scopus: A Comparative Analysis. Scientometrics 2016, 106, 213–228. [Google Scholar] [CrossRef]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Littlewood, A.; Marshall, C.; Metzendorf, M.I.; Noel-Storr, A.; Rader, T.; Shokraneh, F.; Thomas, J.; et al. Searching for and Selecting Studies. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019; pp. 67–108. [Google Scholar] [CrossRef]
- Poli, R. An Analysis of Publications on Particle Swarm Optimisation Applications; Technical Report CSM-469; Department of Computer Science, University of Essex: Essex, UK, 2007; ISSN 1744-8050. [Google Scholar]
- Chen, C.; Jin, J. Surface Acoustic Wave Vapor Sensor with Graphene Interdigital Transducer for TNT Detection. Sens. Imaging 2020, 21, 24. [Google Scholar] [CrossRef]
- Jabbarzadeh, F.; Habibzadeh-Sharif, A. High Performance Dielectric Loaded Graphene Plasmonic Waveguide for Refractive Index Sensing. Opt. Commun. 2021, 479, 126419. [Google Scholar] [CrossRef]
- Qian, L.; Thiruppathi, A.R.; Elmahdy, R.; van der Zalm, J.; Chen, A. Graphene-Oxide-Based Electrochemical Sensors for the Sensitive Detection of Pharmaceutical Drug Naproxen. Sensors 2020, 20, 1252. [Google Scholar] [CrossRef]
- Maharana, P.K.; Padhy, P.; Jha, R. On the Field Enhancement and Performance of an Ultra-Stable SPR Biosensor Based on Graphene. IEEE Photonics Technol. Lett. 2013, 25, 2156–2158. [Google Scholar] [CrossRef]
- Islam, M.S.; Kouzani, A.Z. Variable Incidence Angle Subwavelength Grating SPR Graphene Biosensor. In Proceedings of the 35th Annual International Conference of the IEEE EMBS, Osaka, Japan, 3–7 July 2013; pp. 3024–3027. [Google Scholar] [CrossRef]
- Nayak, J.K.; Maharana, P.K.; Jha, R. Dielectric Over-Layer Assisted Graphene, Its Oxide and MoS2 Based Fibre Optic Sensor with High Field Enhancement. J. Phys. D Appl. Phys. 2017, 50, 415105. [Google Scholar] [CrossRef]
- Meshginqalam, B.; Ahmadi, M.T.; Sabatyan, A.; Ismail, R. The Effect of Molecular Adsorption on Electro-Optical Properties of Graphene-Based Sensors. Plasmonics 2016, 12, 1193–1198. [Google Scholar] [CrossRef]
- Sharma, A.K.; Dominic, A. Influence of Chemical Potential on Graphene-Based SPR Sensor’s Performance. IEEE Photonics Technol. Lett. 2018, 30, 95–98. [Google Scholar] [CrossRef]
- Conceicao, Y.G.; Cruz, A.F.S.; Amaral, P.R.; Lopes, P.C.; Del Rosso, T.; Dmitriev, V.; Costa, K.Q. Numerical Analysis of a Graphene-Based SPR Sensor by the Finite Element Method. In Proceedings of the 2019 SBMO/IEEE MTT-S International Microwave and Optoelectronics Conference (IMOC), Aveiro, Portugal, 10–14 November 2019. [Google Scholar] [CrossRef]
- Liang, C.; Niu, G.; Chen, X.; Zhou, Z.; Yi, Z.; Ye, X.; Duan, T.; Yi, Y.; Xiao, S. Tunable Triple-Band Graphene Refractive Index Sensor with Good Angle-Polarization Tolerance. Opt. Commun. 2018, 436, 57–62. [Google Scholar] [CrossRef]
- Rouf, H.K.; Haque, T. Sensitivity Enhancement of Graphene-MoSe2–Based SPR Sensor Using Ti Adhesion Layer for Detecting Biological Analytes. Plasmonics 2021, 16, 1945–1954. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly Sensitive Graphene Biosensors Based on Surface Plasmon Resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef]
- Islam, M.S.; Kouzani, A.Z.; Dai, X.J.; Michalski, W.P.; Gholamhosseini, H. Comparison of Performance Parameters for Conventional and Localized Surface Plasmon Resonance Graphene Biosensors. In Proceedings of the 33rd Annual International Conference of the IEEE EMBS, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar] [CrossRef]
- Islam, M.S.; Kouzani, A.Z. Variable Incidence Angle Localized Surface Plasmon Resonance Graphene Biosensor. In Proceedings of the 2011 IEEE/ICME International Conference on Complex Medical Engineering, Harbin, China, 22–25 May 2011. [Google Scholar] [CrossRef]
- Islam, M.S.; Kouzani, A.Z.; Dai, X.J.; Michalski, W.P.; Gholamhosseini, H. Design and Analysis of a Multilayer Localized Surface Plasmon Resonance Graphene Biosensor. J. Biomed. Nanotechnol. 2012, 8, 380–393. [Google Scholar] [CrossRef]
- Islam, M.S.; Kouzani, A.Z.; Dai, X.J.; Schiretz, H.F.; Lim, Y.C.; Michalski, W.P. Sensitivity Analysis of a Sub-Wavelength Localized Surface Plasmon Resonance Graphene Biosensor. In Proceedings of the 2012 International Conference on Complex Medical Engineering, Kobe, Japan, 1–4 July 2012. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, C.; Yu, J.; Cao, H.; Li, S.; Jia, W. Design of Infrared Surface Plasmon Resonance Sensors Based on Graphene Ribbon Arrays. Opt. Laser Technol. 2014, 59, 99–103. [Google Scholar] [CrossRef]
- Maharana, P.K.; Srivastava, T.; Jha, R. Low Index Dielectric Mediated Surface Plasmon Resonance Sensor Based on Graphene for Near Infrared Measurements. J. Phys. D Appl. Phys. 2014, 47, 385102. [Google Scholar] [CrossRef]
- Islam, M.S.; Kouzani, A.Z. Numerical Investigation of a Grating and Graphene-Based Multilayer Surface Plasmon Resonance Biosensor. J. Mod. Opt. 2014, 61, 1209–1218. [Google Scholar] [CrossRef]
- Toloue, H.; Centeno, A. Numerical Analysis on DNA-Sensor Based on Copper-Graphene Surface Plasmon Resonance. In Proceedings of the 2015 International Conference on Smart Sensors and Application (ICSSA), Kuala Lumpur, Malaysia, 26–28 May 2015. [Google Scholar] [CrossRef]
- Verma, A.; Prakash, A.; Tripathi, R. Sensitivity Enhancement of Surface Plasmon Resonance Biosensor Using Graphene and Air Gap. Opt. Commun. 2015, 357, 106–112. [Google Scholar] [CrossRef]
- Wu, L.; Ling, Z.; Jiang, L.; Guo, J.; Dai, X.; Xiang, Y.; Fan, D. Long-Range Surface Plasmon with Graphene for Enhancing the Sensitivity and Detection Accuracy of Biosensor. IEEE Photonics J. 2016, 8, 4801409. [Google Scholar] [CrossRef]
- Verma, A.; Prakash, A.; Tripathi, R. Comparative Study of a Surface Plasmon Resonance Biosensor Based on Metamaterial and Graphene. Silicon 2016, 9, 309–320. [Google Scholar] [CrossRef]
- An, G.; Li, S.; Wang, H.; Zhang, X. Metal Oxide-Graphene-Based Quasi-D-Shaped Optical Fiber Plasmonic Biosensor. IEEE Photonics J. 2017, 9, 6803909. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, S. Tunable Terahertz Plasmonics Sensor Using Doped Graphene. IEEE Photonics J. 2017. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, B.D. High Sensitivity Intensity-Interrogated Bloch Surface Wave Biosensor with Graphene. IEEE Sens. J. 2018, 18, 106–113. [Google Scholar] [CrossRef]
- Farmani, A.; Mir, A. Graphene Sensor Based on Surface Plasmon Resonance for Optical Scanning. IEEE Photonics Technol. Lett. 2019, 31, 643–646. [Google Scholar] [CrossRef]
- Hossain, M.M.; Talukder, M.A. Gate-Controlled Graphene Surface Plasmon Resonance Glucose Sensor. Opt. Commun. 2021, 493, 126994. [Google Scholar] [CrossRef]
- Behboudi Amlashi, S.; Khalily, M.; Singh, V.; Xiao, P.; Carey, J.D.; Tafazolli, R. Surface Electromagnetic Performance Analysis of a Graphene-Based Terahertz Sensor Using a Novel Spectroscopy Technique. IEEE J. Sel. Areas Commun. 2021, 39, 1797–1814. [Google Scholar] [CrossRef]
- Mostufa, S.; Akib, T.B.A.; Rana, M.M.; Mehedi, I.M.; Al-Saggaf, U.M.; Alsaggaf, A.U.; Alsaggaf, M.U.; Alam, M.S. Numerical Approach to Design the Graphene-Based Multilayered Surface Plasmon Resonance Biosensor for the Rapid Detection of the Novel Coronavirus. Opt. Contin. 2022, 1, 494–512. [Google Scholar] [CrossRef]
- Ishtiak, K.M.; Imam, S.A.; Khosru, Q.D.M. Graphene-Based Surface Plasmon Resonance Sensor for Water Salinity Concentration Detection Using Multiple Light Source Techniques. IEEE Access 2023, 11, 130601–130617. [Google Scholar] [CrossRef]
- Jpt, H. Cochrane Handbook for Systematic Reviews of Interventions. 2008. Available online: http://www.cochrane-handbook.org (accessed on 15 January 2024).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group*. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Gough, D.; Thomas, J.; Oliver, S. An Introduction to Systematic Reviews; Sage Publications Ltd.: New York, NY, USA, 2017. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]


| P | Population | Biosensors using surface plasmon resonance (SPR) technology. |
| I | Intervention | Incorporation of graphene into SPR biosensors. |
| C | Comparison | SPR biosensors without graphene or using alternative materials. |
| O | Outcomes | Improved sensitivity, specificity, and stability of SPR-based detection. |
| S | Study Design | Evaluation of recent research articles and studies on graphene-enhanced SPR biosensors. |
| Database | Query | Results |
|---|---|---|
| Scopus Web of Sciences PubMed IEEE Xplore | (“Surface plasmon resonance”) AND (“Graphene biosensor” OR “Graphene-based biosensor” OR “Graphene sensor” OR “Graphene-based sensor”) | 59 33 5 23 |
| Reference | Specific Intervention | Target | Sensitivity Improvement | Specificity Improvement | Stability |
|---|---|---|---|---|---|
| Maharana et al. [50] | Incorporation of a monolayer of graphene on silver to enhance field and performance | Detection and identification of different biomolecules of carbon-based ring structure | Improved (22%) | Unspecified | Ultra stable |
| Islam et al. [51] | Incorporation of a periodic array of subwavelength grating on top of a layer of graphene sheet in the multilayer SPR biosensor | Biomolecular interactions of cDNA-ssDNA | Improved | Not specified | Enhanced |
| Nayak et al. [52] | Incorporation of graphene, graphene oxide, and molybdenum disulfide as sensing layers in an SPR-based biosensor. | Not applicable | Improved (202.2 nm/RIU) | Not specified | Enhanced |
| Meshingqalam et al. [53] | Modeling the effect of molecular adsorption on the electro-optical properties of graphene-based sensors for SPR detection. | Molecules like graphene–beryllium, graphene–hydrogen, and graphene–calcium, which impact the electro-optical properties of the sensor. | Improved | High | Enhanced |
| Sharma et al. [54] | Incorporation of a graphene monolayer as a protective and enhancing layer in the SPR sensor. | H2O and D2O samples. | Enhanced | Enhanced | Stable |
| Conceicao et al. [55] | Numerical analysis of a graphene-based SPR sensor using the Finite Element Method. | Fluorescent nanoparticles and other compounds in a microfluidic channel | Enhanced | Improved | Enhanced |
| Liang et al. [56] | Design of a tunable triple-band graphene refractive index sensor with good angle-polarization tolerance | Refractive index changes in the surrounding medium, useful for detecting gases, liquids, or mixed solutions | Improved (11.560 nm/RIU) | Enhanced | Stable |
| Rouf et al. [57] | The study presents a graphene–molybdenum-enhanced SPR biosensor incorporating a silver metallic layer and a titanium adhesion layer to improve performance and chemical stability. | Biological analytes including glucose, the stroma of the cornea, blood plasma, and DNA templates of the hepatitis B virus | Improved (2.42 times) | Improved | Improved |
| Wu et al. [58] | Use of graphene sheet coated above a gold thin film | Biomolecules with carbon-based ring structures, such as single-stranded DNA | Improved (25%) | Enhanced | Stable |
| Islam et al. [59] | Incorporation of a graphene sheet on top of a thin gold film in an LSPR biosensor | Biomolecular interactions of biotin–streptavidin | Improved | Enhanced | Improved |
| Islam et al. [60] | Introduction of an additional graphene sheet layer on top of a gold thin film in a variable incidence angle LSPR biosensor. | Biotin–streptavidin interaction | Improved (linear) | Improved | Improved |
| Islam et al. [61] | Introduction of an additional graphene sheet layer on top of a gold layer in a multilayer LSPR biosensor for enhanced sensitivity and detection accuracy. | Streptavidin (biotin–streptavidin interactions) | Enhanced | Improved | Improved |
| Islam et al. [62] | Introduction of a periodic array of dielectric grating on top of a graphene layer to improve the sensitivity of an LSPR biosensor for monitoring biomolecular interactions of biotin–streptavidin. | Biotin–streptavidin interactions | Significant | Enhanced | Improved |
| Wu et al. [63] | Development of a SPR biosensor using a graphene ribbon array on a quartz substrate for infrared wavelength detection. | Detection of refractive index changes in gases and low-refractive-index materials in aqueous environments. | Improved (4720 nm/RIU) | Enhanced | Stable |
| Maharana et al. [64] | Development of a low-index dielectric (Teflon)-mediated SPR sensor based on graphene in a dielectric–metal–dielectric configuration for near-infrared measurements. | Specific biomolecules detectable in the near-infrared spectrum | Improved (50%) | Enhanced | Improved |
| Islam et al. [65] | Integration of a periodic array of subwavelength grating on top of a layer of graphene sheet to improve sensitivity for DNA hybridization detection. | Biomolecular interactions, specifically focusing on the binding of biotin–streptavidin | Improved (18%) | Enhanced | Improved |
| Toloue et al. [66] | The study incorporates graphene layers on a conventional copper SPR biosensor to enhance sensitivity. This is based on the high adsorption efficiency of graphene due to π-stacking interaction with carbon-based ring biomolecules like single-stranded DNA. | DNA molecules | Significant | Not specified | Improved |
| Verma et al. [67] | Use of graphene and an air gap as dielectric layers in the SPR biosensor | Detection of Pseudomonas and Pseudomonas-like bacteria | Improved (2.35 times) | Not specified | Improved |
| Wu et al. [68] | Incorporation of a graphene layer on the metal surface of a LRSPR biosensor. | Enhancing the sensitivity and detection accuracy of biosensors for chemical examination, medical diagnosis, and biological detection. | Significant (Nearly tenfold) | Significant | Improved |
| Verma et al. [69] | The study proposes to use graphene/metamaterial film to enhance the adsorption of biomolecules. The film of graphene/metamaterial is coated on a gold film in the conventional SPR biosensor. | Biomolecule detection | Improved (750 nm: 54.75°/RIU) | Significant | Enhanced |
| An et al. [70] | The study involves the use of chemically stable graphene and indium tin oxide (ITO) layers outside the fiber structure to realize a simple detection mechanism. | Refractive index sensing for biomolecules, water quality analysis, and other analytes. | Improved (1069–3906 nm/RIU) | Enhanced | Improved |
| Huang et al. [71] | Use of continuous suspended monolayer graphene (MLG) and graphene/insulator stacks (GIS) for SPR-based THz plasmonic sensing | Enhancing detection accuracy and sensitivity for gas sensing applications | Significant (Up to 51.0°/RIU) | Higher | Consistent |
| Lin et al. [72] | Use of graphene as a defect layer attached to the surface of a one-dimensional photonic crystal (1DPC) to enhance biosensor performance. SPR biosensors rely on the excitation of surface plasmons on a metal layer, and BSW biosensors use a 1DPC to excite Bloch Surface Waves | The target for the biosensor is not explicitly mentioned, but it is designed to enhance sensitivity and detection accuracy, likely for various biomolecules. | Improved (3.5 times) | Not specified | Stable |
| Farmani et al. [73] | Use of a monolayer of chemical vapor deposition (CVD) graphene as the sensing layer. | High-resolution detection of refractive index changes in environmental monitoring applications, including temperature sensing and photodetectors for atomic force microscopy | Highest | Improved | Stable |
| Hossain et al. [74] | Gate-controlled graphene SPR glucose sensor | Detection of glucose in blood samples | Improved (21.4%) | Enhanced | Consistent |
| Behboudi et al. [75] | Use of a graphene-based metasurface for THz sensing | Wide range of biological tissues and chemical compounds. | Improved (1.5 THz/Permittivity) | Enhanced | High |
| Mostufa et al. [76] | Incorporation of a graphene-based multilayered structure (BK7/tungsten disulfide/gold/barium titanate/graphene) for an SPR biosensor designed for rapid detection of the novel coronavirus (COVID-19). | Virus spike receptor-binding domain (RBD) and interactions with monoclonal antibodies (mAbs). | Improved (230.77°/RIU) | High | Consistent |
| Ishtiak et al. [77] | Incorporation of graphene for enhanced sensitivity in water salinity detection using SPR. | The concentration of salinity in water | Improved (397.1°/RIU) | Enhanced | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tene, T.; Bellucci, S.; Arias Arias, F.; Carrera Almendariz, L.S.; Flores Huilcapi, A.G.; Vacacela Gomez, C. Role of Graphene in Surface Plasmon Resonance-Based Biosensors. Sensors 2024, 24, 4670. https://doi.org/10.3390/s24144670
Tene T, Bellucci S, Arias Arias F, Carrera Almendariz LS, Flores Huilcapi AG, Vacacela Gomez C. Role of Graphene in Surface Plasmon Resonance-Based Biosensors. Sensors. 2024; 24(14):4670. https://doi.org/10.3390/s24144670
Chicago/Turabian StyleTene, Talia, Stefano Bellucci, Fabian Arias Arias, Luis Santiago Carrera Almendariz, Ana Gabriela Flores Huilcapi, and Cristian Vacacela Gomez. 2024. "Role of Graphene in Surface Plasmon Resonance-Based Biosensors" Sensors 24, no. 14: 4670. https://doi.org/10.3390/s24144670
APA StyleTene, T., Bellucci, S., Arias Arias, F., Carrera Almendariz, L. S., Flores Huilcapi, A. G., & Vacacela Gomez, C. (2024). Role of Graphene in Surface Plasmon Resonance-Based Biosensors. Sensors, 24(14), 4670. https://doi.org/10.3390/s24144670










