Combined Impact of Heart Rate Sensor Placements with Respiratory Rate and Minute Ventilation on Oxygen Uptake Prediction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
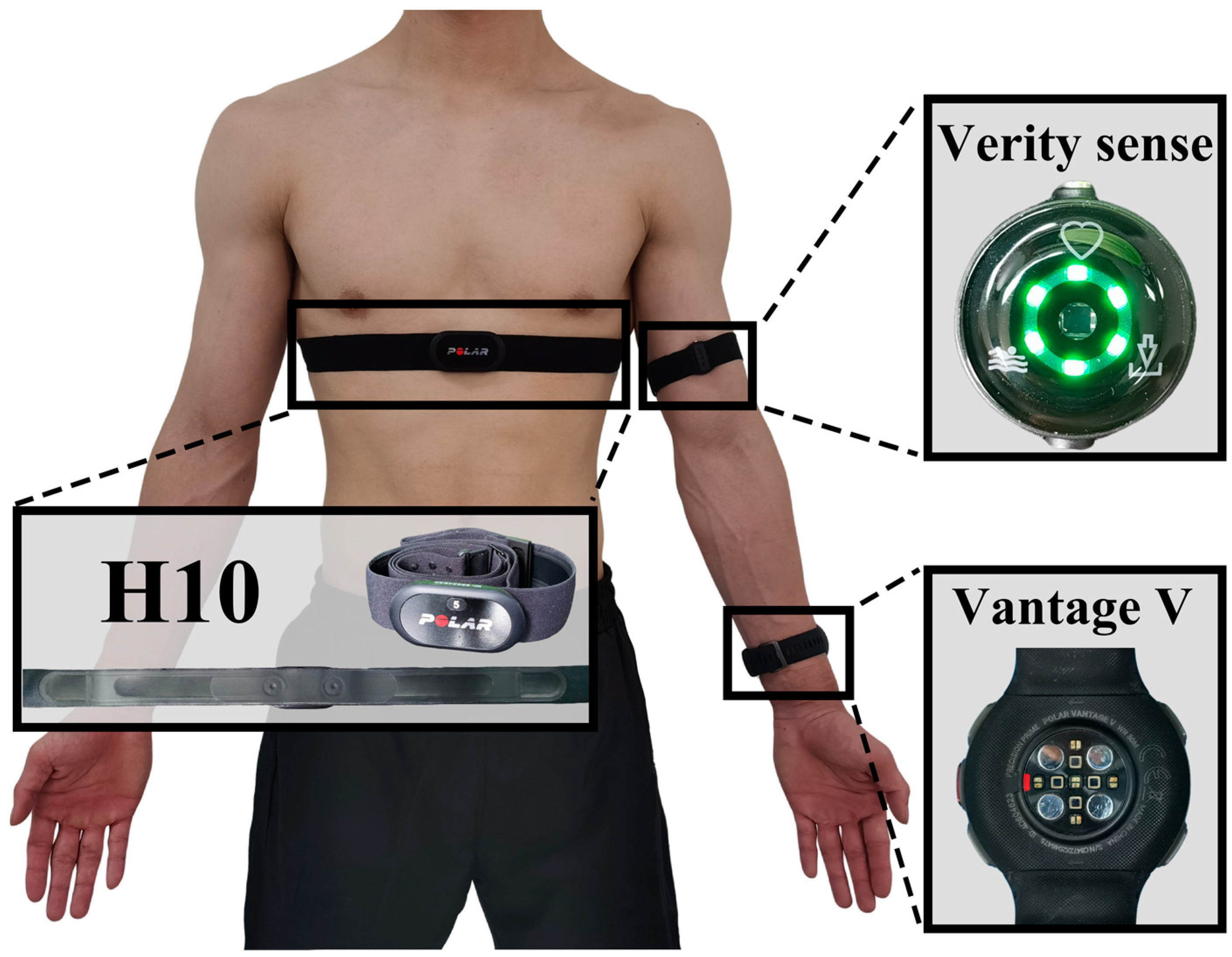
2.3. Data Collection
- The Polar H10 uses a 1000 Hz sample rate to gather data for internal sensor calculations and algorithms. Subsequently, the sensor outputs the processed data at a 130 Hz sampling rate. The H10 transmits HR data once per second;
- The Polar Vantage V utilizes PPG and provides HR samples at a rate of 1 Hz. According to Polar, the internal sampling rate of the Vantage V is considerably higher, and the 1 Hz HR data is derived from this higher-rate sampling;
- The Polar Verity Sense utilizes PPG, with a sampling rate of 135 Hz and a resolution of 22 bits.
2.4. Data Processing and Model Construction
2.4.1. Data Standardization
2.4.2. Building of Backpropagation Neural Network (BPNN)
- From heart rate () only, named ;
- From heart rate () and respiratory rate (), named ;
- From heart rate () and minute ventilation () named ;
- From heart rate (), respiratory rate (), and minute ventilation (), named .
- (1)
- Forward Propagation
- (2)
- Backward Propagation
2.4.3. Training Parameter Setting
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics and Exercise Responses
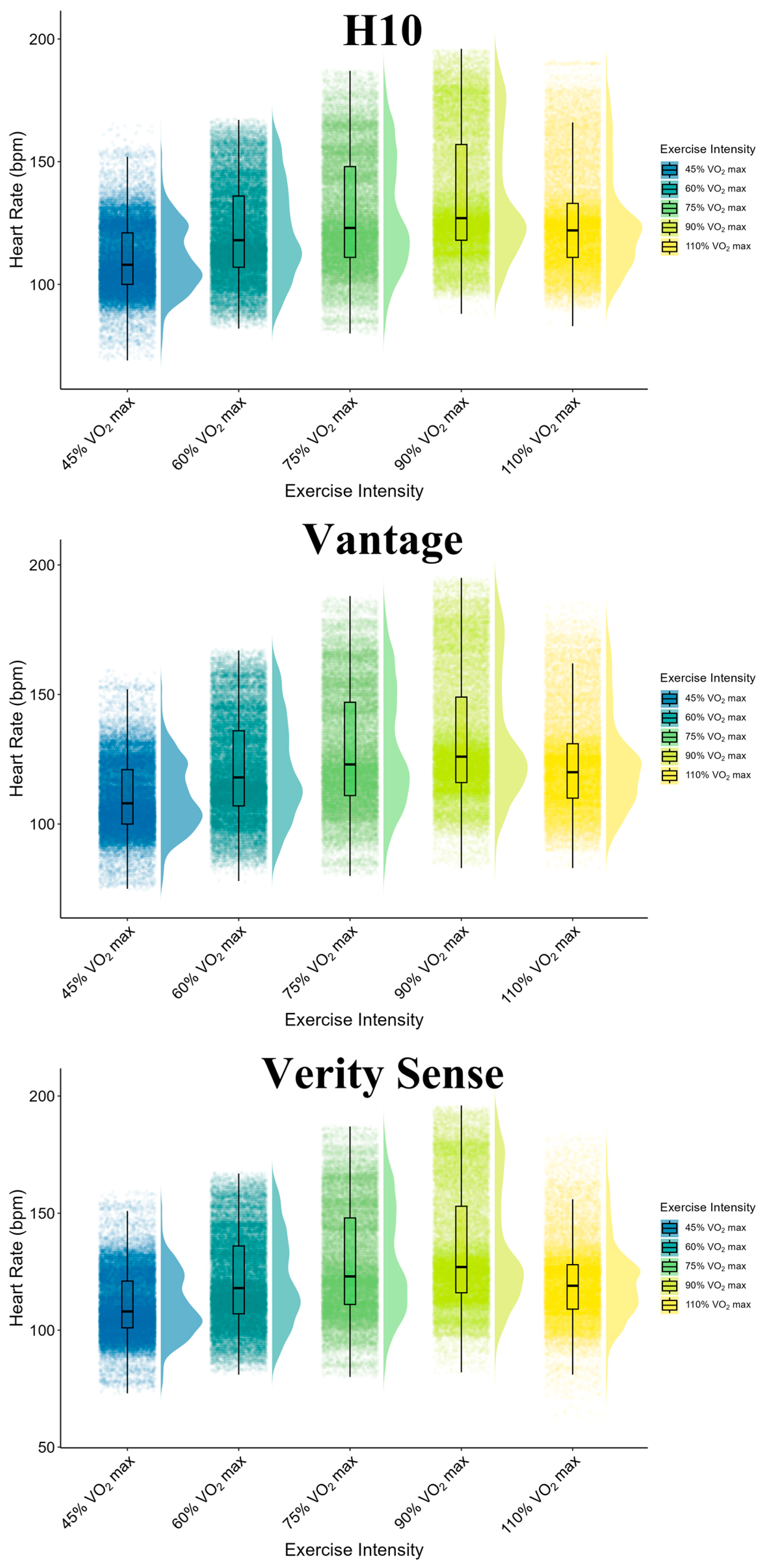
3.2. Descriptive of Heart Rate Measurements: H10, Vantage and Verity Sense
3.3. Accuracy of Different Sensor Positions: Vantage vs. Verity Sense
3.4. Impact of Sensor Placements on Prediction across Varied Exercise Intensities
3.5. Comprehensive Oxygen Consumption Prediction Accuracy with Heart Rate Positioning: Overall Exercise Intensities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spurr, G.B.; Prentice, A.M.; Murgatroyd, P.R.; Goldberg, G.R.; Reina, J.C.; Christman, N.T. Energy expenditure from minute-by-minute heart-rate recording: Comparison with indirect calorimetry. Am. J. Clin. Nutr. 1988, 48, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Powers, S.K.; Dodd, S.; Beadle, R.E. Oxygen uptake kinetics in trained athletes differing in VO2max. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 54, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Burnley, M.; Jones, A. Oxygen uptake kinetics as a determinant of sports performance. Eur. J. Sport Sci. 2007, 7, 63–79. [Google Scholar] [CrossRef]
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol. 2008, 586, 35–44. [Google Scholar] [CrossRef]
- Jones, A.M.; Carter, H. The effect of endurance training on parameters of aerobic fitness. Sports Med. 2000, 29, 373–386. [Google Scholar] [CrossRef]
- Alexander, N.B.; Dengel, D.R.; Olson, R.J.; Krajewski, K.M. Oxygen-uptake (VO2) kinetics and functional mobility performance in impaired older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 734–739. [Google Scholar] [CrossRef]
- Fahling, M.; Persson, P.B. Oxygen sensing, uptake, delivery, consumption and related disorders. Acta Physiol. 2012, 205, 191–193. [Google Scholar] [CrossRef]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2012, 33, 2917–2927. [Google Scholar]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- Brunner-La Rocca, H.P.; Weilenmann, D.; Schalcher, C.; Schlumpf, M.; Follath, F.; Candinas, R.; Kiowski, W. Prognostic significance of oxygen uptake kinetics during low level exercise in patients with heart failure. Am. J. Cardiol. 1999, 84, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Kurpad, A.V.; Raj, R.; Maruthy, K.N.; Vaz, M. A simple method of measuring total daily energy expenditure and physical activity level from the heart rate in adult men. Eur. J. Clin. Nutr. 2006, 60, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, P.G.; Green, D.J.; Etxebarria, N.; Pyne, D.B.; Saunders, P.U.; Minahan, C.L. Validation of heart rate monitor-based predictions of oxygen uptake and energy expenditure. J. Strength Cond. Res. 2009, 23, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Wicks, J.R.; Oldridge, N.B.; Nielsen, L.K.; Vickers, C.E. HR index—A simple method for the prediction of oxygen uptake. Med. Sci. Sports Exerc. 2011, 43, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Li, J.; Li, J.; Shan, W.; Yan, H.; Lu, Y. Estimation of heart rate using regression models and artificial neural network in middle-aged adults. Front. Physiol. 2021, 12, 742754. [Google Scholar] [CrossRef] [PubMed]
- Kalahasty, G.; Ellenbogen, K.A. Simpler is better: New lessons learned from the 12-lead electrocardiogram. Circulation 2010, 121, 617–619. [Google Scholar] [CrossRef]
- Lin, B.S.; Wong, A.M.; Tseng, K.C. Community-Based ECG Monitoring System for Patients with Cardiovascular Diseases. J. Med. Syst. 2016, 40, 80. [Google Scholar] [CrossRef]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar]
- Mu, S.; Liao, S.; Tao, K.; Shen, Y. Intelligent fatigue detection based on hierarchical multi-scale ECG representations and HRV measures. Biomed. Signal Process. Control. 2024, 92, 106127. [Google Scholar] [CrossRef]
- Elgendi, M.; Fletcher, R.; Yongbo, L.; Newton, H.; Lovell, N.; Abbott, D.; Kenneth, L.; Ward, R. The use of photoplethysmography for assessing hypertension. NPJ Digit. Med. 2019, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.; Prentice, A.M.; Coward, W.A.; Ceesay, S.M.; Strain, J.J.; McKenna, P.G.; Nevin, G.B.; Barker, M.E.; Hickey, R.J. Simultaneous measurement of free-living energy expenditure by the doubly labeled water method and heart-rate monitoring. Am. J. Clin. Nutr. 1990, 52, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Schantz, P.; Salier Eriksson, J.; Rosdahl, H. The heart rate method for estimating oxygen uptake: Analyses of reproducibility using a range of heart rates from commuter walking. Eur. J. Appl. Physiol. 2019, 119, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Assah, F.K.; Ekelund, U.; Brage, S.; Corder, K.; Wright, A.; Mbanya, J.C.; Wareham, N.J. Predicting physical activity energy expenditure using accelerometry in adults from sub-Sahara Africa. Obesity 2009, 17, 1588–1595. [Google Scholar] [CrossRef]
- Sirichana, W.; Dolezal, B.A.; Neufeld, E.V.; Wang, X.; Cooper, C.B. Wrist-worn triaxial accelerometry predicts the energy expenditure of non-vigorous daily physical activities. J. Sci. Med. Sport 2017, 20, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, A.; Kettunen, J.; Martinmäki, K.; Saalasti, S.; Rusko, H. On-and off-dynamics and respiration rate enhance the accuracy of heart rate based VO2 estimation. Med. Sci. Sports Exerc. 2004, 36, S253. [Google Scholar]
- Yan, Y.; Chen, Q. Energy Expenditure Estimation of Tabata by Combining Acceleration and Heart Rate. Front. Public Health 2021, 9, 804471. [Google Scholar] [CrossRef]
- Keytel, L.R.; Goedecke, J.H.; Noakes, T.D.; Hiiloskorpi, H.; Laukkanen, R.; van der Merwe, L.; Lambert, E.V. Prediction of energy expenditure from heart rate monitoring during submaximal exercise. J. Sports Sci. 2005, 23, 289–297. [Google Scholar] [CrossRef]
- Beltrame, T.; Amelard, R.; Villar, R.; Shafiee, M.J.; Wong, A.; Hughson, R.L. Estimating oxygen uptake and energy expenditure during treadmill walking by neural network analysis of easy-to-obtain inputs. J. Appl. Physiol. 2016, 121, 1226–1233. [Google Scholar] [CrossRef]
- Zignoli, A.; Fornasiero, A.; Ragni, M.; Pellegrini, B.; Schena, F.; Biral, F.; Laursen, P.B. Estimating an individual’s oxygen uptake during cycling exercise with a recurrent neural network trained from easy-to-obtain inputs: A pilot study. PLoS ONE 2020, 15, e0229466. [Google Scholar] [CrossRef]
- Beltrame, T.; Amelard, R.; Wong, A.; Hughson, R.L. Extracting aerobic system dynamics during unsupervised activities of daily living using wearable sensor machine learning models. J. Appl. Physiol. 2018, 124, 473–481. [Google Scholar] [CrossRef]
- Staudenmayer, J.; Pober, D.; Crouter, S.; Bassett, D.; Freedson, P. An artificial neural network to estimate physical activity energy expenditure and identify physical activity type from an accelerometer. J. Appl. Physiol. 2009, 107, 1300–1307. [Google Scholar] [CrossRef]
- Lu, K.; Yang, L.; Seoane, F.; Abtahi, F.; Forsman, M.; Lindecrantz, K. Fusion of Heart Rate, Respiration and Motion Measurements from a Wearable Sensor System to Enhance Energy Expenditure Estimation. Sensors 2018, 18, 3092. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Huang, Y.; Shen, Y.; Sun, L. Automated stress recognition using supervised learning classifiers by interactive virtual reality scenes. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2060–2066. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Schubert, M.M.; Clark, A.; De La Rosa, A.B. The Polar ((R)) OH1 Optical Heart Rate Sensor is Valid during Moderate-Vigorous Exercise. Sports Med. Int. Open 2018, 2, E67–E70. [Google Scholar] [CrossRef]
- Climstein, M.; Alder, J.L.; Brooker, A.M.; Cartwright, E.J.; Kemp-Smith, K.; Simas, V.; Furness, J. Reliability of the Polar Vantage M Sports Watch when Measuring Heart Rate at Different Treadmill Exercise Intensities. Sports 2020, 8, 117. [Google Scholar] [CrossRef]
- Alzahrani, A.; Hu, S.; Azorin-Peris, V.; Barrett, L.; Esliger, D.; Hayes, M.; Akbare, S.; Achart, J.; Kuoch, S. A multi-channel opto-electronic sensor to accurately monitor heart rate against motion artefact during exercise. Sensors 2015, 15, 25681–25702. [Google Scholar] [CrossRef]
- Armstrong, R.B. Magnitude and distribution of muscle blood flow in conscious animals during locomotory exercise. Med. Sci. Sports Exerc. 1988, 20, S119–S123. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.L.; Ellerby, D.J. Partitioning locomotor energy use among and within muscles. Muscle blood flow as a measure of muscle oxygen consumption. J. Exp. Biol. 2006, 209, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-Ananta, T.; Ajmal; Ramella-Roman, J.C.; McShane, M.J.; Cote, G.L. Sources of Inaccuracy in Photoplethysmography for Continuous Cardiovascular Monitoring. Biosensors 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.F.; Stergiou, P.; Fung, T.S.; Katz, L. Comparison of Polar M600 Optical Heart Rate and ECG Heart Rate during Exercise. Med. Sci. Sports Exerc. 2017, 49, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Olstad, B.H.; Zinner, C. Validation of the Polar OH1 and M600 optical heart rate sensors during front crawl swim training. PLoS ONE 2020, 15, e0231522. [Google Scholar] [CrossRef]
- Leonard, W.R. Measuring human energy expenditure: What have we learned from the flex-heart rate method? Am. J. Hum. Biol. 2003, 15, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Hedge, E.T.; Amelard, R.; Hughson, R.L. Prediction of oxygen uptake kinetics during heavy-intensity cycling exercise by machine learning analysis. J. Appl. Physiol. 2023, 134, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Amelard, R.; Hedge, E.T.; Hughson, R.L. Temporal convolutional networks predict dynamic oxygen uptake response from wearable sensors across exercise intensities. NPJ Digit. Med. 2021, 4, 156. [Google Scholar] [CrossRef] [PubMed]
- Montain, S.J.; Coyle, E.F. Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J. Appl. Physiol. 1992, 73, 1340–1350. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, A.E. Heart rate monitoring: Applications and limitations. Sports Med. 2003, 33, 517–538. [Google Scholar] [CrossRef]
- Lamberts, R.P.; Lambert, M.I. Day-to-day variation in heart rate at different levels of submaximal exertion: Implications for monitoring training. J. Strength Cond. Res. 2009, 23, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, H.; Ju, K.; Shin, K.; Lee, M.; Shelley, K.; Chon, K.H. Can Photoplethysmography Variability Serve as an Alternative Approach to Obtain Heart Rate Variability Information? J. Clin. Monit. Comput. 2008, 22, 23–29. [Google Scholar] [CrossRef]
- Sarkar, S.; Pahuja, S.K. ICT Systems and Sustainability. In Effects of Measurement Site on Heart Rate Variability Derived from Photoplethysmography; Tuba, M., Akashe, S., Joshi, A., Eds.; Springer Nature: Singapore, 2023; pp. 487–492. [Google Scholar]
- Yuda, E.; Shibata, M.; Ogata, Y.; Ueda, N.; Yambe, T.; Yoshizawa, M.; Hayano, J. Pulse rate variability: A new biomarker, not a surrogate for heart rate variability. J. Physiol. Anthropol. 2020, 39, 21. [Google Scholar] [CrossRef]


| Measurement | Male (n = 16) | Female (n = 12) |
|---|---|---|
| Age (y) | 19 ± 0 | 19 ± 1 |
| Body mass (kg) | 70.7 ± 5.7 | 55.6 ± 3.9 |
| Height (cm) | 180.6 ± 5.1 | 166.3 ± 4.1 |
| BMI (kg/m2) | 21.7 ± 1.8 | 20.2 ± 1.5 |
| Body fat percentage (%) | 10.1 ± 2.7 | 18.8 ± 3.7 |
| () | 47.3 ± 4.7 | 39.5 ± 4.1 |
| Exercises and Devices | Mean Difference ± SD | Mean Relative Error ± SD | Mean Absolute Error ± SD | Concordance Correlation Coefficient (95% CI) | Pearson Correlation |
|---|---|---|---|---|---|
| 45% | |||||
| Vantage | 0.16 ± 3.79 | 0.06 ± 3.54 | 2.59 ± 2.40 | 0.96 (0.96–0.96) | 0.96 |
| Verity Sense | 0.06 ± 2.99 | 0.00 ± 3.66 | 1.95 ± 2.00 | 0.98 (0.98–0.98) | 0.98 |
| 60% | |||||
| Vantage | 0.15 ± 3.55 | 0.09 ± 3.08 | 2.19 ± 2.18 | 0.95 (0.98–0.98) | 0.98 |
| Verity Sense | 0.28 ± 4.36 | 0.18 ± 3.30 | 1.83 ± 2.75 | 0.97 (0.97–0.97) | 0.97 |
| 75% | |||||
| Vantage | −0.04 ± 3.42 | −0.08 ± 2.84 | 1.92 ± 2.09 | 0.99 (0.99–0.99) | 0.99 |
| Verity Sense | 0.04 ± 2.90 | 0.01 ± 2.40 | 1.52 ± 1.85 | 0.99 (0.99–0.99) | 0.99 |
| 95% | |||||
| Vantage | 2.88 ± 13.29 | 1.64 ± 7.95 | 3.62 ± 7.26 | 0.85 (0.85–0.85) | 0.86 |
| Verity Sense | 1.94 ± 10.42 | 1.17 ± 6.55 | 2.83 ± 6.06 | 0.91 (0.91–0.91) | 0.91 |
| 110% | |||||
| Vantage | 2.33 ± 11.62 | 1.35 ± 7.3 | 3.49 ± 6.56 | 0.81 (0.81–0.82) | 0.82 |
| Verity Sense | 5.29 ± 17.56 | 3.18 ± 10.78 | 4.79 ± 10.17 | 0.54 (0.53–0.54) | 0.57 |
| Model | Devices | R2 | MAE (mL/min) | MAPE (%) | |
|---|---|---|---|---|---|
| 45% | H10 | 0.27 | 204.85 | 21.83% | |
| Vantage | 0.27 | 205.20 | 21.82% | ||
| Verity Sense | 0.26 | 204.91 | 21.81% | ||
| H10 | 0.27 | 205.50 | 21.71% | ||
| Vantage | 0.26 | 206.35 | 21.90% | ||
| Verity Sense | 0.26 | 205.96 | 21.88% | ||
| H10 | 0.85 | 88.98 | 9.29% | ||
| Vantage | 0.86 | 89.14 | 9.31% | ||
| Verity Sense | 0.86 | 89.01 | 9.30% | ||
| H10 | 0.91 | 68.89 | 7.12% | ||
| Vantage | 0.91 | 68.50 | 7.00% | ||
| Verity Sense | 0.91 | 68.46 | 7.00% | ||
| 60% | H10 | 0.52 | 244.11 | 22.39% | |
| Vantage | 0.51 | 247.76 | 22.68% | ||
| Verity Sense | 0.50 | 251.31 | 22.96% | ||
| H10 | 0.55 | 231.71 | 21.39% | ||
| Vantage | 0.54 | 235.89 | 21.72% | ||
| Verity Sense | 0.53 | 238.77 | 21.92% | ||
| H10 | 0.89 | 120.45 | 11.10% | ||
| Vantage | 0.88 | 120.65 | 11.12% | ||
| Verity Sense | 0.88 | 120.75 | 11.12% | ||
| H10 | 0.92 | 97.16 | 8.98% | ||
| Vantage | 0.92 | 100.54 | 9.22% | ||
| Verity Sense | 0.92 | 100.84 | 9.22% | ||
| 75% | H10 | 0.74 | 242.43 | 19.29% | |
| Vantage | 0.72 | 254.52 | 20.17% | ||
| Verity Sense | 0.73 | 249.75 | 19.81% | ||
| H10 | 0.75 | 238.43 | 18.86% | ||
| Vantage | 0.72 | 250.88 | 19.78% | ||
| Verity Sense | 0.73 | 246.33 | 19.45% | ||
| H10 | 0.92 | 139.40 | 11.14% | ||
| Vantage | 0.92 | 140.92 | 11.29% | ||
| Verity Sense | 0.92 | 140.55 | 11.25% | ||
| H10 | 0.96 | 102.87 | 8.30% | ||
| Vantage | 0.95 | 105.48 | 8.53% | ||
| Verity Sense | 0.95 | 104.91 | 8.47% | ||
| 95% | H10 | 0.73 | 317.35 | 23.48% | |
| Vantage | 0.59 | 367.81 | 25.75% | ||
| Verity Sense | 0.62 | 355.60 | 25.38% | ||
| H10 | 0.74 | 289.51 | 19.87% | ||
| Vantage | 0.58 | 345.66 | 22.30% | ||
| Verity Sense | 0.63 | 329.40 | 21.74% | ||
| H10 | 0.88 | 215.10 | 15.37% | ||
| Vantage | 0.87 | 227.47 | 16.00% | ||
| Verity Sense | 0.87 | 223.89 | 15.87% | ||
| H10 | 0.92 | 165.27 | 12.57% | ||
| Vantage | 0.88 | 200.27 | 14.31% | ||
| Verity Sense | 0.90 | 187.88 | 13.75% | ||
| 110% | H10 | 0.67 | 216.25 | 18.44% | |
| Vantage | 0.35 | 267.62 | 20.71% | ||
| Verity Sense | 0.06 | 315.36 | 22.62% | ||
| H10 | 0.68 | 221.55 | 19.39% | ||
| Vantage | 0.37 | 276.45 | 22.01% | ||
| Verity Sense | 0.06 | 325.27 | 23.71% | ||
| H10 | 0.82 | 162.12 | 13.63% | ||
| Vantage | 0.80 | 171.47 | 14.25% | ||
| Verity Sense | 0.78 | 176.67 | 14.43% | ||
| H10 | 0.83 | 154.89 | 12.87% | ||
| Vantage | 0.79 | 174.08 | 14.02% | ||
| Verity Sense | 0.75 | 182.67 | 14.25% |
| Devices | R2 | MAE (mL/min) | MAPE (%) | %-Increase in MAE | |
|---|---|---|---|---|---|
| H10 | 0.65 | 278.66 | 28.78% | --- | |
| Vantage | 0.56 | 300.85 | 30.01% | 7.96% | |
| Verity Sense | 0.54 | 302.57 | 30.01% | 8.58% | |
| H10 | 0.66 | 276.90 | 29.29% | --- | |
| Vantage | 0.57 | 298.33 | 30.49% | 7.74% | |
| Verity Sense | 0.55 | 300.29 | 30.51% | 8.45% | |
| H10 | 0.84 | 188.76 | 17.98% | --- | |
| Vantage | 0.83 | 194.13 | 18.26% | 2.85% | |
| Verity Sense | 0.82 | 195.68 | 18.27% | 3.67% | |
| H10 | 0.87 | 165.28 | 15.91% | --- | |
| Vantage | 0.86 | 175.22 | 16.44% | 6.02% | |
| Verity Sense | 0.85 | 175.01 | 16.37% | 5.89% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Yang, J.; Tao, K.; Li, X.; Xu, H.; Qiu, J. Combined Impact of Heart Rate Sensor Placements with Respiratory Rate and Minute Ventilation on Oxygen Uptake Prediction. Sensors 2024, 24, 5412. https://doi.org/10.3390/s24165412
Lu Z, Yang J, Tao K, Li X, Xu H, Qiu J. Combined Impact of Heart Rate Sensor Placements with Respiratory Rate and Minute Ventilation on Oxygen Uptake Prediction. Sensors. 2024; 24(16):5412. https://doi.org/10.3390/s24165412
Chicago/Turabian StyleLu, Zhihui, Junchao Yang, Kuan Tao, Xiangxin Li, Haoqi Xu, and Junqiang Qiu. 2024. "Combined Impact of Heart Rate Sensor Placements with Respiratory Rate and Minute Ventilation on Oxygen Uptake Prediction" Sensors 24, no. 16: 5412. https://doi.org/10.3390/s24165412





