SPRi Biosensor for Simultaneous Determination of HIF-1α, Angiopoietin-2, and Interleukin-1β in Blood Plasma
Abstract
:1. Introduction
2. Experimental
2.1. Material and Reagents
2.2. SPRi Apparatus
2.3. Biological Material
2.4. Procedures
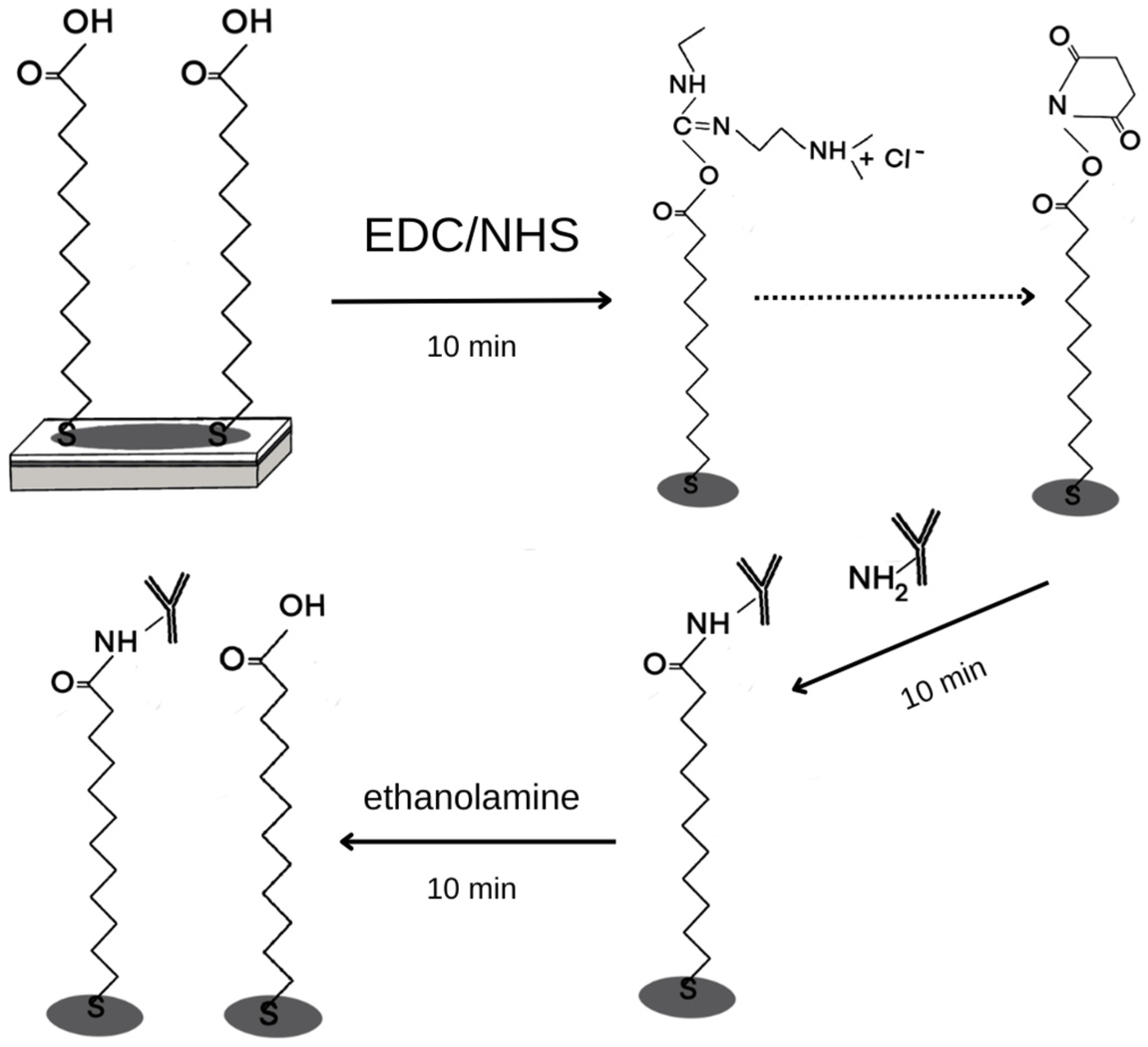
2.4.1. Chip Preparation
2.4.2. SPRi Measurement
3. Results and Discussion of Biosensor Formation and Its Validation
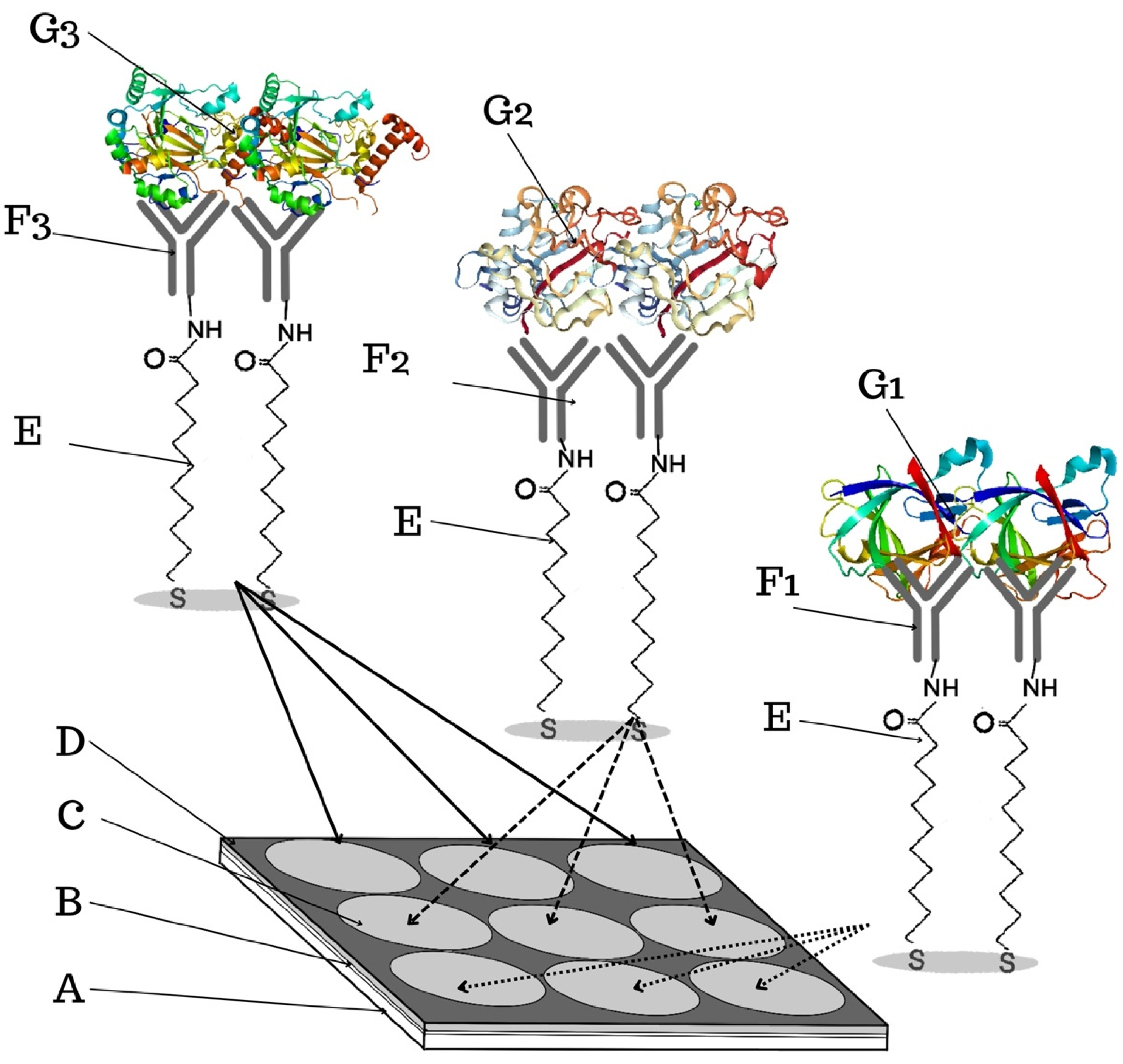
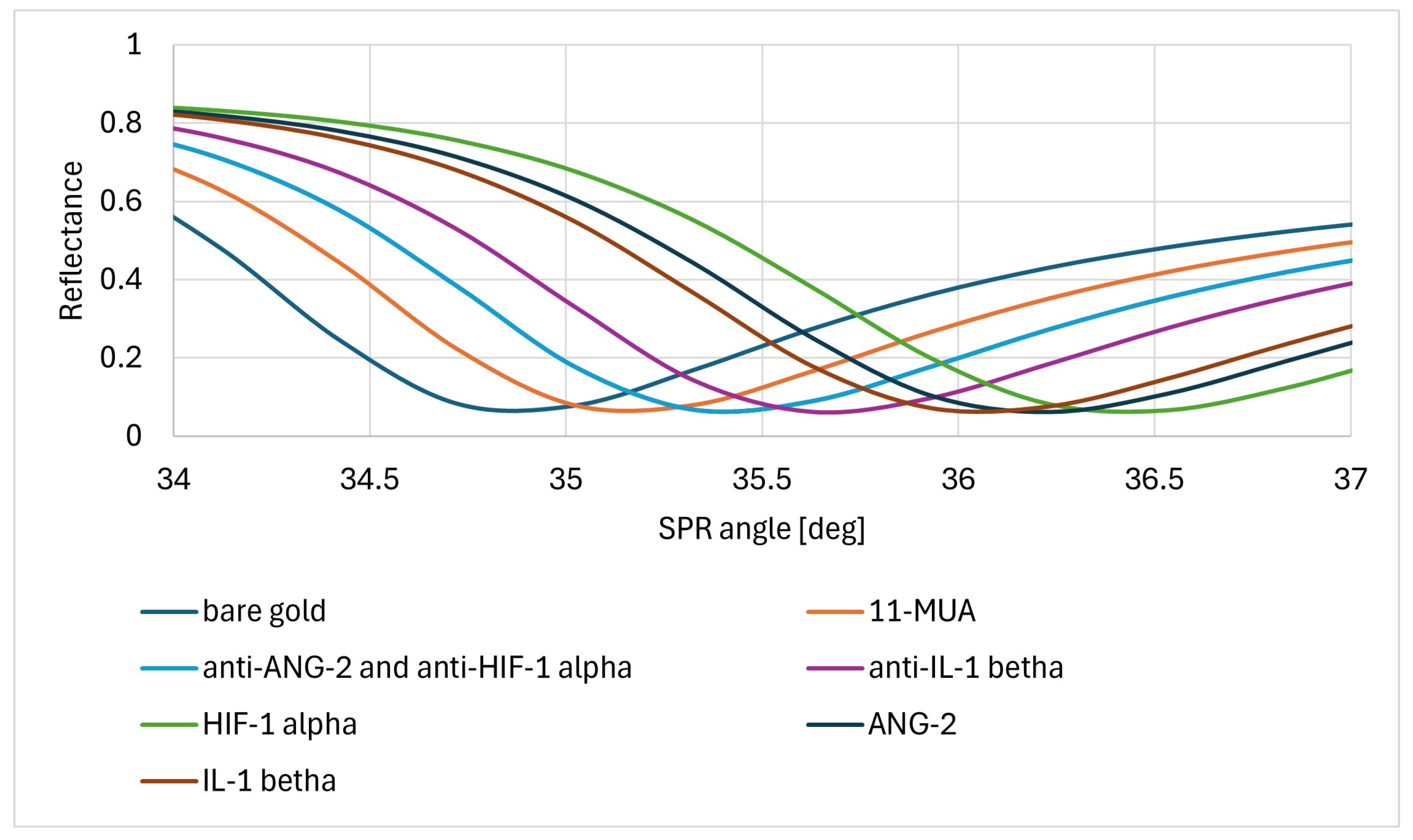
3.1. Formation of Layers on the Biosensor Surface
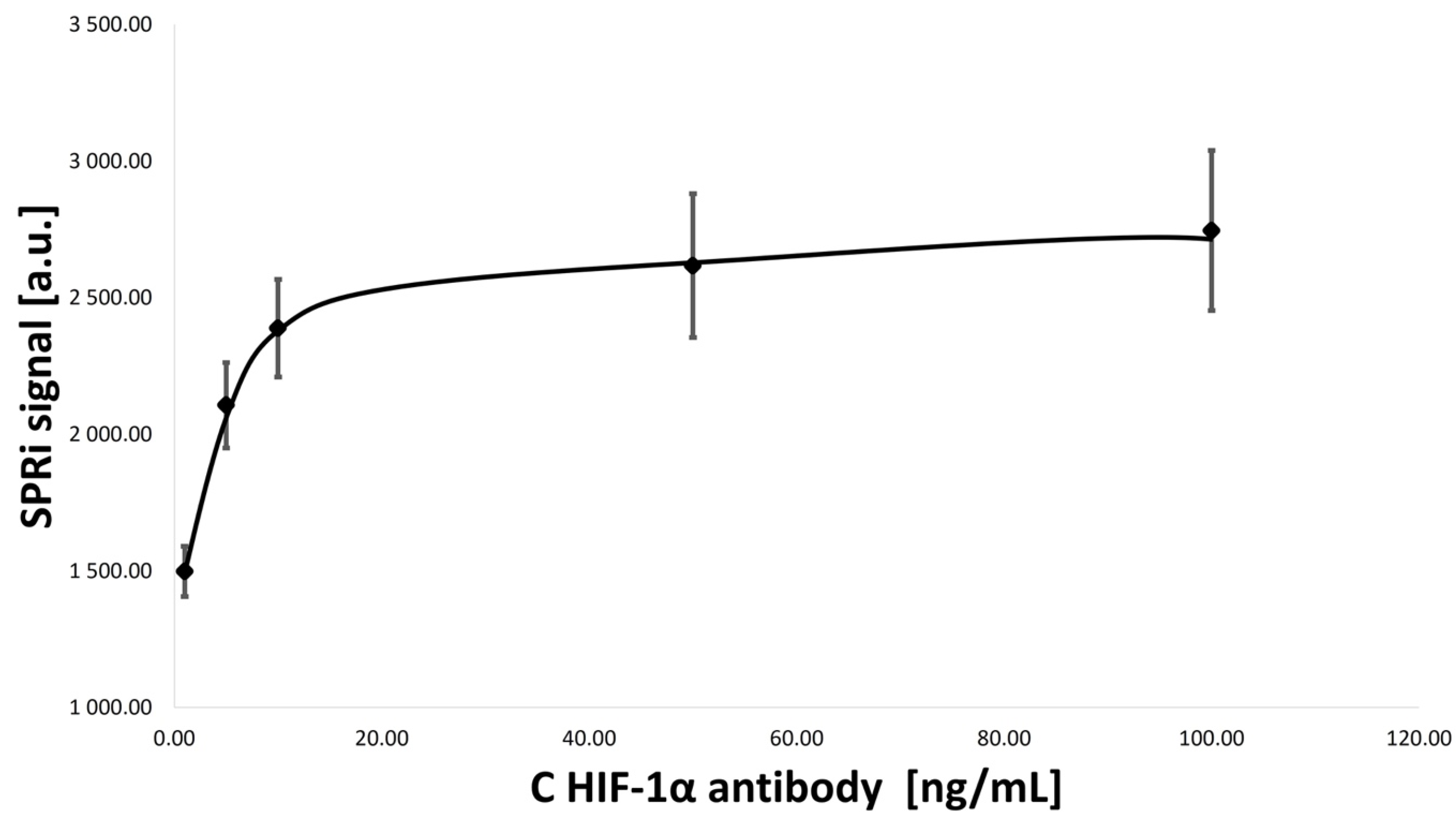
3.2. Optimization of Concentrations of Ligand–Antibody Monolayers
- HIF-1α antibody concentration: 1.00, 5.00, 10.00, 50.00, and 100.00 ng/mL;
- ANG-2 antibody concentration: 1.00, 5.00, 10.00, 15.00, and 20.00 ug/mL;
- IL-1β antibody concentration: 5.00, 10.00, 50.00, 100.00, and 1000.00 ng/mL.
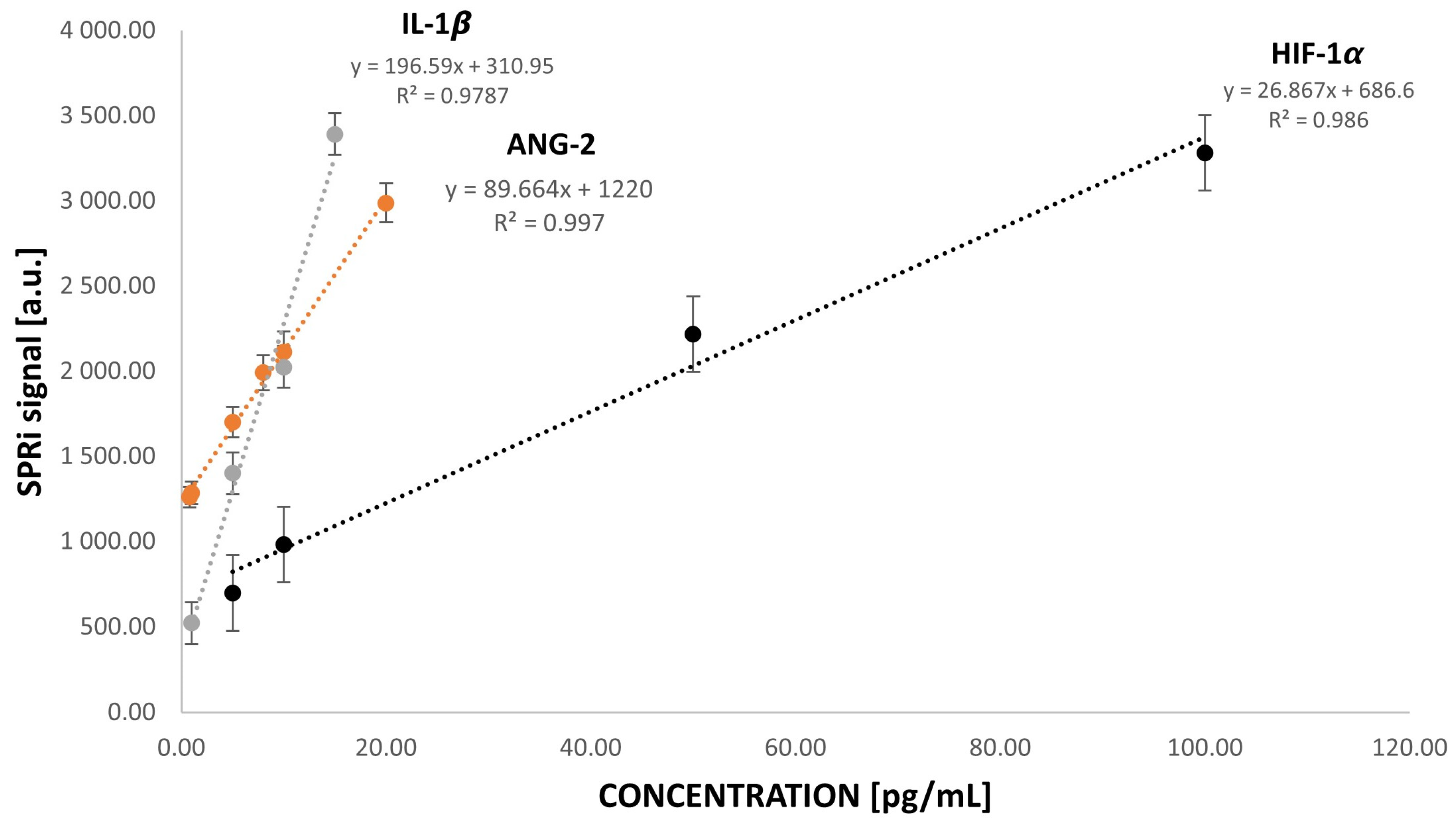
3.3. Analytical Response to HIF-1α, ANG-2, and IL-1β Concentrations
3.4. Precision, Accuracy, and Repeatability of the Biosensor
3.5. The Influence of Interferences on the Analytical Signal
3.6. Determination in Biological Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-H.; Fang, W.-G. Hypoxia-inducible factor-1 in tumour angiogenesis. World J. Gastroenterol. 2004, 10, 1082. [Google Scholar] [CrossRef]
- Singh, R.P.; Agarwal, R. Tumor Angiogenesis: A Potential Target In Cancer Control by Phytochemicals. Curr. Cancer Drug Targets 2003, 3, 205–217. [Google Scholar] [CrossRef]
- Czajka, I. Angiogeneza nowotworowa. Endokrynol. Pol. 2004, 4, 454–455. [Google Scholar]
- Szala, S.; Markowska, J. Naczynia Okołonowotworowe jako Cele Terapii Przeciwnowotworowej; Markowska, J., Ed.; Ginekologia Onkologiczna; Wydawnictwo Medyczne Urban & Partner: Wrocław, Poland, 2006; pp. 44–57. [Google Scholar]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down regulation of HIF-1α in cancer progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, A.; Carico, E. Role of HIF-1 in Cancer Progression: Novel Insights. A Review. Curr. Mol. Med. 2018, 18, 343–351. [Google Scholar] [CrossRef]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and Beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef]
- Available online: https://commons.wikimedia.org/wiki/File:Protein_HIF1A_PDB_1h2k.png# (accessed on 22 June 2024).
- Hu, B.; Cheng, S.Y. Angiopoietin-2: Development of inhibitors for cancer therapy. Curr. Oncol. Rep. 2009, 11, 111–116. [Google Scholar] [CrossRef]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef]
- Park, J.H.; Park, K.J.; Kim, Y.S.; Sheen, S.S.; Lee, K.S.; Lee, H.N.; Oh, Y.J.; Hwang, S.C. Serum angiopoietin-2 as a clinical marker for lung cancer. Chest 2007, 132, 200–206. [Google Scholar] [CrossRef]
- Kurzyk, A. Angiogenesis-possibilities, problems and perspectives. Postępy Biochem. 2015, 61, 25–34. [Google Scholar] [PubMed]
- Munakata, S.; Ueyama, T.; Ishihara, H. Angiopoietin-2 as a Prognostic Factor in Patients with Incurable Stage IV Colorectal Cancer. J. Gastrointest. Cancer 2021, 52, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.S.; Rhyu, K.H.; Kang, J.M.; Lee, D.G.; Yu, H.C.; Yeum, J.H.; Koh, G.Y.; Tarnawski, A.S. Overexpression of VEGF and angiopoietin 2: A key tohigh vascularity of hepatocellular carcinoma? Mod. Pathol. 2003, 16, 552–557. [Google Scholar] [CrossRef]
- Koga, K.; Todaka, T.; Morioka, M.; Hamada, J.; Kai, Y.; Yano, S.; Okamura, A.; Takakura, N.; Suda, T.; Ushio, Y. Expression of angiopoietin-2in human glioma cells and its role for angiogenesis. Cancer Res. 2001, 61, 6248–625459. [Google Scholar]
- Oka, N.; Yamamoto, Y.; Takahashi, M.; Nishitani, M.; Kanayama, H.-O.; Kagawa, S. Expression of angiopoietin-1 and -2, and its clinical significance in human bladder cancer. BJU Int. 2005, 95, 660–663. [Google Scholar] [CrossRef]
- Available online: https://www.sinobiological.com/resource/angiopoietin-2/proteins (accessed on 22 June 2024).
- Carmi, Y.; Dotan, S.; Rider, P.; Kaplanov, I.; White, M.R.; Baron, R.; Voronov, E. The Role of IL-1 in the Early Tumor Cell-Induced Angiogenic Response. J. Immunol. 2013, 190, 3500–3509. [Google Scholar] [CrossRef] [PubMed]
- Mohr, T.; Haudek-Prinz, V.; Slany, A.; Grillari, J.; Micksche, M.; Gerner, C. Proteome profiling in IL-1β and VEGF-activated human umbilical vein endothelial cells delineates the interlink between inflammation and angiogenesis. PLoS ONE 2017, 12, e0179065. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef]
- Jung, Y.J.; Isaacs, J.S.; Lee, S.; Trepel, J.; Neckers, L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003, 17, 2115–2117. [Google Scholar] [CrossRef]
- Kaler, P.; Augenlicht, L.; Klampfer, L. Macrophage-derived IL-1β stimulates Wnt signaling and growth of colon cancer cells: A crosstalk interrupted by vitamin D3. Oncogene 2009, 28, 3892–3902. [Google Scholar] [CrossRef]
- Matanic, D.; Beg-Zec, Z.; Stojanović, D.; Matakorić, N.; Flego, V.; Milevoj-Ribic, F. Cytokines in Patients with Lung Cancer. Scand. J. Immunol. 2003, 57, 173–178. [Google Scholar] [CrossRef]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti–PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. USA 2018, 116, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Bermudez-Fernandez, S.; Fernández-García, B.; Atienza, S.; Beridze, N.; Escaf, S.; Vizoso, F.J. Analysis of the Expression of Interleukins, Interferon β, and Nuclear Factor-κ B in Prostate Cancer and their Relationship with Biochemical Recurrence. J. Immunother. 2014, 37, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Woolery, K.T.; Hoffman, M.S.; Kraft, J.; Nicosia, S.V.; Kumar, A.; Kruk, P.A. Urinary interleukin-1β levels among gynecological patients. J. Ovarian Res. 2014, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, L.; Jiang, H.; Li, Q.; Wang-Gillam, A.; Yu, J.; Head, R.; Liu, J.; Ruzinova, M.B.; Lim, K.-H. Tumor–Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer. Cancer Res. 2018, 78, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, M.; Chamlati, J.M.; Hennenlotter, J.; Todenhöfer, T.; Lütfrenk, T.; Jersinovic, S.; Tsaur, I.; Stenzl, A.; Rausch, S. Interleukin-1β/Interleukin (IL)-1-Receptor-Antagonist (IL1-RA) Axis in Invasive Bladder Cancer—An Exploratory Analysis of Clinical and Tumor Biological Significance. Int. J. Mol. Sci. 2024, 25, 2447. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Jiang, J.R.; Ma, X.K.; Chen, J.; Li, H.P.; Li, X.; Wu, X.Y.; Huang, M.S.; Lin, Q. Prognostic value of serum HIF-1α change following transarterial chemoembolization in hepatocellular carcinoma. Clin. Exp. Med. 2021, 21, 109–120. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Ikeda, M.; Shimizu, S. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br. J. Cancer 2013, 108, 2063–2069. [Google Scholar] [CrossRef]
- Available online: https://commons.wikimedia.org/wiki/File:31BI.png# (accessed on 22 June 2024).
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Ho, H.P.; Kong, S.K.; Kabashin, A.V. Phase-sensitive surface plasmon resonance biosensors: Methodology, instrumentation and applications. Ann. Phys. 2012, 524, 637–662. [Google Scholar] [CrossRef]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent Advances in Optical Biosensors for Sensing Applications: A Review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Szarvas, T.; Jäger, T.; Droste, F. Serum Levels of Angiogenic Factors and their Prognostic Relevance in Bladder Cancer. Pathol. Oncol. Res. 2009, 15, 193–201. [Google Scholar] [CrossRef]
- Dmytryk, V.; Luhovska, T.; Yakovlev, P.; Savchuk, O.; Ostapchenko, L.; Halenova, T.; Raksha, N.; Tomchuk, V.; Vovk, T. Elevated levels of proinflammatory and anti-inflammatory cytokines in patients with bladder cancer depending on a tumor stage. Boll. Soc. Ital. Biol. Sper. 2020, 93. [Google Scholar] [CrossRef]




| HIF-1α | ANG-2 | IL-1β | |
|---|---|---|---|
| LOD [pg/mL] | 0.09 | 0.01 | 0.01 |
| LOQ [pg/mL] | 0.27 | 0.039 | 0.02 |
| Concentration of the Standard Solution HIF-1α [pg/mL] | SD [pg/mL] | Cmean [pg/mL] | CV [%] |
| 5.00 | 0.72 | 5.14 | 14.09 |
| 10.00 | 1.91 | 12.20 | 8.28 |
| 50.00 | 1.83 | 44.85 | 4.08 |
| 100.00 | 6.43 | 75.04 | 8.57 |
| Concentration of the standard solution ANG-2 [pg/mL] | SD [pg/mL] | Cmean [pg/mL] | CV [%] |
| 1.00 | 0.13 | 1.93 | 6.83 |
| 5.00 | 0.18 | 6.26 | 2.85 |
| 10.00 | 1.18 | 12.41 | 9.51 |
| 20.00 | 1.14 | 20.08 | 5.70 |
| Concentration of the standard solution IL-1β [pg/mL] | SD [pg/mL] | Cmean [pg/mL] | CV [%] |
| 1.00 | 0.20 | 1.19 | 16.94 |
| 5.00 | 0.46 | 4.73 | 9.66 |
| 10.00 | 0.91 | 10.16 | 8.92 |
| 15.00 | 0.54 | 15.005 | 3.62 |
| Sample Number | Concentration of HIF-1α [ng/mL] | Concentration of ANG-2 [ng/mL] | Concentration of IL-1β [ng/mL] |
|---|---|---|---|
| 1 | 6.667 | 0.143 | 0.208 |
| 2 | 0.655 | 0.134 | 0.187 |
| 3 | 5.983 | 0.088 | 0.190 |
| 4 | 2.321 | 0.129 | 0.247 |
| 5 | 5.565 | 0.129 | 0.117 |
| 6 | 2.400 | 0.093 | 0.084 |
| 7 | 1.442 | 0.136 | 0.167 |
| 8 | 6.526 | 0.144 | 0.187 |
| 9 | 2.492 | 0.178 | 0.165 |
| 10 | 7.910 | 0.151 | 0.186 |
| 11 | 4.335 | 0.080 | 0.100 |
| 12 | 6.085 | 0.086 | 0.163 |
| 13 | 4.973 | 0.120 | 0.062 |
| 14 | 2.575 | 0.118 | 0.150 |
| 15 | 9.391 | 0.087 | 0.092 |
| 16 | 7.885 | 0.154 | 0.070 |
| 17 | 1.376 | 0.155 | 0.057 |
| 18 | 5.008 | 0.102 | 0.184 |
| 1C | 1.074 | 0.037 | 0.053 |
| 2C | 0.445 | 0.024 | 0.014 |
| 3C | 0.897 | 0.049 | 0.006 |
| Mean [ng/mL] | 4.644 | 0.124 | 0.145 |
| Meancontrol [ng/mL] | 0.805 | 0.036 | 0.024 |
| SD [ng/mL] | 2.573 | 0.029 | 0.055 |
| SDcontrol [ng/mL] | 0.324 | 0.013 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielinska, Z.; Oldak, L.; Guszcz, T.; Hermanowicz, A.; Gorodkiewicz, E. SPRi Biosensor for Simultaneous Determination of HIF-1α, Angiopoietin-2, and Interleukin-1β in Blood Plasma. Sensors 2024, 24, 5481. https://doi.org/10.3390/s24175481
Zielinska Z, Oldak L, Guszcz T, Hermanowicz A, Gorodkiewicz E. SPRi Biosensor for Simultaneous Determination of HIF-1α, Angiopoietin-2, and Interleukin-1β in Blood Plasma. Sensors. 2024; 24(17):5481. https://doi.org/10.3390/s24175481
Chicago/Turabian StyleZielinska, Zuzanna, Lukasz Oldak, Tomasz Guszcz, Adam Hermanowicz, and Ewa Gorodkiewicz. 2024. "SPRi Biosensor for Simultaneous Determination of HIF-1α, Angiopoietin-2, and Interleukin-1β in Blood Plasma" Sensors 24, no. 17: 5481. https://doi.org/10.3390/s24175481





