Design and Analysis of a Contact Piezo Microphone for Recording Tracheal Breathing Sounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Microphone Design and Performance Analysis
2.2. Test 1: Frequency Response Analysis
2.3. Test 2: Tracheal and Lung Sounds Recording Analysis
2.4. Pre-Processing and Signal Analysis
2.5. Statistical Analysis
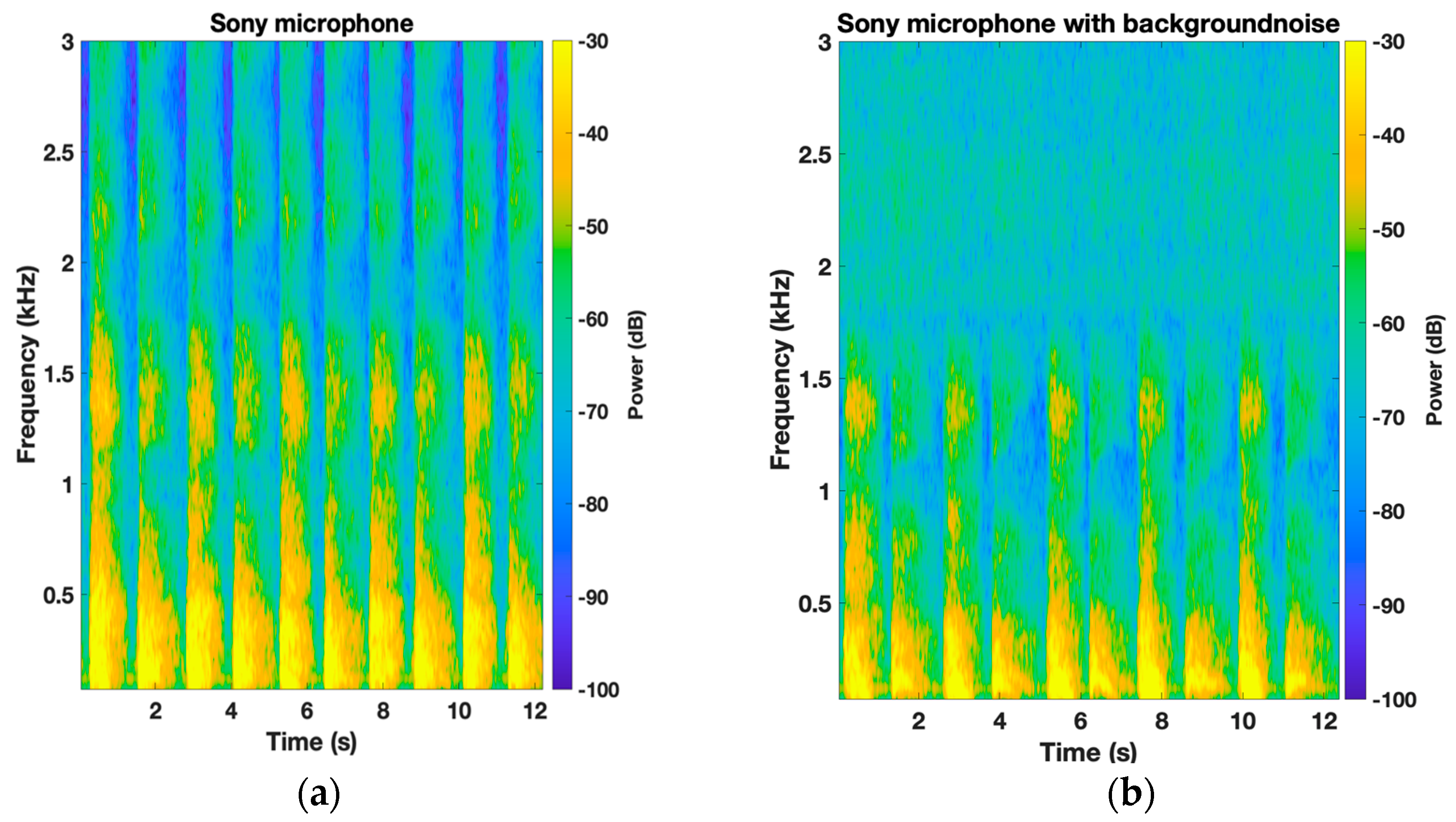
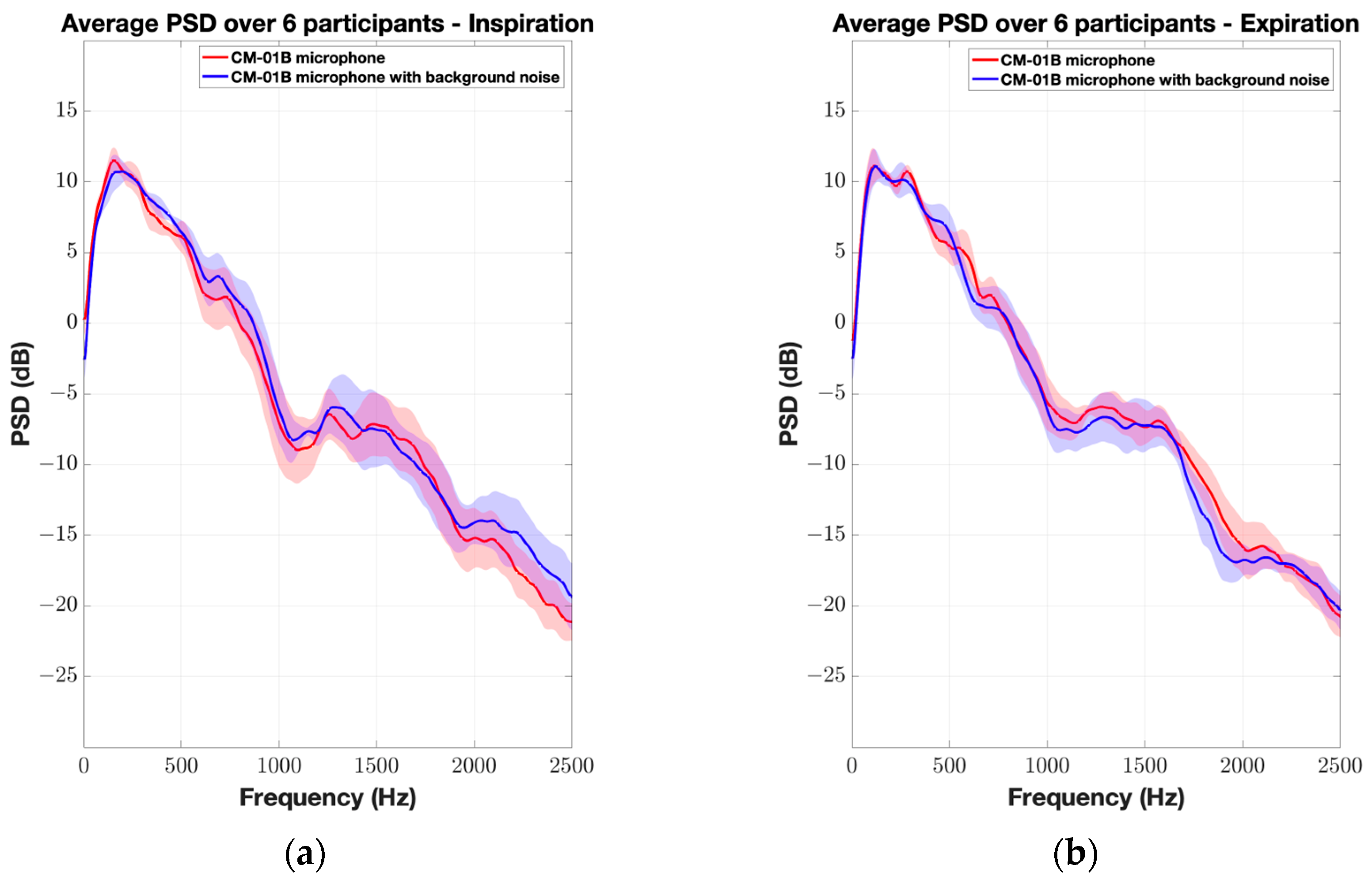
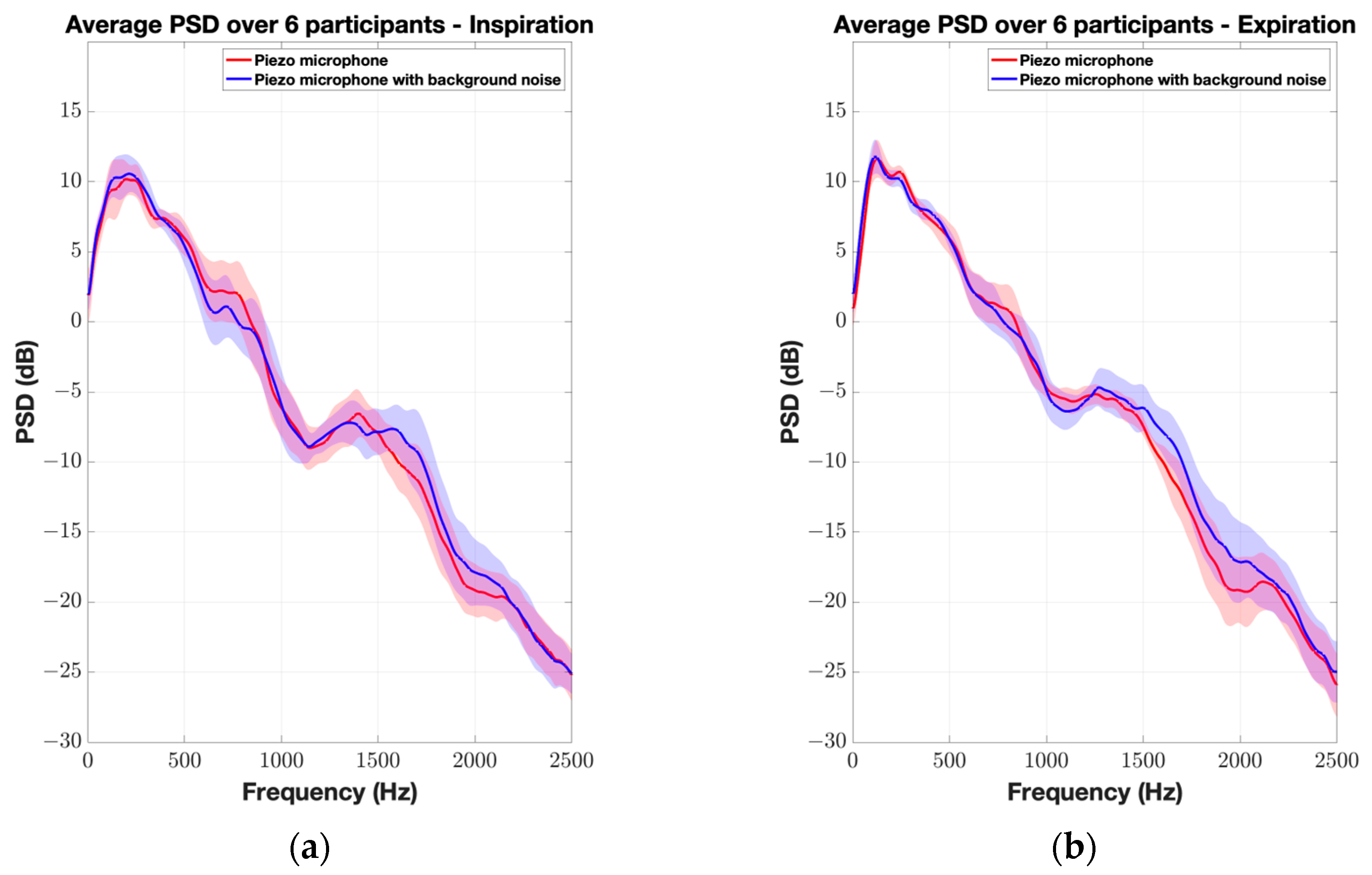
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sola-Soler, J.; Fiz, J.A.; Torres, A.; Jane, R. Identification of Obstructive Sleep Apnea patients from tracheal breath sound analysis during wakefulness in polysomnographic studies. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC, Chicago, IL, USA, 26–30 August 2014; pp. 4232–4235. [Google Scholar]
- Elwali, A.; Moussavi, Z. Obstructive Sleep Apnea Screening and Airway Structure Characterization During Wakefulness Using Tracheal Breathing Sounds. Ann Biomed Eng. 2017, 45, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Hayashi, M.; Ohshima, E.; Nishikata, N.; Shinohara, T. Validation of a new system of tracheal sound analysis for the diagnosis of sleep apnea-hypopnea syndrome. Sleep 2004, 27, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Rauhala, E.; Hasan, J.; Kulkas, A.; Saastamoinen, A.; Huupponen, E.; Cameron, F.; Himanen, S.L. Compressed tracheal sound analysis in screening of sleep-disordered breathing. Clin. Neurophysiol. 2008, 119, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, A.; Montazeri, A.; Azarbarzin, A.; Moussavi, Z. Respiratory flow-sound relationship during both wakefulness and sleep and its variation in relation to sleep apnea. Ann. Biomed. Eng. 2013, 41, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Kulkas, A.; Huupponen, E.; Virkkala, J.; Tenhunen, M.; Saastamoinen, A.; Rauhala, E.; Himanen, S.L. New tracheal sound feature for apnoea analysis. Med. Biol. Eng. Comput. 2009, 47, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, P.; Nathoo, A.R.; Richardson, H.D. Breath sounds. Thorax 1971, 26, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Sattar, F.; Goh, D.Y.T. Automatic wheeze detection using histograms of sample entropy. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS’08—“Personalized Healthcare through Technology”, Vancouver, BC, Canada, 20–24 August 2008; pp. 1890–1893. [Google Scholar]
- Taplidou, S.A.; Hadjileontiadis, L.J. Wheeze detection based on time-frequency analysis of breath sounds. Comput. Biol. Med. 2007, 37, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Pourazad, M.T.; Moussavi, Z.; Thomas, G. Heart sound cancellation from lung sound recordings using time-frequency filtering. Med. Biol. Eng. Comput. 2006, 44, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, H.; Kraman, S.S.; Wodicka, G.R. Respiratory sounds. Advances beyond the stethoscope. Am. J. Respir. Crit. Care Med. 1997, 156 Pt 1, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, Z. Anatomy and Physiology of Respiratory System BT—Fundamentals of Respiratory System and Sounds Analysis; Moussavi, Z., Ed.; Springer International Publishing: Cham, Switzerland, 2006; pp. 1–8. [Google Scholar] [CrossRef]
- Gavriely, N.; Cugell, D.W. Breath Sounds Methodology; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Penzel, T.; Sabil, A.K. The use of tracheal sounds for the diagnosis of sleep apnoea. Breathe 2017, 13, e37–e45. [Google Scholar] [CrossRef] [PubMed]
- Joyashiki, T.; Wada, C. Validation of a body-conducted sound sensor for respiratory sound monitoring and a comparison with several sensors. Sensors 2020, 20, 942. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, H.; Kraman, S.S.; DeFrain, P.D.; Wodicka, G.R. Measurement of respiratory acoustical signals: Comparison of sensors. Chest 1993, 104, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Kraman, S.S.; Wodicka, G.R.; Pressler, G.A.; Pasterkamp, H. Comparison of lung sound transducers using a bioacoustic transducer testing system. J. Appl. Physiol. 2006, 101, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, S.H.; Chae, S.M.; Hwang, K.H.; Cho, M.J.; Im, J.J. Detection and Analysis of Breathing Sounds from Trachea. In Proceedings of the—2018 11th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics, CISP-BMEI 2018, Beijing, China, 13–15 October 2018; pp. 1–5. [Google Scholar]
- Gupta, P.; Wen, H.; Daruwalla, A.; Moghimi, M.J.; Ayazi, F. Hermetically-Encapsulated Unidirectional Accelerometer Contact Microphone for Wearable Applications. In Proceedings of IEEE Sensors; IEEE: New York, NY, USA, 2019; pp. 1–4. [Google Scholar]








| Sensitivity (Measured at 1 kHz-94 dB SPL) | Frequency Range (Linear) | Sensor Type (Test Involved) | |
|---|---|---|---|
| Piezo-electric microphone | −50 dB (3.2 mv/Pa) | (20–5000) Hz | Piezo-electric disc (Tests 1 and 2) |
| CM-01B | −60 dB (0.9 mv/Pa) | (8–2500) Hz | Piezo-electric (Tests 1 and 2) |
| Brüel and Kjær type 4948-B | −57 dB (1.4 mv/Pa) | (5–20,000) Hz | Surface microphone (Test 1) |
| Sony ECM-77B | −52 dB (2 mv/Pa) | (40–20,000) Hz | Condenser microphone (Test 2) |
| 200–599 Hz | 600–999 Hz | 1000–1399 Hz | 1400–1799 Hz | 1800–2199 Hz | 2200–2599 Hz | 2600–3000 Hz | |
|---|---|---|---|---|---|---|---|
| Sony microphone | 0.33 ± 1.43 dB (0.67) | 0.42 ± 1.74 dB (0.54) | 0.46 ± 1.91 dB (0.81) | 0.18 ± 3.63 dB (0.68) | 4.80 ± 6.82 dB (0.006) * | 4.07 ± 6.68 dB (0.03) * | 2.80 ± 5.7 dB (0.24) |
| CM-01B microphone | 0.12 ± 1.63 dB (0.69) | 1.07 ± 2.15 dB (0.26) | 1.03 ± 2.81 dB (0.32) | 0.28 ± 2.52 dB (0.82) | 1.15 ± 3.42 dB (0.11) | 2.81 ± 4.32 dB (0.02) * | 1.42 ± 4.50 dB (0.33) |
| Piezo microphone | 0.10 ± 1.19 dB (0.85) | 0.81 ± 2.36 dB (0.73) | 0.23 ± 2.95 dB (0.78) | 0.80 ± 3.20 dB (0.53) | 1.5 ± 3.08 dB (0.36) | 0.04 ± 2.10 dB (0.71) | 0.17 ± 2.19 dB (0.87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, W.; Moussavi, Z. Design and Analysis of a Contact Piezo Microphone for Recording Tracheal Breathing Sounds. Sensors 2024, 24, 5511. https://doi.org/10.3390/s24175511
Ashraf W, Moussavi Z. Design and Analysis of a Contact Piezo Microphone for Recording Tracheal Breathing Sounds. Sensors. 2024; 24(17):5511. https://doi.org/10.3390/s24175511
Chicago/Turabian StyleAshraf, Walid, and Zahra Moussavi. 2024. "Design and Analysis of a Contact Piezo Microphone for Recording Tracheal Breathing Sounds" Sensors 24, no. 17: 5511. https://doi.org/10.3390/s24175511
APA StyleAshraf, W., & Moussavi, Z. (2024). Design and Analysis of a Contact Piezo Microphone for Recording Tracheal Breathing Sounds. Sensors, 24(17), 5511. https://doi.org/10.3390/s24175511







