The Cumulative Impacts of Fatigue during Overload Training Can Be Tracked Using Field-Based Monitoring of Running Stride Interval Correlations
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Training Intervention
2.4. Field Testing
2.5. Short Recovery and Stress Scale
2.6. Running Performance
2.7. IMU Data Processing
2.8. Statistical Analysis
3. Results
3.1. Participant Flow, Demographics, and Compliance
3.2. DFA α Outcomes
3.3. Questionnaire and Performance Outcomes
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neupert, E.; Gupta, L.; Holder, T.; Jobson, S.A. Athlete monitoring practices in elite sport in the United Kingdom. J. Sports Sci. 2022, 40, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Coutts, A.J.; Crowcroft, S.; Kempton, S. Developing athlete monitoring systems: Theoretical basis and practical applications. In Recovery and Well-Being in Sport and Exercise, 1st ed.; Kellmann, M., Beckmann, J., Eds.; Routledge: Oxfordshire, UK, 2021; pp. 17–31. [Google Scholar]
- McGuigan, H.E.; Hassmén, P.; Rosic, N.; Stevens, C.J. Monitoring of training in high-performance athletes: What do practitioners do? J. Sport Exerc. Sci. 2021, 5, 121–129. [Google Scholar] [CrossRef]
- Carroll, T.J.; Taylor, J.L.; Gandevia, S.C. Recovery of central and peripheral neuromuscular fatigue after exercise. J. Appl. Physiol. 2017, 122, 1068–1076. [Google Scholar] [CrossRef]
- McMorris, T.; Barwood, M.; Corbett, J. Central fatigue theory and endurance exercise: Toward an interoceptive model. Neurosci. Biobehav. Rev. 2018, 93, 93–107. [Google Scholar] [CrossRef]
- Willy, R.W. Innovations and pitfalls in the use of wearable devices in the prevention and rehabilitation of running related injuries. Phys. Ther. Sport 2018, 29, 26–33. [Google Scholar] [CrossRef]
- Meardon, S.A.; Hamill, J.; Derrick, T.R. Running injury and stride time variability over a prolonged run. Gait Posture 2011, 33, 36–40. [Google Scholar] [CrossRef]
- Fuller, J.T.; Bellenger, C.R.; Thewlis, D.; Arnold, J.; Thomson, R.L.; Tsiros, M.D.; Robertson, E.Y.; Buckley, J.D. Tracking performance changes with running-stride variability when athletes are functionally overreached. Int. J. Sports Physiol. Perform. 2017, 12, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, C.R.; Arnold, J.B.; Buckley, J.D.; Thewlis, D.; Fuller, J.T. Detrended fluctuation analysis detects altered coordination of running gait in athletes following a heavy period of training. J. Sci. Med. Sport 2019, 22, 294–299. [Google Scholar] [CrossRef]
- Gates, D.H.; Dingwell, J.B. Peripheral neuropathy does not alter the fractal dynamics of stride intervals of gait. J. Appl. Physiol. 2007, 102, 965–971. [Google Scholar] [CrossRef][Green Version]
- Hausdorff, J.M.; Mitchell, S.L.; Firtion, R.; Peng, C.K.; Cudkowicz, M.E.; Wei, J.Y.; Goldberger, A.L. Altered fractal dynamics of gait: Reduced stride-interval correlations with aging and Huntington’s disease. J. Appl. Physiol. 1997, 82, 262–269. [Google Scholar] [CrossRef]
- Mo, S.; Chow, D.H.K. Stride-to-stride variability and complexity between novice and experienced runners during a prolonged run at anaerobic threshold speed. Gait Posture 2018, 64, 7–11. [Google Scholar] [CrossRef]
- Fuller, J.T.; Thewlis, D.; Wills, J.A.; Buckley, J.D.; Arnold, J.B.; Doyle, E.; Doyle, T.L.; Bellenger, C.R. Optimizing wearable device and testing parameters to monitor running-stride long-range correlations for fatigue management in field settings. Int. J. Sports Physiol. Perform. 2023, 19, 207–211. [Google Scholar] [CrossRef]
- Willwacher, S.; Kurz, M.; Robbin, J.; Thelen, M.; Hamill, J.; Kelly, L.; Mai, P. Running-related biomechanical risk factors for overuse injuries in distance runners: A systematic review considering injury specificity and the potentials for future research. Sports Med. 2022, 52, 1863–1877. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Halson, S.L.; Bridge, M.W.; Meeusen, R.; Busschaert, B.; Gleeson, M.; Jones, D.A.; Jeukendrup, A.E. Time course of performance changes and fatigue markers during intensified training in trained cyclists. J. Appl. Physiol. 2002, 93, 947–956. [Google Scholar] [CrossRef]
- Kölling, S.; Schaffran, P.; Bibbey, A.; Drew, M.; Raysmith, B.; Nässi, A.; Kellmann, M. Validation of the Acute Recovery and Stress Scale (ARSS) and the Short Recovery and Stress Scale (SRSS) in three English-speaking regions. J. Sports Sci. 2020, 38, 130–139. [Google Scholar] [CrossRef]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef]
- Le Meur, Y.; Pichon, A.; Schaal, K.; Schmitt, L.; Louis, J.; Gueneron, J.; Vidal, P.P.; Hausswirth, C. Evidence of parasympathetic hyperactivity in functionally overreached athletes. Med. Sci. Sports Exerc. 2013, 45, 2061–2071. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, Physio- Toolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef]
- Jordan, K.; Challis, J.H.; Newell, K.M. Long range correlations in the stride interval of running. Gait Posture 2006, 24, 120–125. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- A New View of Statistics. Available online: http://www.sportsci.org/resource/stats/ (accessed on 20 February 2024).
- Hausdorff, J.M.; Ashkenazy, Y.; Peng, C.K.; Ivanov, P.C.; Stanley, H.E.; Goldberger, A.L. When human walking becomes random walking: Fractal analysis and modeling of gait rhythm fluctuations. Physica A 2001, 302, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.C.; Baudendistel, S.T.; Lipat, A.L.; White, T.A.; Raffegeau, T.E.; Hass, C.J. Walking indoors, outdoors, and on a treadmill: Gait differences in healthy young and older adults. Gait Posture 2021, 90, 468–474. [Google Scholar] [CrossRef]
- Terrier, P.; Dériaz, O. Kinematic variability, fractal dynamics and local dynamic stability of treadmill walking. J. Neuroeng. Rehabil. 2011, 8, 12. [Google Scholar] [CrossRef]
- Natera, A.O.W.; Jennings, J.; Oakley, A.J.; Jones, T.W. Influence of environmental conditions on performance and heart rate responses to the 30-15 incremental fitness test in rugby union athletes. J. Strength Cond. Res. 2019, 33, 486–491. [Google Scholar] [CrossRef]
- Nakayama, Y.; Kudo, K.; Ohtsuki, T. Variability and fluctuation in running gait cycle of trained runners and non-runners. Gait Posture 2010, 31, 331–335. [Google Scholar] [CrossRef]
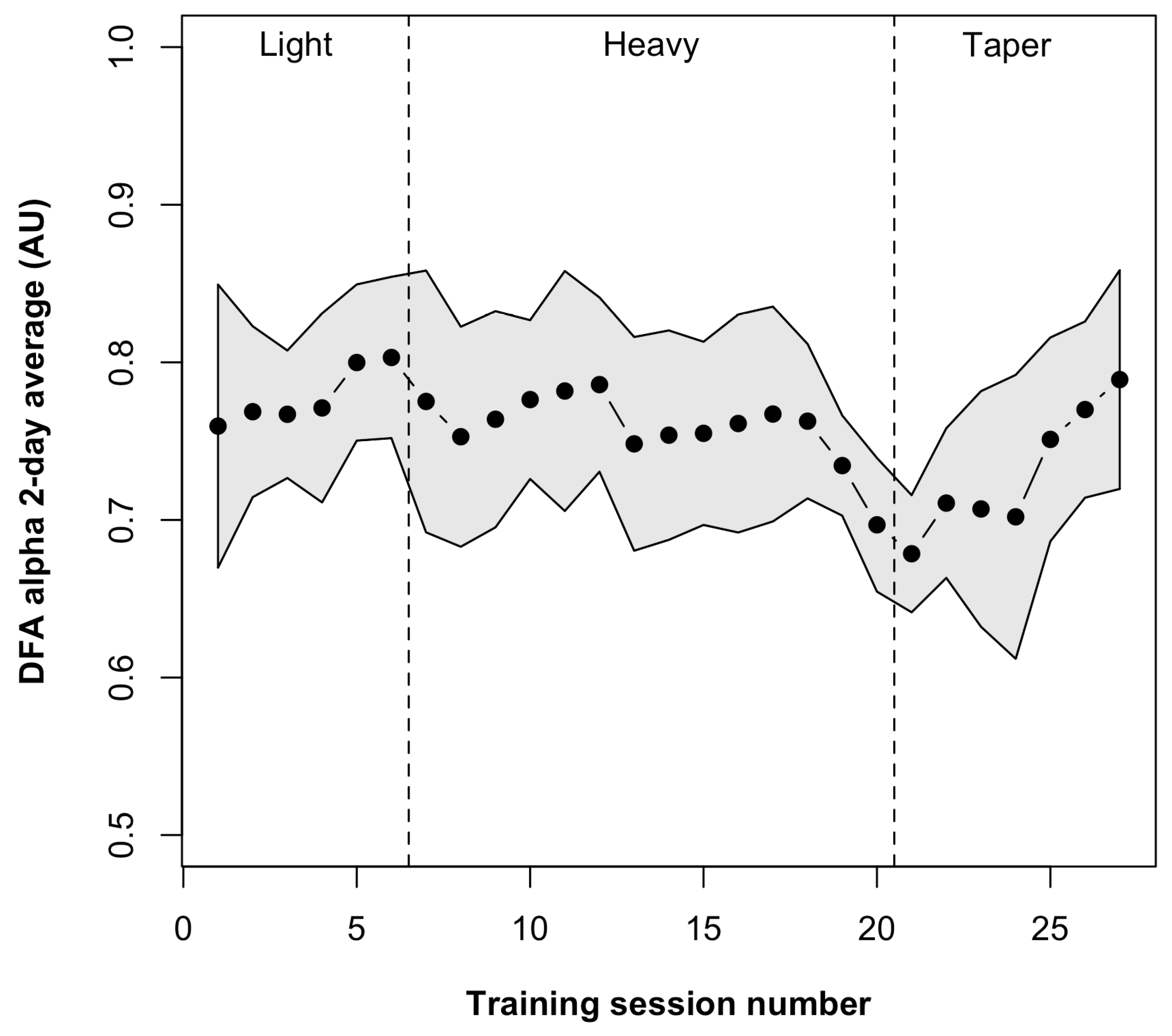
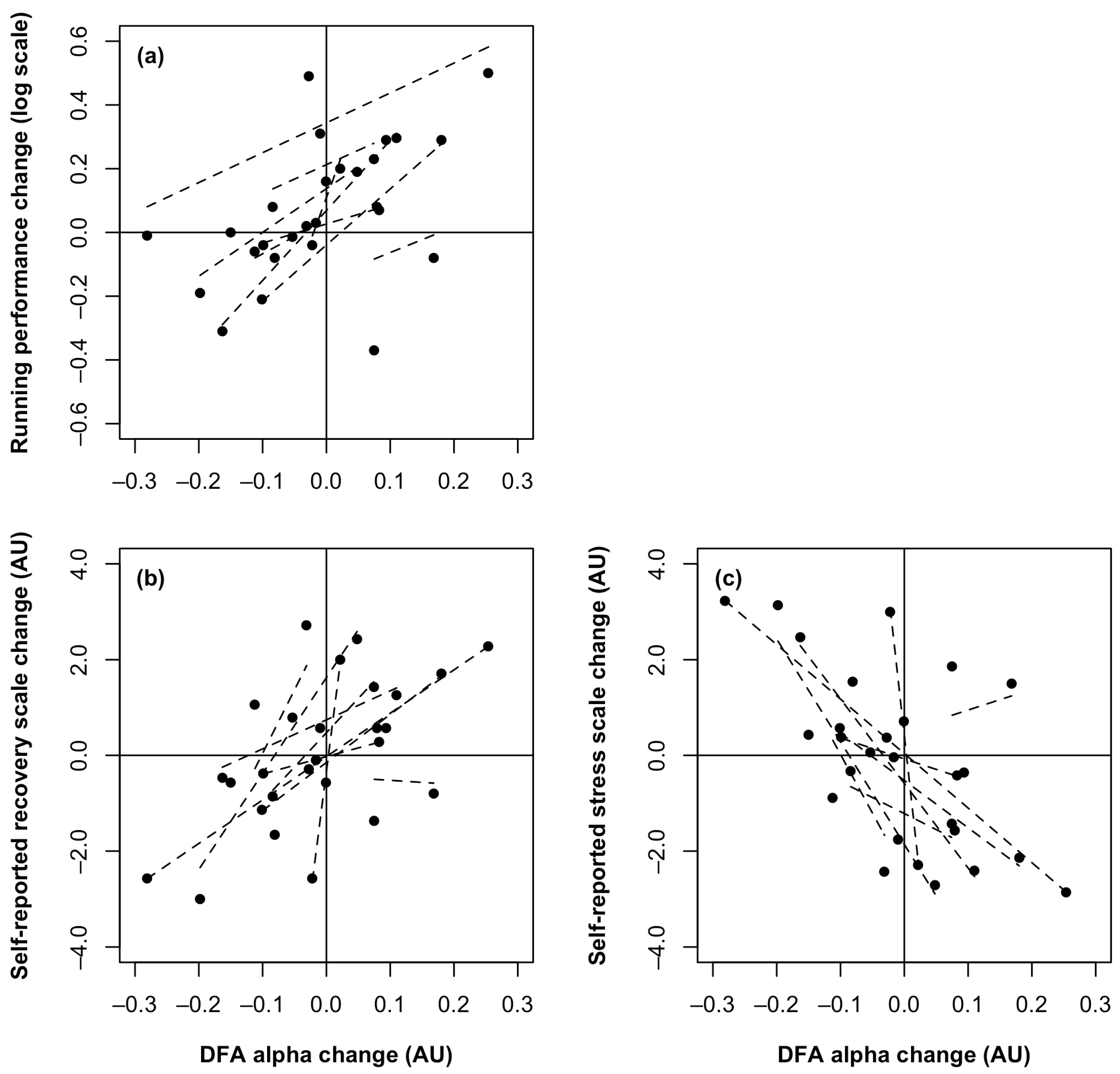
| Outcome | Post-Baseline Mean (SD) * | Post-Heavy Mean (SD) * | Post-Taper Mean (SD) * | p-Value Main Effect |
|---|---|---|---|---|
| DFA α (AU) | 0.80 (0.08) | 0.70 (0.07) 1 | 0.79 (0.10) 2 | 0.011 |
| RTE distance (log scale) | 1.705 (0.369) | 1.576 (0.357) 1 | 1.808 (0.375) 2 | 0.007 |
| RTE RPE (6–20 scale) | 20 (19–20) | 19 (19–20) | 20 (19–20) | 0.717 |
| SRSS recovery (0–6 scale) | 4.5 (0.8) | 2.9 (0.9) 1 | 4.5 (0.7) 2 | <0.001 |
| SRSS stress (0–6 scale) | 1.8 (1.1) | 3.6 (0.9) 1 | 1.7 (0.6) 2 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuller, J.T.; Doyle, T.L.A.; Doyle, E.W.; Arnold, J.B.; Buckley, J.D.; Wills, J.A.; Thewlis, D.; Bellenger, C.R. The Cumulative Impacts of Fatigue during Overload Training Can Be Tracked Using Field-Based Monitoring of Running Stride Interval Correlations. Sensors 2024, 24, 5538. https://doi.org/10.3390/s24175538
Fuller JT, Doyle TLA, Doyle EW, Arnold JB, Buckley JD, Wills JA, Thewlis D, Bellenger CR. The Cumulative Impacts of Fatigue during Overload Training Can Be Tracked Using Field-Based Monitoring of Running Stride Interval Correlations. Sensors. 2024; 24(17):5538. https://doi.org/10.3390/s24175538
Chicago/Turabian StyleFuller, Joel Thomas, Tim Leo Atherton Doyle, Eoin William Doyle, John Bradley Arnold, Jonathan David Buckley, Jodie Anne Wills, Dominic Thewlis, and Clint Ronald Bellenger. 2024. "The Cumulative Impacts of Fatigue during Overload Training Can Be Tracked Using Field-Based Monitoring of Running Stride Interval Correlations" Sensors 24, no. 17: 5538. https://doi.org/10.3390/s24175538
APA StyleFuller, J. T., Doyle, T. L. A., Doyle, E. W., Arnold, J. B., Buckley, J. D., Wills, J. A., Thewlis, D., & Bellenger, C. R. (2024). The Cumulative Impacts of Fatigue during Overload Training Can Be Tracked Using Field-Based Monitoring of Running Stride Interval Correlations. Sensors, 24(17), 5538. https://doi.org/10.3390/s24175538










