Virtual Needle Insertion with Enhanced Haptic Feedback for Guidance and Needle–Tissue Interaction Forces
Abstract
:1. Introduction
1.1. Related Work and Contributions
- The effect of enhancing force feedback in the axial and/or radial directions of the needle on the users’ ability to efficiently perceive needle–tissue interaction and guide the needle to the target, respectively.
- The effect of enhanced force feedback on the degree of reliance on CT scans (visual feedback) to guide the needle to the target.
- The effect of real-time visual feedback compared to enhanced real-time haptic feedback on user performance.
1.2. Scope of the Study
2. Materials and Methods
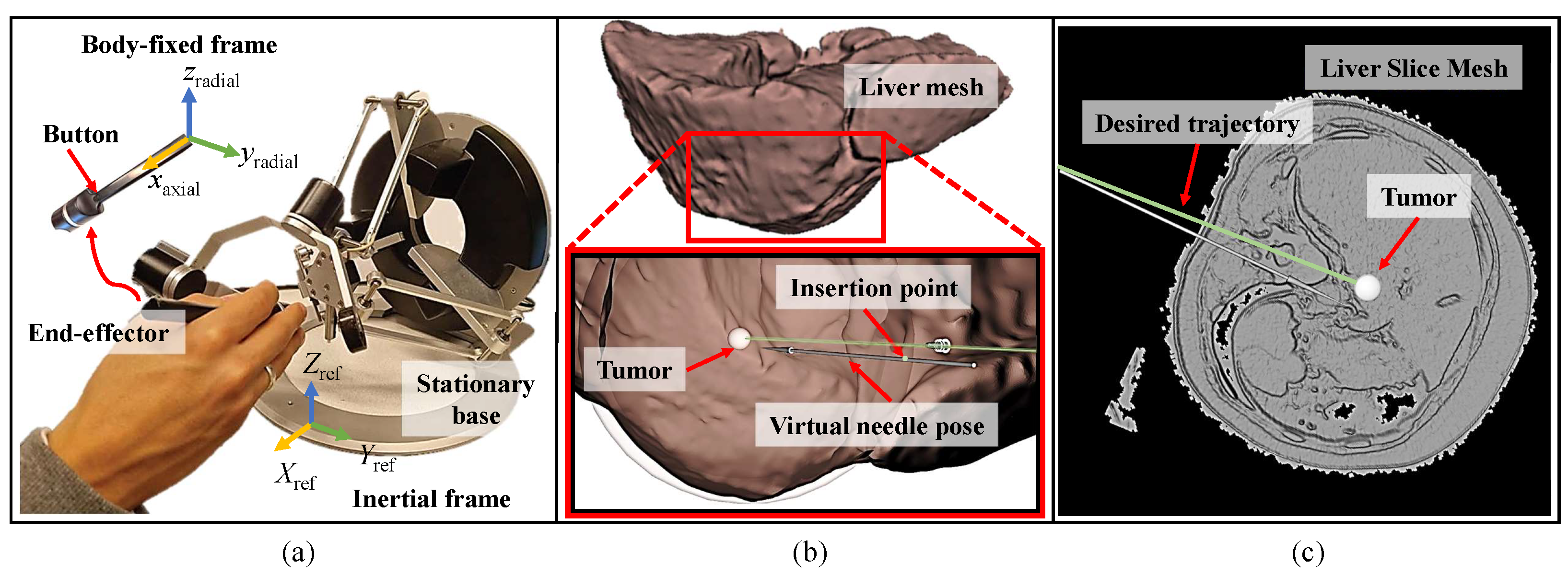
2.1. Setup and Simulation Environment
2.2. Visual Feedback Strategy
2.3. Haptic Feedback Strategy
2.3.1. Radial Force Feedback
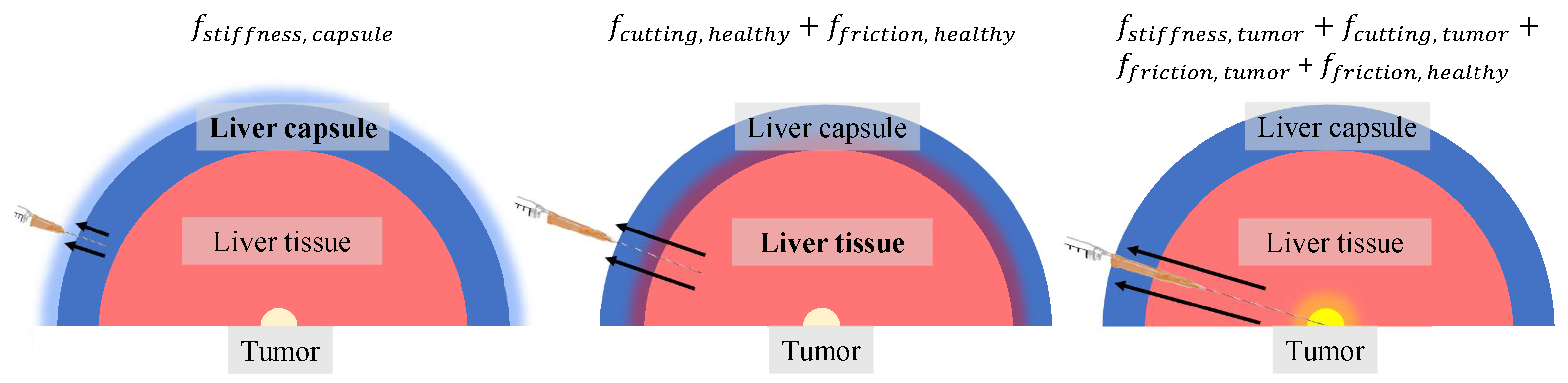
2.3.2. Axial Force Feedback

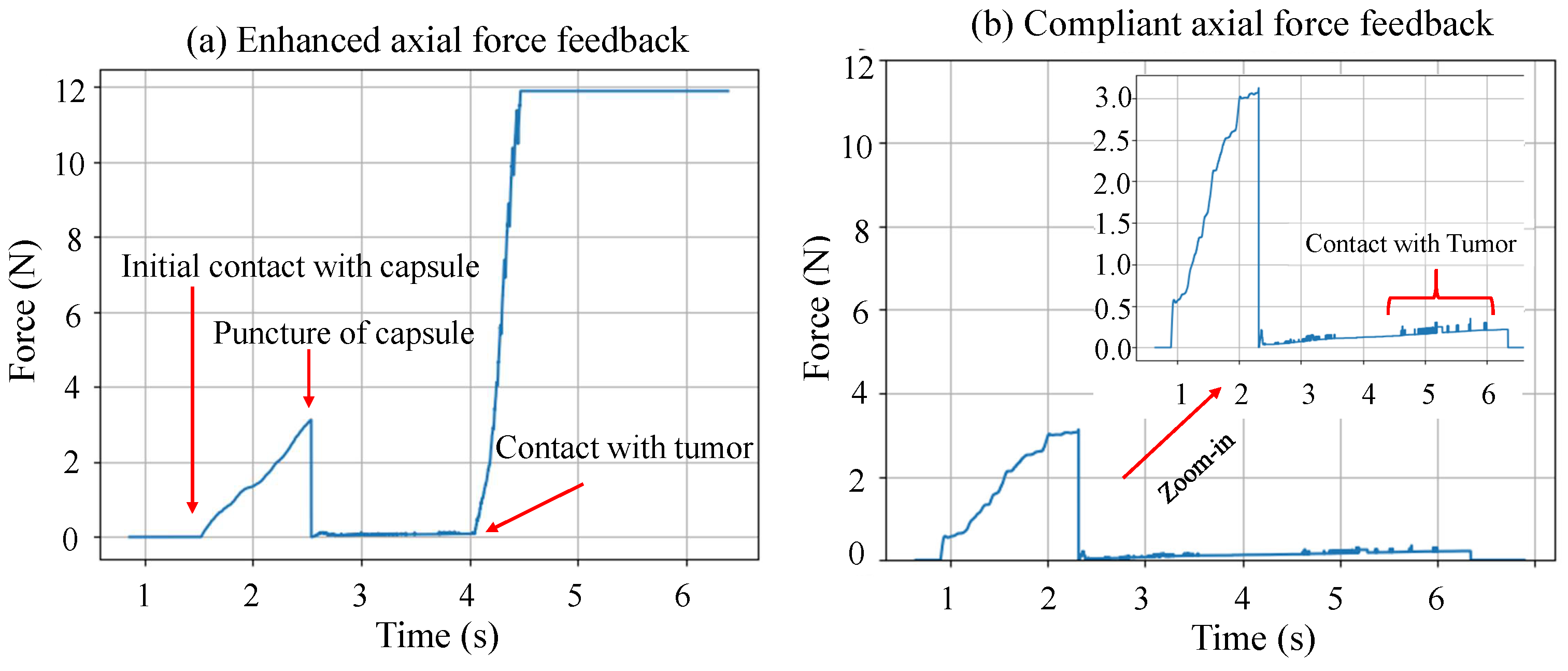
2.3.3. Modes of Operation
2.4. Experimental Design
- Baseline (BL) scenario, compliant radial force feedback with intermittent visual CT-like scan feedback provided upon request. The axial forces were not enhanced and are similar to what the interventionalist would feel in an insertion procedure. The simulated tumor exhibited a slightly greater magnitude in terms of friction and cutting forces compared to liver tissue.
- Real-time (RT) scenario, real-time and continuous visual feedback. The participant did not need to request scans, since they were automatically displayed. Axial and radial forces were implemented exactly like the baseline scenario.
- Enhanced sensing (ES) scenario, enhanced axial forces. The axial forces were magnified when the needle made contact with the tumor. All other aspects of haptic and visual feedback were provided in the same way as the baseline scenario.
- Enhanced guidance (EG) scenario, enhanced radial forces. The radial forces were amplified throughout the entire scenario. All other aspects of haptic and visual feedback were provided in the same way as the baseline scenario.
- Enhanced sensing and guidance (ESG) scenario, enhanced axial and radial forces. The axial and radial forces were amplified (combination of EG and ES). Visual feedback was provided upon request.
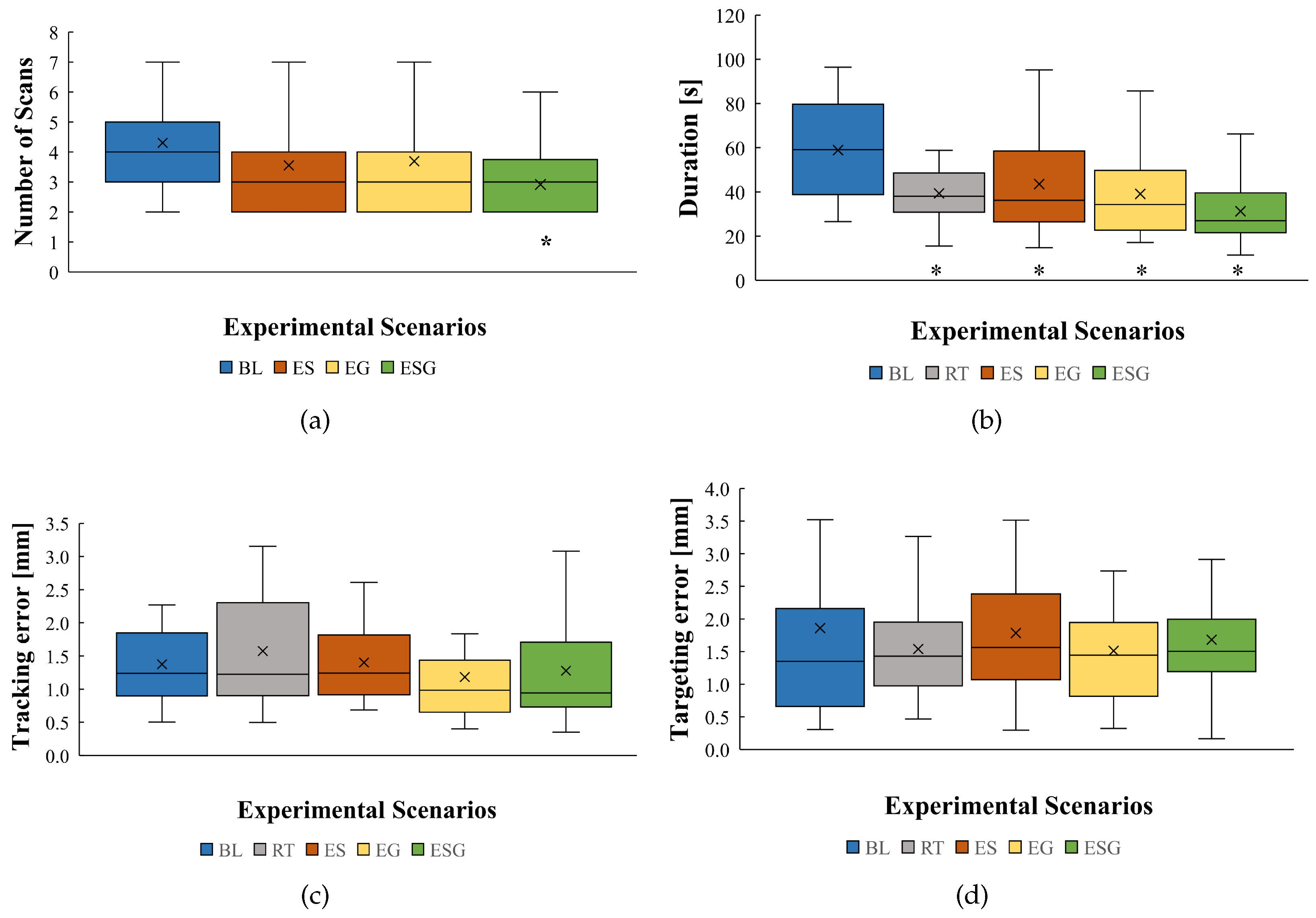
3. Results
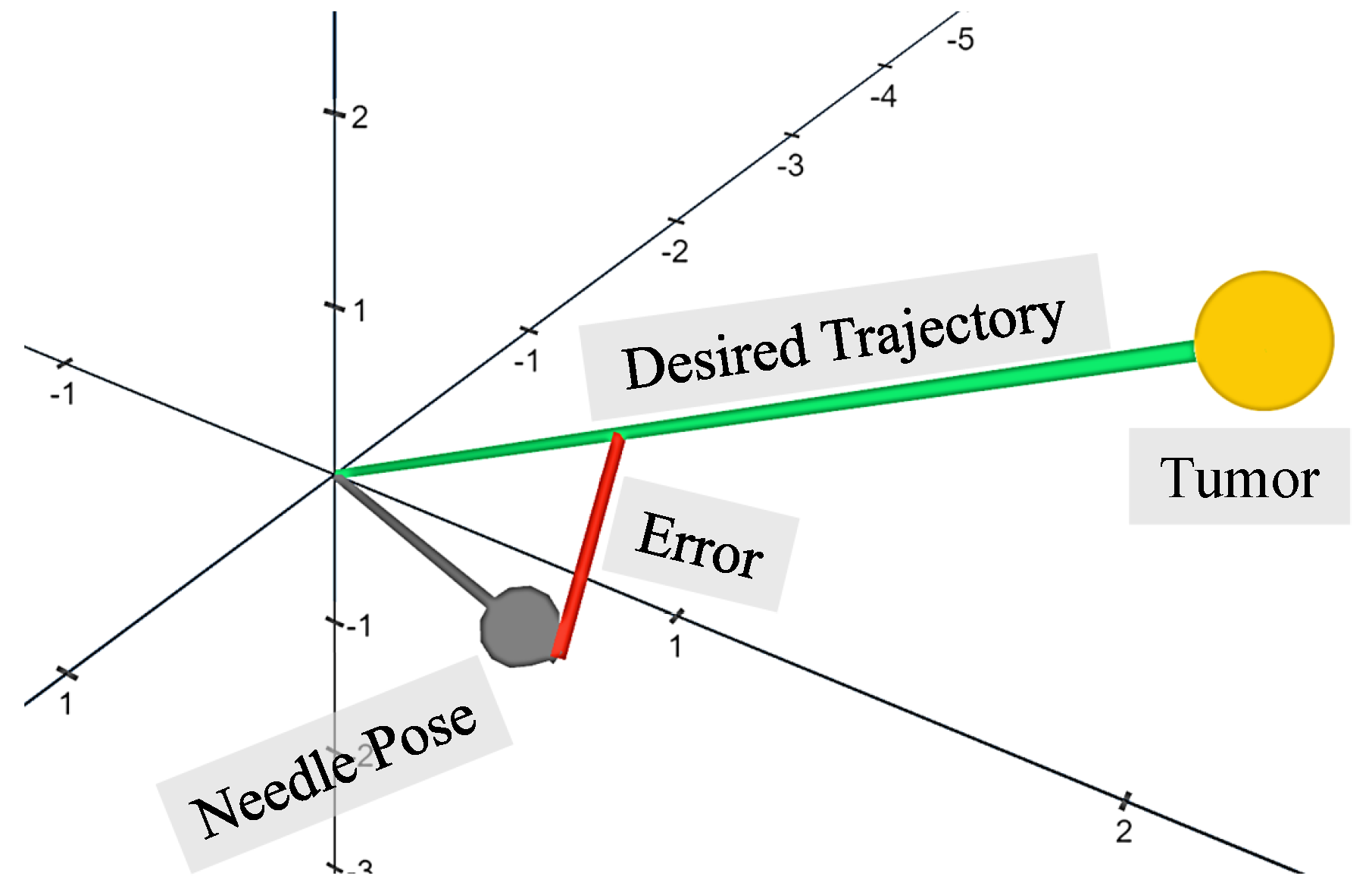
3.1. Performance Evaluation
3.2. Questionnaire Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| ROS | Robot Operating System |
| HSD | Honestly significant difference |
| DoF | Degrees of freedom |
| PD | Proportional–derivative |
Appendix A

References
- Al Knawy, B.; Shiffman, M. Percutaneous liver biopsy in clinical practice. Liver Int. 2007, 27, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Gala, K.B.; Chandra, D.; Shetty, N.S.; Agarwal, U.; Bansal, H.; Shariq, M.; Pendse, H.A.; Janu, A.; Mandava, R.; Kulkarni, S.S. Imaging Recommendations for Image-Guided Biopsy in Oncology. Indian J. Med. Paediatr. Oncol. 2023, 44, 334–342. [Google Scholar] [CrossRef]
- Patel, R.V.; Atashzar, S.F.; Tavakoli, M. Haptic Feedback and Force-Based Teleoperation in Surgical Robotics. Proc. IEEE 2022, 110, 1012–1027. [Google Scholar] [CrossRef]
- Aggravi, M.; Estima, D.A.L.; Krupa, A.; Misra, S.; Pacchierotti, C. Haptic Teleoperation of Flexible Needles Combining 3D Ultrasound Guidance and Needle Tip Force Feedback. IEEE Robot. Autom. Lett. 2021, 6, 4859–4866. [Google Scholar] [CrossRef]
- Thai, M.T.; Phan, P.T.; Hoang, T.T.; Wong, S.; Lovell, N.H.; Do, T.N. Advanced Intelligent Systems for Surgical Robotics. Adv. Intell. Syst. 2020, 2, 1900138. [Google Scholar] [CrossRef]
- Chevrie, J.; Krupa, A.; Babel, M. Real-time Teleoperation of Flexible Beveled-tip Needle Insertion using Haptic Force Feedback and 3D Ultrasound Guidance. In Proceedings of the proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; pp. 2700–2706. [Google Scholar]
- Mieling, R.; Stapper, C.; Gerlach, S.; Neidhardt, M.; Latus, S.; Gromniak, M.; Breitfeld, P.; Schlaefer, A. Proximity-Based Haptic Feedback for Collaborative Robotic Needle Insertion. In Haptics: Science, Technology, Applications. EuroHaptics 2022; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2022; Volume 13235, pp. 301–309. [Google Scholar]
- Dagnino, G.; Liu, J.; Abdelaziz, M.E.M.K.; Chi, W.; Riga, C.; Yang, G.Z. Haptic Feedback and Dynamic Active Constraints for Robot-Assisted Endovascular Catheterization. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 1770–1775. [Google Scholar]
- Okamura, A.; Simone, C.; O’Leary, M. Force Modeling for Needle Insertion Into Soft Tissue. IEEE Trans. Biomed. Eng. 2004, 51, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Mieling, R.; Neidhardt, M.; Latus, S.; Stapper, C.; Gerlach, S.; Kniep, I.; Heinemann, A.; Ondruschka, B.; Schlaefer, A. Collaborative Robotic Biopsy with Trajectory Guidance and Needle Tip Force Feedback. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), London, UK, 29 May–2 June 2023; pp. 6893–6900. [Google Scholar]
- Saudrais, C.; Rubbert, L.; Bonnefoy, L.; Zhu, R.; Schneegans, H.; Baur, C.; Mescheder, U.; Renaud, P. Experimental Evaluation of Needle Tip Force Sensing Associated to Tactile Feedback for Improving Needle Remote Insertion. In New Trends in Medical and Service Robotics. MESROB 2020; Springer International Publishing: Cham, Switzerland, 2021; Volume 93, pp. 136–142. [Google Scholar]
- De Lorenzo, D.; Koseki, Y.; De Momi, E.; Chinzei, K.; Okamura, A.M. Coaxial Needle Insertion Assistant With Enhanced Force Feedback. IEEE Trans. Biomed. Eng. 2013, 60, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Washio, T.; Chinzei, K. Needle Force Sensor, Robust and Sensitive Detection of the Instant of Needle Puncture. In Medical Image Computing and Computer-Assisted Intervention—(MICCAI); Springer: Berlin/Heidelberg, Germany, 2004; Volume 3217, pp. 113–120. [Google Scholar]
- Chadda, R.; Wismath, S.; Hessinger, M.; Schäfer, N.; Schlaefer, A.; Kupnik, M. Needle Tip Force Sensor for Medical Applications. In Proceedings of the IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Kumar, S.; Shrikanth, V.; Amrutur, B.; Asokan, S.; Bobji, M.S. Detecting stages of needle penetration into tissues through force estimation at needle tip using fiber Bragg grating sensors. J. Biomed. Opt. 2016, 21, 127009. [Google Scholar] [CrossRef] [PubMed]
- Meli, L.; Pacchierotti, C.; Prattichizzo, D. Experimental evaluation of magnified haptic feedback for robot-assisted needle insertion and palpation. Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13, e1809. [Google Scholar] [CrossRef] [PubMed]
- Abayazid, M.; Pacchierotti, C.; Moreira, P.; Alterovitz, R.; Prattichizzo, D.; Misra, S. Experimental evaluation of co-manipulated ultrasound-guided flexible needle steering. Int. J. Med. Robot. Comput. Assist. Surg. 2016, 12, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Gerovich, O.; Marayong, P.; Okamura, A.M. The effect of visual and haptic feedback on computer-assisted needle insertion. Comput. Aided Surg. 2004, 9, 243–249. [Google Scholar] [PubMed]
- Wang, Z.; Reed, I.; Fey, A.M. Toward Intuitive Teleoperation in Surgery: Human-Centric Evaluation of Teleoperation Algorithms for Robotic Needle Steering. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Brisbane, Australia, 21–25 May 2018; pp. 5799–5806. [Google Scholar]
- Rangarajan, K.; Davis, H.; Pucher, P.H. Systematic Review of Virtual Haptics in Surgical Simulation: A Valid Educational Tool? J. Surg. Educ. 2020, 77, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Bilic, P.; Christ, P.; Li, H.B.; Vorontsov, E.; Ben-Cohen, A.; Kaissis, G.; Szeskin, A.; Jacobs, C.; Mamani, G.E.H.; Chartrand, G.; et al. The Liver Tumor Segmentation Benchmark (LiTS). Med. Image Anal. 2023, 84, 102680. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, J.; Wang, B.; Zhu, X.; Shi, X.; Ding, Y. Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: A population-based study. Cancer Manag. Res. 2018, 10, 4401–4410. [Google Scholar] [CrossRef] [PubMed]
- Neagu, A.V.; Betianu, C.I. Hepatic Pseudolesions and How to Spot Them. In Proceedings of the European Congress of Radiology—ECR: 2020, Virtual, 15–19 July 2020. [Google Scholar]
- Van Gerwen, D.J.; Dankelman, J.; Van Den Dobbelsteen, J.J. Needle–tissue interaction forces—A survey of experimental data. Med. Eng. Phys. 2012, 34, 665–680. [Google Scholar] [CrossRef] [PubMed]
| Type | Mechanical Properties | Experimental Scenario | ||||
|---|---|---|---|---|---|---|
| BL | RT | ES | EG | ESG | ||
| Axial: Liver Capsule | (N/m) | 100 | ||||
| Axial: Liver Tissue | (Ns/m) | 5 | ||||
| (N) | 0.8 | |||||
| (N) | 1 | |||||
| c (Ns/m) | 10 | |||||
| Axial: Tumor | (N/m) | 0 | 0 | 1000 | 0 | 1000 |
| (Ns/m) | 7 | 7 | 10 | 7 | 10 | |
| (N) | 0.9 | 0.9 | 1.4 | 0.9 | 1.4 | |
| (N) | 1.1 | 1.1 | 1.8 | 1.1 | 1.8 | |
| c (Ns/m) | 10.5 | 10.5 | 15 | 10.5 | 15 | |
| Radial | 100 | 100 | 100 | 1000 | 1000 | |
| 30 | 30 | 30 | 70 | 50 | ||
| Scenarios | RT | ES | EG | ESG |
|---|---|---|---|---|
| Improved performance compared to baseline | 4.3 | 3.7 | 4.3 | 4.5 |
| Easy to navigate to target | 4.5 | 3.6 | 4.2 | 4.4 |
| Confidence level increase after experiment | 4.5 | 4 | 4.1 | 4.5 |
| Overall score | 4.4 | 3.8 | 4.2 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, M.; Dresscher, D.; Abayazid, M. Virtual Needle Insertion with Enhanced Haptic Feedback for Guidance and Needle–Tissue Interaction Forces. Sensors 2024, 24, 5560. https://doi.org/10.3390/s24175560
Selim M, Dresscher D, Abayazid M. Virtual Needle Insertion with Enhanced Haptic Feedback for Guidance and Needle–Tissue Interaction Forces. Sensors. 2024; 24(17):5560. https://doi.org/10.3390/s24175560
Chicago/Turabian StyleSelim, Mostafa, Douwe Dresscher, and Momen Abayazid. 2024. "Virtual Needle Insertion with Enhanced Haptic Feedback for Guidance and Needle–Tissue Interaction Forces" Sensors 24, no. 17: 5560. https://doi.org/10.3390/s24175560





