Comparing Device-Generated and Calculated Bioimpedance Variables in Community-Dwelling Older Adults
Abstract
:1. Introduction
2. Materials and Methods
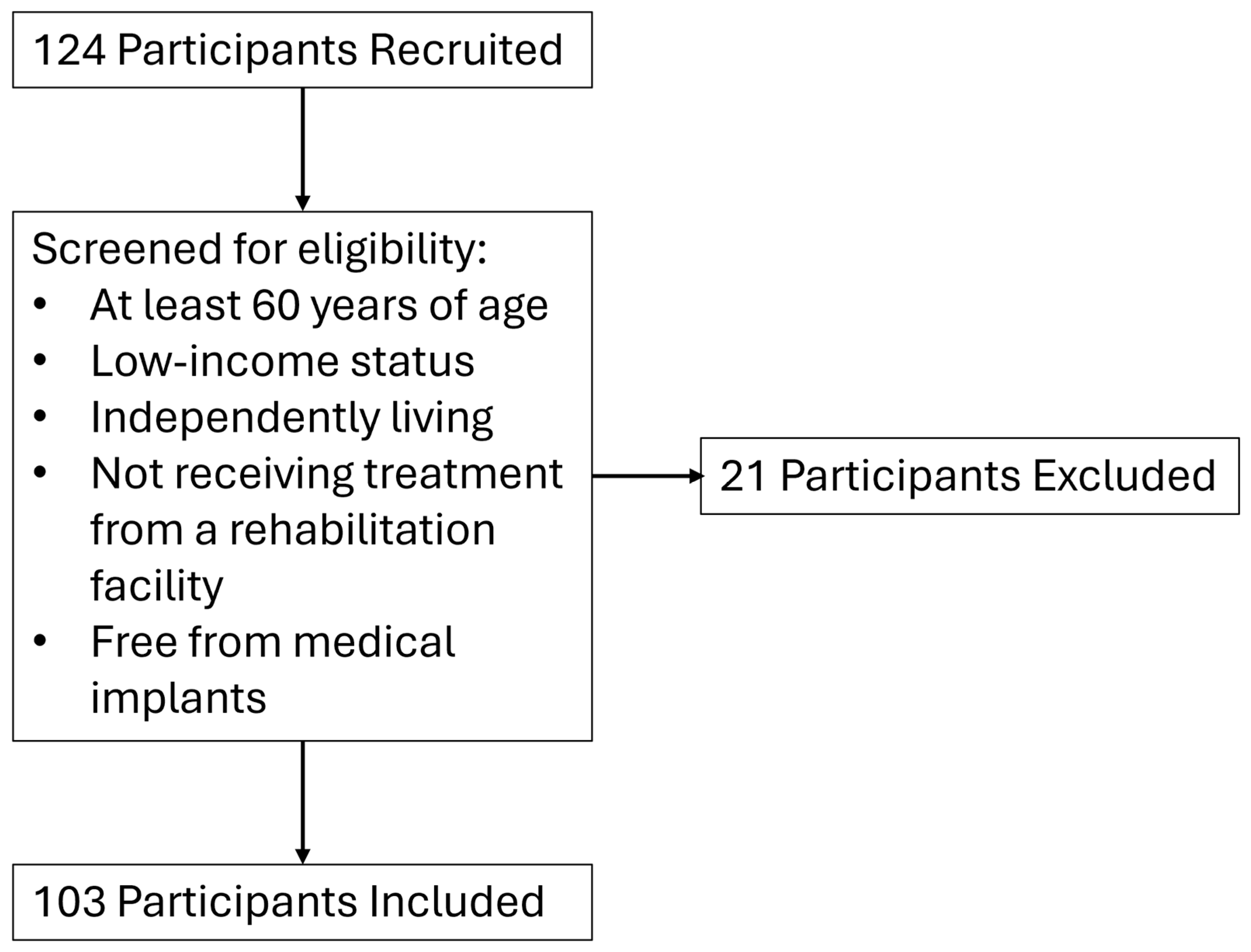
2.1. Participants
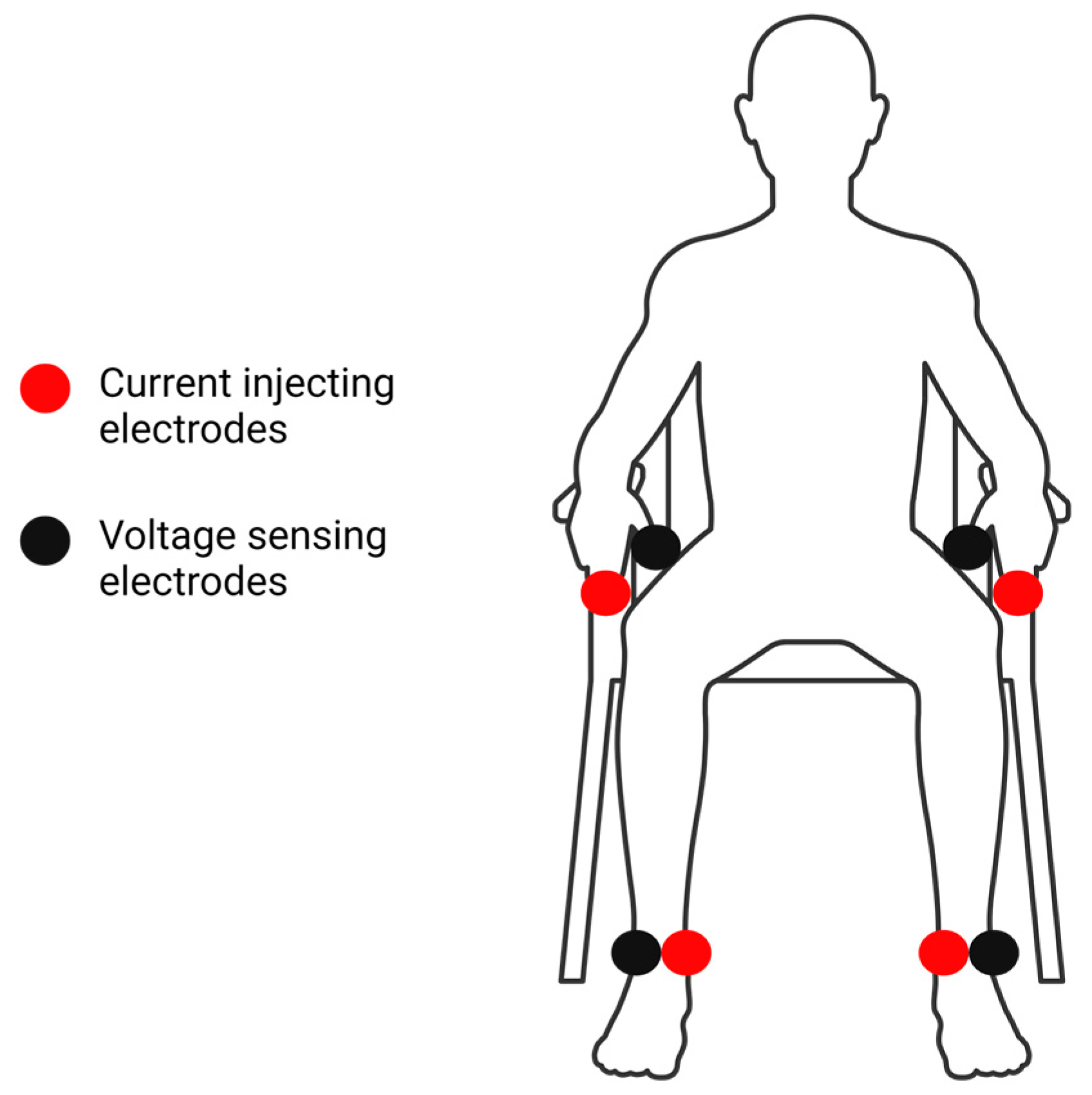
2.2. Bioelectrical Impedance Analysis
2.3. Phase Angle Calculations
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Whole-Body PhA
3.3. Segmental BIA Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uemura, K.; Doi, T.; Tsutsumimoto, K.; Nakakubo, S.; Kim, M.J.; Kurita, S.; Ishii, H.; Shimada, H. Predictivity of bioimpedance phase angle for incident disability in older adults. J. Cachexia Sarcopenia Muscle 2020, 11, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Walter-Kroker, A.; Kroker, A.; Mattiucci-Guehlke, M.; Glaab, T. A practical guide to bioelectrical impedance analysis using the example of chronic obstructive pulmonary disease. Nutr. J. 2011, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C.; Brantlov, S. Bioimpedance basics and phase angle fundamentals. Rev. Endocr. Metab. Disord. 2023, 24, 381–391. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobaus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Chumlea, W.C.; Roche, A.F. Bioelectric impedance phase-angle and body-composition. Am. J. Clin. Nutr. 1988, 48, 16–23. [Google Scholar] [CrossRef]
- Rinniella, E.; Cintoni, M.; Addolorato, G.; Triarico, S.; Ruggiero, A.; Perna, A.; Silvestri, G.; Gasbarrini, A.; Mele, M. Phase angle and impedance ratio: Two specular ways to analyze body composition. Ann. Clin. Nutr. 2018, 1, 1–5. [Google Scholar]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F.; Lis, C.G. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer 2008, 8, 249. [Google Scholar] [CrossRef]
- Ceolin, J.; de Borba, E.L.; Mundstock, E.; de Oliveira, J.R.; Mattiello, R.; Bodanese, L.C. Phase angle of bioimpedance as a marker of inflammation in cardiovascular diseases: A systematic review. Nutrition 2023, 112, 112064. [Google Scholar] [CrossRef]
- de Borba, E.L.; Ceolin, J.; Ziegelmann, P.K.; Bodanese, L.C.; Goncalves, M.R.; Canon-Montanez, W.; Mattiello, R. Phase angle of bioimpedance at 50 kHz is associated with cardiovascular diseases: Systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 1366–1373. [Google Scholar] [CrossRef]
- Jun, M.H.; Kim, S.; Ku, B.; Cho, J.; Kim, K.; Yoo, H.R.; Kim, J.U. Glucose-independent segmental phase angles from multi-frequency bioimpedance analysis to discriminate diabetes mellitus. Sci. Rep. 2018, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C. Editorial Comment: Phase angle from bioimpedance measurements as a surrogate of cardiovascular disease. Eur. J. Clin. Nutr. 2022, 76, 1364–1365. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C. Segmental bioelectrical impedance analysis: An update. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Bracco, D.; Thiebaud, D.; Chiolero, R.L.; Landry, M.; Burckhardt, P.; Schutz, Y. Segmental body composition assessed by bioelectrical impedance analysis and DEXA in humans. J. Appl. Physiol. 1996, 81, 2580–2587. [Google Scholar] [CrossRef]
- Mally, K.; Dettbarn, K.; Dittmar, M. Influence of electrode position on segmental resistance, reactance, and phase angle values. Int. J. Body Compos. Res. 2011, 9, 25–31. [Google Scholar]
- Malavolti, M.; Mussi, C.; Poli, M.; Fantuzzi, A.L.; Salvioli, G.; Battistini, N.; Bedogni, G. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann. Hum. Biol. 2003, 30, 380–391. [Google Scholar] [CrossRef]
- Sartorio, A.; Malavolti, M.; Agosti, F.; Marinone, P.G.; Caiti, O.; Battistini, N.; Bedogni, G. Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2005, 59, 155–160. [Google Scholar] [CrossRef]
- Jensen, B.; Braun, W.; Both, M.; Gallagher, D.; Clark, P.; González, D.L.; Klückmann, K.; Bosy-Westphal, A. Configuration of bioelectrical impedance measurements affects results for phase angle. Med. Eng. Phys. 2020, 84, 10–15. [Google Scholar] [CrossRef]
- Thiamwong, L.; Stout, J.R.; Park, J.H.; Yan, X. Technology-based fall risk assessments for older adults in low-income settings: Protocol for a cross-sectional study. JMIR Res. Protoc. 2021, 10, e27381. [Google Scholar] [CrossRef]
- Poverty Thresholds. Available online: https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-poverty-thresholds.html (accessed on 18 March 2024).
- Ding, Y.; Chang, L.; Zhang, H.; Wang, S. Predictive value of phase angle in sarcopenia in patients on maintenance hemodialysis. Nutrition 2022, 94, 111527. [Google Scholar] [CrossRef]
- Wu, H.; Ding, P.; Wu, J.; Yang, P.; Tian, Y.; Zhao, Q. Phase angle derived from bioelectrical impedance analysis as a marker for predicting sarcopenia. Front. Nutr. 2022, 9, 1060224. [Google Scholar] [CrossRef]
- Buckinx, F.; Reginster, J.Y.; Dardenne, N.; Croisiser, J.L.; Kaux, J.F.; Beaudart, C.; Slomian, J.; Bruyere, O. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: A cross-sectional study. BMC Musculoskel. Dis. 2015, 16, 1–7. [Google Scholar] [CrossRef]
- Love, J.; Droppmann, D.; Selker, R.; Gallucci, M.; Jentschke, S.; Balci, S.; Seol, H.; Agosti, M. The Jamovi Project, Version 2.4.1. 2023. Available online: https://www.jamovi.org/ (accessed on 29 July 2024).
- R: A Language and Environment for Statistical Computing. Available online: https://cran.r-project.org (accessed on 29 July 2024).
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Gerke, O. Nonparametric limits of agreement in method comparison studies: A simulation study on extreme quantile estimation. Int. J. Environ. Res. Public Health 2020, 17, 8330. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.V. Note on rank biserial correlation. Educ. Psychol. Meas. 1966, 26, 623–631. [Google Scholar] [CrossRef]
- Fuller, N.J.; Elia, M. Potential use of bioelectrical impedance of the ‘whole body’ and of body segments for the assessment of body composition: Comparison with densitometry and anthropometry. Eur. J. Clin. Nutr. 1989, 43, 779–791. [Google Scholar] [PubMed]
- Stewart, S.P.; Bramley, P.N.; Heighton, R.; Green, J.H.; Horsman, A.; Losowsky, M.S.; Smith, M.A. Estimation of body composition from bioelectrical impedance of body segments: Comparison with dual-energy X-ray absorptiometry. Br. J. Nutr. 1993, 69, 645–655. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykken, G.I. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef]
- Lijewski, M.; Burdukiewicz, A.; Pietraszewska, J.; Andrzejewska, J.; Stachon, A. Asymmetry of muscle mass distribution and grip strength in professional handball players. Int. J. Environ. Res. Public Health 2021, 18, 1913. [Google Scholar] [CrossRef]
- Roehrich, L.; Suendermann, S.; Just, I.A.; Knierim, J.; Mulzer, J.; Mueller, M.; Eulert-Grehn, J.J.; Hummel, M.; Starck, C.; Potapov, E.; et al. Safety of bioelectrical impedance analysis in advanced heart failure patients. Pacing Clin. Electrophysiol. 2020, 43, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Dovancescu, S.; Saporito, S.; Herold, I.; Korsten, H.; Aarts, R.; Massimo, M. Monitoring thoracic fluid content using bioelectrical impedance spectroscopy and cole modeling. J. Electr. Bioimpedance 2017, 8, 107–115. [Google Scholar] [CrossRef]
- Sterner, D.A.; Stout, J.R.; Lafontant, K.; Park, J.H.; Fukuda, D.H.; Thiamwong, L. Phase angle and impedance ratio as indicators of physical function and fear of falling in older adult women: Cross-sectional analysis. JMIR Aging 2024, 7, e53975. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | Whole-Body PhADG 1 (°) | Body Mass Index (kg/m2) | Body Weight (kg) | |
|---|---|---|---|---|
| Mean | 75.7 | 5.4 | 26.9 | 73.2 |
| Median | 75.0 | 5.4 | 26.1 | 73.0 |
| Standard deviation | 7.2 | 0.9 | 5.2 | 15.8 |
| Interquartile range | 9.8 | 1.0 | 6.2 | 23.3 |
| Minimum | 60.0 | 3.0 | 18.2 | 42.2 |
| Maximum | 96.0 | 6.9 | 47.9 | 126.6 |
| Agreement | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Method | Mean ± SD | Median (IQR) | 2.5th Quantile | 97.5th Quantile | rs | Mean Difference | 95% Confidence Interval | p | Effect Size |
| PhADG | 5.38 ± 0.80 | 5.40 (1.0) | 0.002 | 0.05 | 0.97 | −0.004 | (−0.011, 0.003) | 0.26 | −0.13 |
| Right Side PhACalc | 5.41 ± 0.75 | 5.40 (1.0) | |||||||
| PhADG | 5.38 ± 0.80 | 5.40 (1.0) | 0.02 | 1.88 | 0.69 | 0.11 | (0.023, 0.174) | 0.01 | 0.29 |
| Left Side PhACalc | 5.51 ± 1.50 | 5.40 (1.1) | |||||||
| PhADG | 5.38 ± 0.80 | 5.40 (1.0) | 0.02 | 0.91 | 0.79 | 0.11 | (0.067, 0.141) | <0.001 | 0.54 |
| Combined Sides PhACalc | 5.40 ± 0.91 | 5.36 (1.0) | |||||||
| Correlation | Mean Differences | ||||||
|---|---|---|---|---|---|---|---|
| Variable | rs | p | Wilcoxon W | Mean Difference | SE | p | Effect Size |
| Impedance (Z) | 0.95 | <0.001 | 1780 | −5.05 | 2.75 | 0.01 | −0.32 |
| Resistance (R) | 0.95 | <0.001 | 1780 | −4.78 | 2.82 | 0.01 | −0.32 |
| Reactance (Xc) | 0.84 | <0.001 | 2794 | 0.40 | 1.08 | 0.27 | −0.13 |
| PhACalc | 0.75 | <0.001 | 3425 | 0.11 | 0.14 | 0.01 | 0.30 |
| Correlation | Mean Differences | ||||||
|---|---|---|---|---|---|---|---|
| Variable | rs | p | Wilcoxon W | Mean Difference | SE | p | Effect Size |
| Arms | |||||||
| Impedance (Z) | 0.92 | <0.001 | 1535 | −6.70 | 2.27 | <0.001 | −0.42 |
| Resistance (R) | 0.91 | <0.001 | 1532 | −6.77 | 2.35 | <0.001 | −0.42 |
| Reactance (Xc) | 0.77 | <0.001 | 2937 | 0.35 | 1.08 | 0.22 | 0.14 |
| PhADG | 0.69 | <0.001 | 1662 | −0.19 | 0.24 | 0.001 | −0.37 |
| Legs | |||||||
| Impedance (Z) | 0.96 | <0.001 | 2582 | −0.20 | 1.17 | 0.88 | −0.02 |
| Resistance (R) | 0.96 | <0.001 | 2577 | −0.24 | 1.17 | 0.87 | −0.02 |
| Reactance (Xc) | 0.94 | <0.001 | 2649 | 0.06 | 0.17 | 0.67 | 0.05 |
| PhADG | 0.88 | <0.001 | 2552 | −0.004 | 0.06 | 0.81 | −0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lafontant, K.; Sterner, D.A.; Fukuda, D.H.; Stout, J.R.; Park, J.-H.; Thiamwong, L. Comparing Device-Generated and Calculated Bioimpedance Variables in Community-Dwelling Older Adults. Sensors 2024, 24, 5626. https://doi.org/10.3390/s24175626
Lafontant K, Sterner DA, Fukuda DH, Stout JR, Park J-H, Thiamwong L. Comparing Device-Generated and Calculated Bioimpedance Variables in Community-Dwelling Older Adults. Sensors. 2024; 24(17):5626. https://doi.org/10.3390/s24175626
Chicago/Turabian StyleLafontant, Kworweinski, Danielle A. Sterner, David H. Fukuda, Jeffrey R. Stout, Joon-Hyuk Park, and Ladda Thiamwong. 2024. "Comparing Device-Generated and Calculated Bioimpedance Variables in Community-Dwelling Older Adults" Sensors 24, no. 17: 5626. https://doi.org/10.3390/s24175626







