Screen-Printed Electrodes as Low-Cost Sensors for Breast Cancer Biomarker Detection
Abstract
:1. Introduction
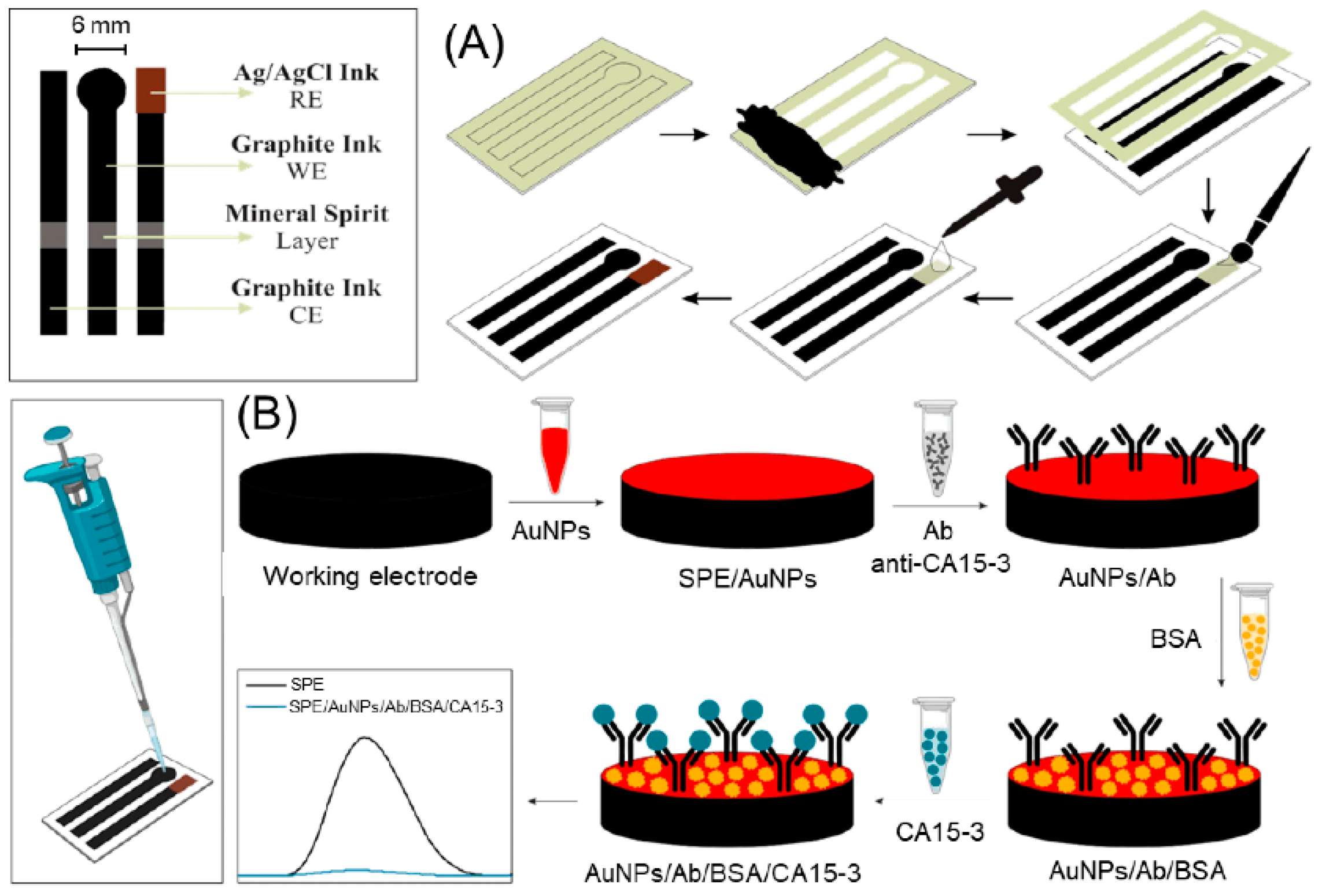
2. Screen-Printed Electrodes: Fundamentals and Fabrication
3. Modification Strategies for SPEs in Breast Cancer Biomarker Detection
3.1. Nanomaterial-Based Screen-Printing Pastes
3.2. Immobilization Strategies
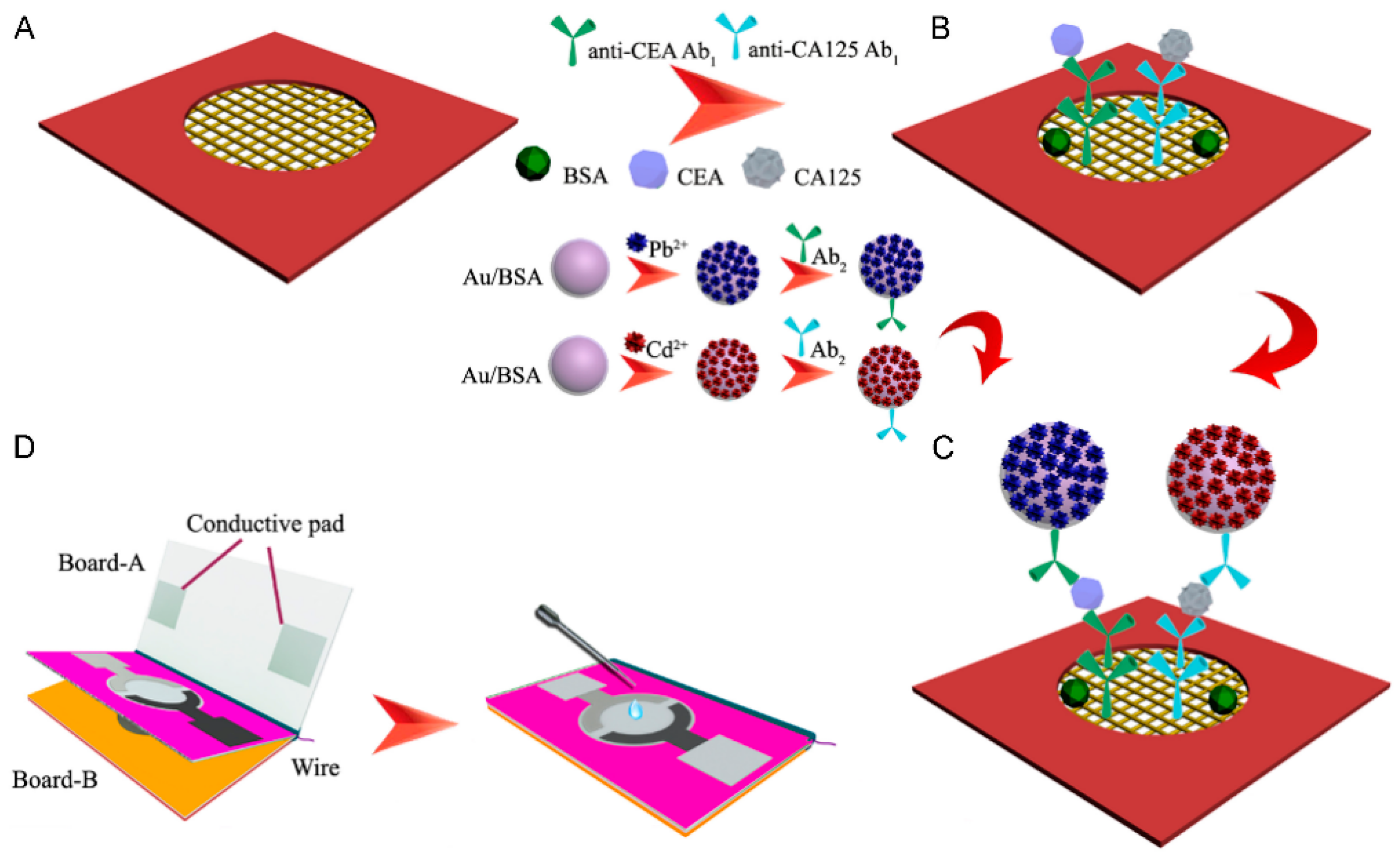
3.3. Multiplex Sensors
4. SPE-Based Biosensors for Breast Cancer Biomarkers
4.1. HER2
4.2. CA15-3
4.3. CEA
4.4. BRCA1
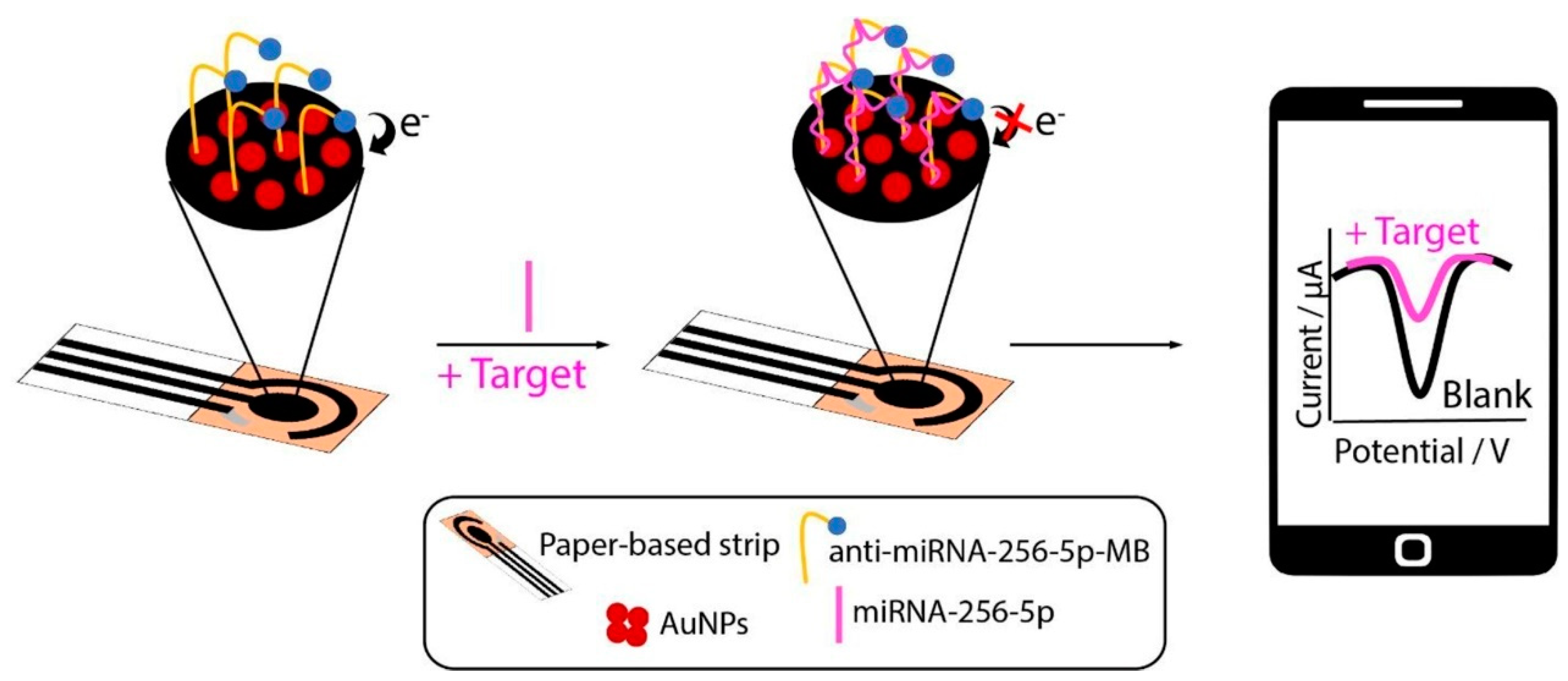
4.5. MicroRNAs
4.6. Metabolite Biomarkers
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coleman, C. Early Detection and Screening for Breast Cancer. Semin. Oncol. Nurs. 2017, 33, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, O.; Yip, C.-H.; Brooks, A.; Cabanes, A.; Caleffi, M.; Dunstan Yataco, J.A.; Gyawali, B.; McCormack, V.; McLaughlin de Anderson, M.; Mehrotra, R.; et al. Breast Cancer Early Detection: A Phased Approach to Implementation. Cancer 2020, 126, 2379–2393. [Google Scholar] [CrossRef] [PubMed]
- Levenson, V.V. Biomarkers for Early Detection of Breast Cancer: What, When, and Where? Biochim. Biophys. Acta (BBA)-Gen. Subj. 2007, 1770, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guan, X.; Fan, Z.; Ching, L.-M.; Li, Y.; Wang, X.; Cao, W.-M.; Liu, D.-X. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef]
- Fehm, T.; Becker, S.; Duerr-Stoerzer, S.; Sotlar, K.; Mueller, V.; Wallwiener, D.; Lane, N.; Solomayer, E.; Uhr, J. Determination of HER2 Status Using Both Serum HER2 Levels and Circulating Tumor Cells in Patients with Recurrent Breast Cancer Whose Primary Tumor Was HER2 Negative or of Unknown HER2 Status. Breast Cancer Res. 2007, 9, R74. [Google Scholar] [CrossRef]
- Ryu, J.M.; Kang, D.; Cho, J.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Lee, S.K.; Kim, Y.J.; Im, Y.-H.; Ahn, J.S.; et al. Prognostic Impact of Elevation of Cancer Antigen 15-3 (CA15-3) in Patients with Early Breast Cancer with Normal Serum CA15-3 Level. J. Breast Cancer 2023, 26, 126–135. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, X.; He, Y.; Liu, C.; Liu, H. Elevated Levels of Serum Tumor Markers CEA and CA15-3 Are Prognostic Parameters for Different Molecular Subtypes of Breast Cancer. PLoS ONE 2015, 10, e0133830. [Google Scholar] [CrossRef]
- Fang, C.; Cao, Y.; Liu, X.; Zeng, X.-T.; Li, Y. Serum CA125 Is a Predictive Marker for Breast Cancer Outcomes and Correlates with Molecular Subtypes. Oncotarget 2017, 8, 63963–63970. [Google Scholar] [CrossRef]
- Moreno, M.; Bontkes, H.J.; Scheper, R.J.; Kenemans, P.; Verheijen, R.H.M.; von Mensdorff-Pouilly, S. High Level of MUC1 in Serum of Ovarian and Breast Cancer Patients Inhibits huHMFG-1 Dependent Cell-Mediated Cytotoxicity (ADCC). Cancer Lett. 2007, 257, 47–55. [Google Scholar] [CrossRef]
- Reeves, K.W.; Ness, R.B.; Stone, R.A.; Weissfeld, J.L.; Vogel, V.G.; Powers, R.W.; Modugno, F.; Cauley, J.A. Vascular Endothelial Growth Factor and Breast Cancer Risk. Cancer Causes Control 2009, 20, 375–386. [Google Scholar] [CrossRef]
- Thery, L.; Meddis, A.; Cabel, L.; Proudhon, C.; Latouche, A.; Pierga, J.-Y.; Bidard, F.-C. Circulating Tumor Cells in Early Breast Cancer. JNCI Cancer Spectr. 2019, 3, pkz026. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Airò, F.; Merkoçi, A. Enhanced Gold Nanoparticle Based ELISA for a Breast Cancer Biomarker. Anal. Chem. 2010, 82, 1151–1156. [Google Scholar] [CrossRef]
- Zaha, D.C. Significance of Immunohistochemistry in Breast Cancer. World J. Clin. Oncol. 2014, 5, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Oloomi, M.; Moazzezy, N.; Bouzari, S. Comparing Blood versus Tissue-Based Biomarkers Expression in Breast Cancer Patients. Heliyon 2020, 6, e03728. [Google Scholar] [CrossRef] [PubMed]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-Printed Electrodes for Biosensing: A Review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Rowley-Neale, S.J.; Banks, C.E. Screen-Printed Electrodes: Transitioning the Laboratory in-to-the Field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Moreira, F.T.C.; Ferreira, M.J.M.S.; Puga, J.R.T.; Sales, M.G.F. Screen-Printed Electrode Produced by Printed-Circuit Board Technology. Application to Cancer Biomarker Detection by Means of Plastic Antibody as Sensing Material. Sens. Actuators B Chem. 2016, 223, 927–935. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hossain, M.M.; Safavieh, M.; Wong, Y.L.; Rahman, I.A.; Zourob, M.; Tamiya, E. Toward the Development of Smart and Low Cost Point-of-Care Biosensors Based on Screen Printed Electrodes. Crit. Rev. Biotechnol. 2016, 36, 495–505. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical Biosensors Based on Nanomodified Screen-Printed Electrodes: Recent Applications in Clinical Analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef]
- Yamanaka, K.; Vestergaard, M.C.; Tamiya, E. Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors 2016, 16, 1761. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T. Recent Developments and Applications of Screen-Printed Electrodes in Environmental Assays—A Review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef]
- Suresh, R.R.; Lakshmanakumar, M.; Arockia Jayalatha, J.B.B.; Rajan, K.S.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of Screen-Printed Electrodes: Opportunities and Challenges. J. Mater. Sci. 2021, 56, 8951–9006. [Google Scholar] [CrossRef]
- Dhanapala, L.; Krause, C.E.; Jones, A.L.; Rusling, J.F. Printed Electrodes in Microfluidic Arrays for Cancer Biomarker Protein Detection. Biosensors 2020, 10, 115. [Google Scholar] [CrossRef]
- Harahsheh, T.; Makableh, Y.F.; Rawashdeh, I.; Al-Fandi, M. Enhanced Aptasensor Performance for Targeted HER2 Breast Cancer Detection by Using Screen-Printed Electrodes Modified with Au Nanoparticles. Biomed. Microdevices 2021, 23, 46. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Oliveira, O.N.; Machado, S.A.S. A Sandwich-Type Electrochemical Immunosensor Based on Au-rGO Composite for CA15-3 Tumor Marker Detection. Microchim. Acta 2021, 189, 38. [Google Scholar] [CrossRef]
- Chakraborty, B.; Das, A.; Mandal, N.; Samanta, N.; Das, N.; Chaudhuri, C.R. Label Free, Electric Field Mediated Ultrasensitive Electrochemical Point-of-Care Device for CEA Detection. Sci. Rep. 2021, 11, 2962. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M. Advances in Electrochemical Biosensor Technologies for the Detection of Nucleic Acid Breast Cancer Biomarkers. Sensors 2023, 23, 4128. [Google Scholar] [CrossRef]
- Erdem, A.; Eksin, E.; Congur, G. Indicator-Free Electrochemical Biosensor for microRNA Detection Based on Carbon Nanofibers Modified Screen Printed Electrodes. J. Electroanal. Chem. 2015, 755, 167–173. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.; Metters, J.P.; Kadara, R.O.; Banks, C.E. The Fabrication, Characterisation and Electrochemical Investigation of Screen-Printed Graphene Electrodes. Phys. Chem. Chem. Phys. 2014, 16, 4598–4611. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mohammadi, S.Z.; Safaei, M.; Tajik, S. Applications of Electrochemical Sensors and Biosensors Based on Modified Screen-Printed Electrodes: A Review. Anal. Methods 2020, 12, 1547–1560. [Google Scholar] [CrossRef]
- Foster, C.W.; Kadara, R.O.; Banks, C.E. Fabricating Screen-Printed Electrochemical Architectures: Successful Design and Fabrication. In Screen-Printing Electrochemical Architectures; Banks, C.E., Foster, C.W., Kadara, R.O., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–33. ISBN 978-3-319-25193-6. [Google Scholar]
- Foster, C.W.; Kadara, R.O.; Banks, C.E. Quality Control/Quality Assurance Analysis of Electrochemical Screen-Printed Sensors. In Screen-Printing Electrochemical Architectures; Banks, C.E., Foster, C.W., Kadara, R.O., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 35–56. ISBN 978-3-319-25193-6. [Google Scholar]
- Rao, V.K.; Sharma, M.K.; Pandey, P.; Sekhar, K. Comparison of Different Carbon Ink Based Screen-Printed Electrodes towards Amperometric Immunosensing. World J. Microbiol. Biotechnol. 2006, 22, 1135–1143. [Google Scholar] [CrossRef]
- Chu, Z.; Peng, J.; Jin, W. Advanced Nanomaterial Inks for Screen-Printed Chemical Sensors. Sens. Actuators B Chem. 2017, 243, 919–926. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Costa-García, A.; Muñiz, A.D.L.E. Electrochemical (Bio)Sensors for Pesticides Detection Using Screen-Printed Electrodes. Biosensors 2020, 10, 32. [Google Scholar] [CrossRef]
- Ferreira, L.M.C.; Reis, I.F.; Martins, P.R.; Marcolino-Junior, L.H.; Bergamini, M.F.; Camargo, J.R.; Janegitz, B.C.; Vicentini, F.C. Using Low-Cost Disposable Immunosensor Based on Flexible PET Screen-Printed Electrode Modified with Carbon Black and Gold Nanoparticles for Sensitive Detection of SARS-CoV-2. Talanta Open 2023, 7, 100201. [Google Scholar] [CrossRef]
- Michalska, A.; Kisiel, A.; Maksymiuk, K. Screen-Printed Disposable Reference Electrodes. In Handbook of Reference Electrodes; Inzelt, G., Lewenstam, A., Scholz, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 325–330. ISBN 978-3-642-36188-3. [Google Scholar]
- Obaje, E.A.; Cummins, G.; Schulze, H.; Mahmood, S.; Desmulliez, M.P.Y.; Bachmann, T.T. Carbon Screen-Printed Electrodes on Ceramic Substrates for Label-Free Molecular Detection of Antibiotic Resistance. J. Interdiscip. Nanomed. 2016, 1, 93–109. [Google Scholar] [CrossRef]
- Paimard, G.; Ghasali, E.; Baeza, M. Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications. Chemosensors 2023, 11, 113. [Google Scholar] [CrossRef]
- Kadara, R.O.; Jenkinson, N.; Banks, C.E. Screen Printed Recessed Microelectrode Arrays. Sens. Actuators B Chem. 2009, 142, 342–346. [Google Scholar] [CrossRef]
- Tan, F.; Metters, J.P.; Banks, C.E. Electroanalytical Applications of Screen Printed Microelectrode Arrays. Sens. Actuators B Chem. 2013, 181, 454–462. [Google Scholar] [CrossRef]
- Yévenes, C.F.G.; Wongkaew, N.; Ngamchana, S.; Surareungchai, W. Exploring Interdigitated Electrode Arrays Screen-Printed on Paper Substrates for Steady-State Electrochemical Measurements. J. Electrochem. Soc. 2022, 169, 103502. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Furuta, R.H.M.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Arantes, L.M.R.B.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Chaur, M.N.; et al. Screen-Printed Interdigitated Electrodes Modified with Nanostructured Carbon Nano-Onion Films for Detecting the Cancer Biomarker CA19-9. Mater. Sci. Eng. C 2019, 99, 1502–1508. [Google Scholar] [CrossRef]
- Dong, H.; Li, C.-M.; Zhang, Y.-F.; Cao, X.-D.; Gan, Y. Screen-Printed Microfluidic Device for Electrochemical Immunoassay. Lab Chip 2007, 7, 1752–1758. [Google Scholar] [CrossRef]
- Nawaz, M.A.H.; Rauf, S.; Catanante, G.; Nawaz, M.H.; Nunes, G.; Marty, J.L.; Hayat, A. One Step Assembly of Thin Films of Carbon Nanotubes on Screen Printed Interface for Electrochemical Aptasensing of Breast Cancer Biomarker. Sensors 2016, 16, 1651. [Google Scholar] [CrossRef]
- Chan, K.F.; Lim, H.N.; Shams, N.; Jayabal, S.; Pandikumar, A.; Huang, N.M. Fabrication of Graphene/Gold-Modified Screen-Printed Electrode for Detection of Carcinoembryonic Antigen. Mater. Sci. Eng. C 2016, 58, 666–674. [Google Scholar] [CrossRef]
- Lee, S.X.; Lim, H.N.; Ibrahim, I.; Jamil, A.; Pandikumar, A.; Huang, N.M. Horseradish Peroxidase-Labeled Silver/Reduced Graphene Oxide Thin Film-Modified Screen-Printed Electrode for Detection of Carcinoembryonic Antigen. Biosens. Bioelectron. 2017, 89, 673–680. [Google Scholar] [CrossRef]
- Chandra, P.; Singh, J.; Singh, A.; Srivastava, A.; Goyal, R.N.; Shim, Y.B. Gold Nanoparticles and Nanocomposites in Clinical Diagnostics Using Electrochemical Methods. J. Nanoparticles 2013, 2013, 535901. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Jia, Y.; Li, Y.; Wang, P.; Liu, Q.; Xu, Z.; Li, X.; Dong, Y. Sandwich-Type Electrochemical Immunosensor for Sensitive Detection of CEA Based on the Enhanced Effects of Ag NPs@CS Spaced Hemin/rGO. Biosens. Bioelectron. 2019, 126, 785–791. [Google Scholar] [CrossRef]
- Nguyen, V.-A.; Nguyen, H.L.; Nguyen, D.T.; Do, Q.P.; Tran, L.D. Electrosynthesized Poly(1,5-Diaminonaphthalene)/Polypyrrole Nanowires Bilayer as an Immunosensor Platform for Breast Cancer Biomarker CA 15-3. Curr. Appl. Phys. 2017, 17, 1422–1429. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Silva, M.S.V.; Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly Imprinted Electrochemical Sensor for the Point-of-Care Detection of a Breast Cancer Biomarker (CA 15-3). Sens. Actuators B Chem. 2018, 256, 905–912. [Google Scholar] [CrossRef]
- Chocholova, E.; Bertok, T.; Lorencova, L.; Holazova, A.; Farkas, P.; Vikartovska, A.; Bella, V.; Velicova, D.; Kasak, P.; Eckstein, A.A.; et al. Advanced Antifouling Zwitterionic Layer Based Impedimetric HER2 Biosensing in Human Serum: Glycoprofiling as a Novel Approach for Breast Cancer Diagnostics. Sens. Actuators B Chem. 2018, 272, 626–633. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Ferrari, A.G.-M.; Dempsey, N.C.; Banks, C.E. Electroanalytical Overview: Screen-Printed Electrochemical Sensing Platforms for the Detection of Vital Cardiac, Cancer and Inflammatory Biomarkers. Sens. Diagn. 2022, 1, 405–428. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Batistuti, M.R.; Bachour, B.; Mulato, M. Aptasensor Based on Screen-Printed Electrode for Breast Cancer Detection in Undiluted Human Serum. Bioelectrochemistry 2021, 137, 107586. [Google Scholar] [CrossRef]
- Marques, R.C.B.; Costa-Rama, E.; Viswanathan, S.; Nouws, H.P.A.; Costa-García, A.; Delerue-Matos, C.; González-García, M.B. Voltammetric Immunosensor for the Simultaneous Analysis of the Breast Cancer Biomarkers CA 15-3 and HER2-ECD. Sens. Actuators B Chem. 2018, 255, 918–925. [Google Scholar] [CrossRef]
- Al-Khafaji, Q.a.M.; Harris, M.; Tombelli, S.; Laschi, S.; Turner, A.P.F.; Mascini, M.; Marrazza, G. An Electrochemical Immunoassay for HER2 Detection. Electroanalysis 2012, 24, 735–742. [Google Scholar] [CrossRef]
- Tallapragada, S.D.; Layek, K.; Mukherjee, R.; Mistry, K.K.; Ghosh, M. Development of Screen-Printed Electrode Based Immunosensor for the Detection of HER2 Antigen in Human Serum Samples. Bioelectrochemistry 2017, 118, 25–30. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Syahruni, S.; Gaffar, S.; Wyantuti, S.; Yusuf, M.; Subroto, T. An Electrochemical Aptasensor for the Detection of HER2 as a Breast Cancer Biomarker Based on Gold Nanoparticles-Aptamer Bioconjugates. Indones. J. Chem. 2021, 21, 1526–1536. [Google Scholar] [CrossRef]
- Bezerra, G.; Córdula, C.; Campos, D.; Nascimento, G.; Oliveira, N.; Seabra, M.A.; Visani, V.; Lucas, S.; Lopes, I.; Santos, J.; et al. Electrochemical Aptasensor for the Detection of HER2 in Human Serum to Assist in the Diagnosis of Early Stage Breast Cancer. Anal. Bioanal. Chem. 2019, 411, 6667–6676. [Google Scholar] [CrossRef]
- Carvajal, S.; Fera, S.N.; Jones, A.L.; Baldo, T.A.; Mosa, I.M.; Rusling, J.F.; Krause, C.E. Disposable Inkjet-Printed Electrochemical Platform for Detection of Clinically Relevant HER-2 Breast Cancer Biomarker. Biosens. Bioelectron. 2018, 104, 158–162. [Google Scholar] [CrossRef]
- Marques, R.C.B.; Viswanathan, S.; Nouws, H.P.A.; Delerue-Matos, C.; González-García, M.B. Electrochemical Immunosensor for the Analysis of the Breast Cancer Biomarker HER2 ECD. Talanta 2014, 129, 594–599. [Google Scholar] [CrossRef]
- Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Electrochemical Sensing Platforms for HER2-ECD Breast Cancer Biomarker Detection. Electroanalysis 2019, 31, 121–128. [Google Scholar] [CrossRef]
- Ravalli, A.; da Rocha, C.G.; Yamanaka, H.; Marrazza, G. A Label-Free Electrochemical Affisensor for Cancer Marker Detection: The Case of HER2. Bioelectrochemistry 2015, 106, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Wignarajah, S.; Chianella, I.; Tothill, I.E. Development of Electrochemical Immunosensors for HER-1 and HER-2 Analysis in Serum for Breast Cancer Patients. Biosensors 2023, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Ilkhani, H.; Ravalli, A.; Marrazza, G. Design of an Affibody-Based Recognition Strategy for Human Epidermal Growth Factor Receptor 2 (HER2) Detection by Electrochemical Biosensors. Chemosensors 2016, 4, 23. [Google Scholar] [CrossRef]
- Nemeir, I.A.; Mouawad, L.; Saab, J.; Hleihel, W.; Errachid, A.; Zine, N. Electrochemical Impedance Spectroscopy Characterization of Label-Free Biosensors for the Detection of HER2 in Saliva. Proceedings 2020, 60, 17. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C.; Resende, M.A.C.; Ferreira, L.F. Disposable Voltammetric Immunosensor for Determination and Quantification of Biomarker CA 15-3 in Biological Specimens. Analytica 2024, 5, 74–89. [Google Scholar] [CrossRef]
- Thongwattana, N.; Phasuksom, K.; Ariyasajjamongkol, N.; Parinyanitikul, N.; Sirivat, A. Detection of Cancer Biomarker CA15-3 in Serums by Label-Free Immunosensor Based on Multiwall-Carbon Nanotube/Doped-Poly(2-Chloroaniline). Microchem. J. 2024, 206, 111389. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C.; Ferreira, L.F. Disposable Electropolymerized Molecularly Imprinted Electrochemical Sensor for Determination of Breast Cancer Biomarker CA 15-3 in Human Serum Samples. Talanta 2023, 252, 123819. [Google Scholar] [CrossRef]
- de Oliveira, R.A.G.; Materon, E.M.; Melendez, M.E.; Carvalho, A.L.; Faria, R.C. Disposable Microfluidic Immunoarray Device for Sensitive Breast Cancer Biomarker Detection. ACS Appl. Mater. Interfaces 2017, 9, 27433–27440. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.S.; Moreira, F.T.C.; Fernandes, R.; Sales, M.G.F. Sensing CA 15-3 in Point-of-Care by Electropolymerizing O-Phenylenediamine (oPDA) on Au-Screen Printed Electrodes. PLoS ONE 2018, 13, e0196656. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Pereira, C.M.; Silva, A.F.; Sales, M.G.F. Disposable Electrochemical Detection of Breast Cancer Tumour Marker CA 15-3 Using Poly(Toluidine Blue) as Imprinted Polymer Receptor. Biosens. Bioelectron. 2018, 109, 246–254. [Google Scholar] [CrossRef]
- Oliveira, D.; Barcelay, Y.R.; Moreira, F.T.C. An Electrochemically Synthesized Molecularly Imprinted Polymer for Highly Selective Detection of Breast Cancer Biomarker CA 15-3: A Promising Point-of-Care Biosensor. RSC Adv. 2024, 14, 15347–15357. [Google Scholar] [CrossRef]
- Khoshroo, A.; Mazloum-Ardakani, M.; Forat-Yazdi, M. Enhanced Performance of Label-Free Electrochemical Immunosensor for Carbohydrate Antigen 15-3 Based on Catalytic Activity of Cobalt Sulfide/Graphene Nanocomposite. Sens. Actuators B Chem. 2018, 255, 580–587. [Google Scholar] [CrossRef]
- Ma, C.; Li, W.; Kong, Q.; Yang, H.; Bian, Z.; Song, X.; Yu, J.; Yan, M. 3D Origami Electrochemical Immunodevice for Sensitive Point-of-Care Testing Based on Dual-Signal Amplification Strategy. Biosens. Bioelectron. 2015, 63, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-S.; Li, X.-J.; Ma, R.-N.; Shang, L.; Zhang, W.; Zhao, H.-Q.; Jia, L.-P.; Wang, H.-S. A Novel Dual-Signal Output Strategy for POCT of CEA Based on a Smartphone Electrochemical Aptasensing Platform. Microchim. Acta 2024, 191, 407. [Google Scholar] [CrossRef]
- Shamsuddin, S.H.; Gibson, T.D.; Tomlinson, D.C.; McPherson, M.J.; Jayne, D.G.; Millner, P.A. Reagentless Affimer- and Antibody-Based Impedimetric Biosensors for CEA-Detection Using a Novel Non-Conducting Polymer. Biosens. Bioelectron. 2021, 178, 113013. [Google Scholar] [CrossRef]
- Viswanathan, S.; Rani, C.; Vijay Anand, A.; Ho, J.A. Disposable Electrochemical Immunosensor for Carcinoembryonic Antigen Using Ferrocene Liposomes and MWCNT Screen-Printed Electrode. Biosens. Bioelectron. 2009, 24, 1984–1989. [Google Scholar] [CrossRef]
- Pavithra, M.; Muruganand, S.; Parthiban, C. Development of Novel Paper Based Electrochemical Immunosensor with Self-Made Gold Nanoparticle Ink and Quinone Derivate for Highly Sensitive Carcinoembryonic Antigen. Sens. Actuators B Chem. 2018, 257, 496–503. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Luo, J.; Liu, J.; Wang, L.; Fan, Y.; Yan, S.; Yang, Y.; Cai, X. A Novel Label-Free Microfluidic Paper-Based Immunosensor for Highly Sensitive Electrochemical Detection of Carcinoembryonic Antigen. Biosens. Bioelectron. 2016, 83, 319–326. [Google Scholar] [CrossRef]
- Feng, D.; Su, J.; He, G.; Xu, Y.; Wang, C.; Zheng, M.; Qian, Q.; Mi, X. Electrochemical DNA Sensor for Sensitive BRCA1 Detection Based on DNA Tetrahedral-Structured Probe and Poly-Adenine Mediated Gold Nanoparticles. Biosensors 2020, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Karadeniz, H.; Canavar, E.; Erdem, A. Electrochemical Sensing of Label Free DNA Hybridization Related to Breast Cancer 1 Gene at Disposable Sensor Platforms Modified with Single Walled Carbon Nanotubes. Electrochim. Acta 2012, 82, 137–142. [Google Scholar] [CrossRef]
- Raucci, A.; Cimmino, W.; Grosso, S.P.; Normanno, N.; Giordano, A.; Cinti, S. Paper-Based Screen-Printed Electrode to Detect miRNA-652 Associated to Triple-Negative Breast Cancer. Electrochim. Acta 2024, 487, 144205. [Google Scholar] [CrossRef]
- Pimalai, D.; Putnin, T.; Waiwinya, W.; Chotsuwan, C.; Aroonyadet, N.; Japrung, D. Development of Electrochemical Biosensors for Simultaneous Multiplex Detection of microRNA for Breast Cancer Screening. Microchim. Acta 2021, 188, 329. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, T.S.C.R.; Pereira, C.M.; Sales, M.G.F.; Noronha, J.P.; Costa-Rodrigues, J.; Silva, F.; Fernandes, M.H. Sarcosine Oxidase Composite Screen-Printed Electrode for Sarcosine Determination in Biological Samples. Anal. Chim. Acta 2014, 850, 26–32. [Google Scholar] [CrossRef]
- Hu, F.; Liu, T.; Pang, J.; Chu, Z.; Jin, W. Facile Preparation of Porous Co3O4 Nanocubes for Directly Screen-Printing an Ultrasensitive Glutamate Biosensor Microchip. Sens. Actuators B Chem. 2020, 306, 127587. [Google Scholar] [CrossRef]
- Li, M.; Li, D.-W.; Xiu, G.; Long, Y.-T. Applications of Screen-Printed Electrodes in Current Environmental Analysis. Curr. Opin. Electrochem. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Mincu, N.-B.; Lazar, V.; Stan, D.; Mihailescu, C.M.; Iosub, R.; Mateescu, A.L. Screen-Printed Electrodes (SPE) for In Vitro Diagnostic Purpose. Diagnostics 2020, 10, 517. [Google Scholar] [CrossRef]
- Rebelo, T.S.C.R.; Costa, R.; Brandão, A.T.S.C.; Silva, A.F.; Sales, M.G.F.; Pereira, C.M. Molecularly Imprinted Polymer SPE Sensor for Analysis of CA-125 on Serum. Anal. Chim. Acta 2019, 1082, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mpupa, A.; Selahle, S.K.; Mizaikoff, B.; Nomngongo, P.N. Recent Advances in Solid-Phase Extraction (SPE) Based on Molecularly Imprinted Polymers (MIPs) for Analysis of Hormones. Chemosensors 2021, 9, 151. [Google Scholar] [CrossRef]


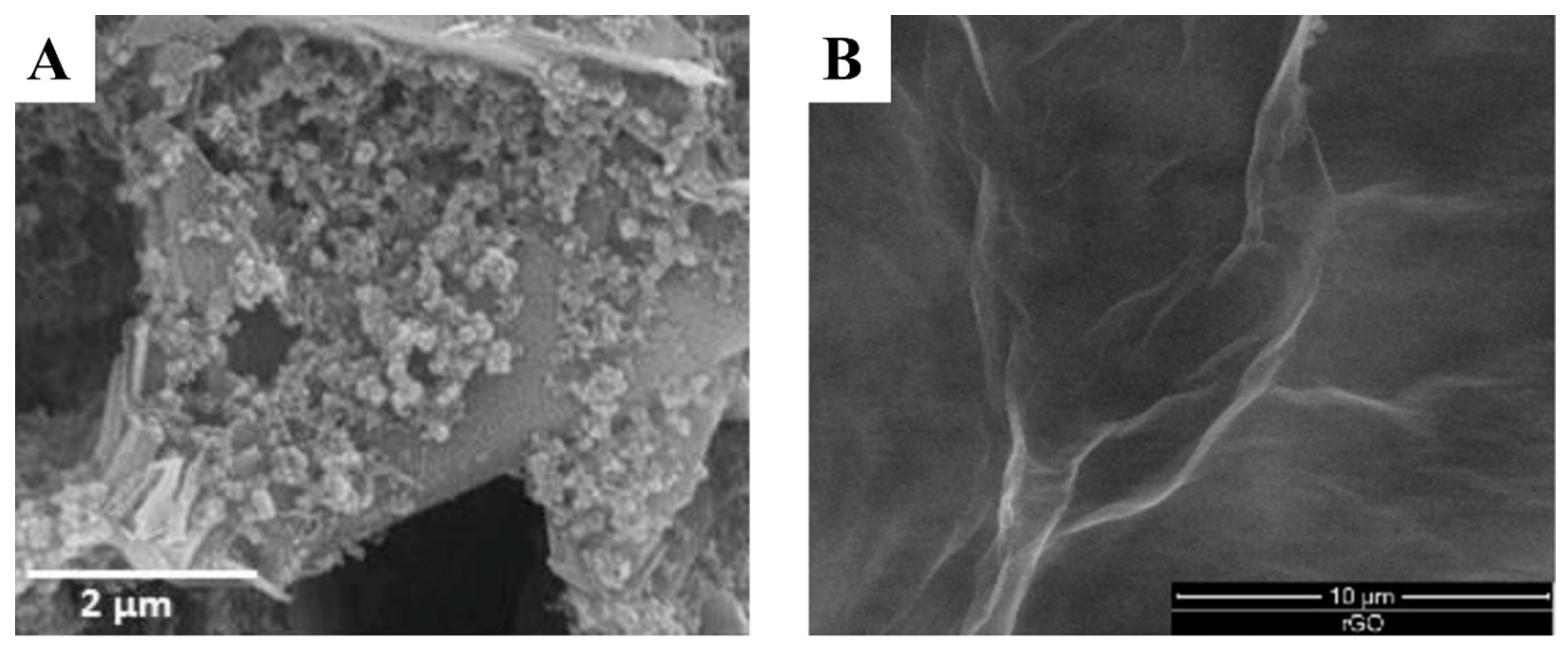

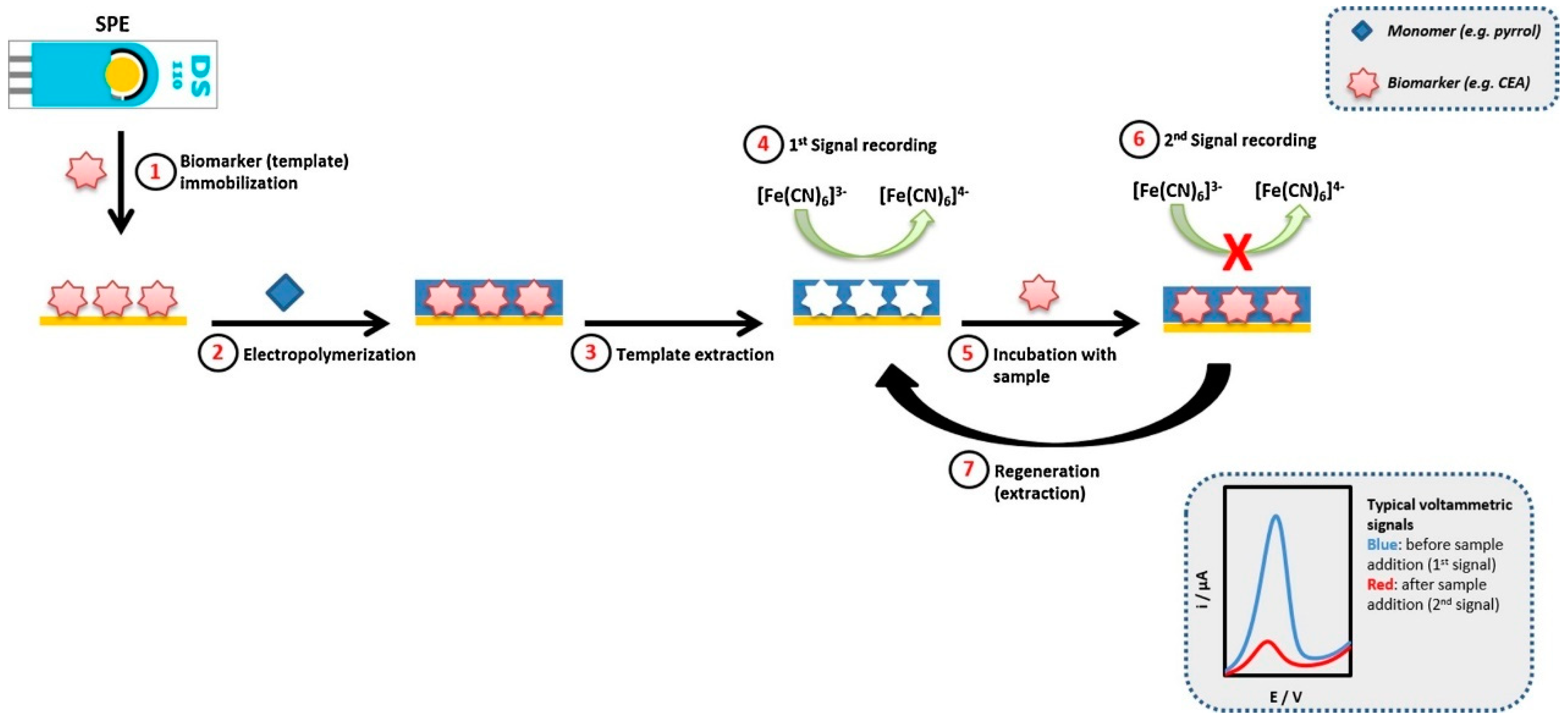

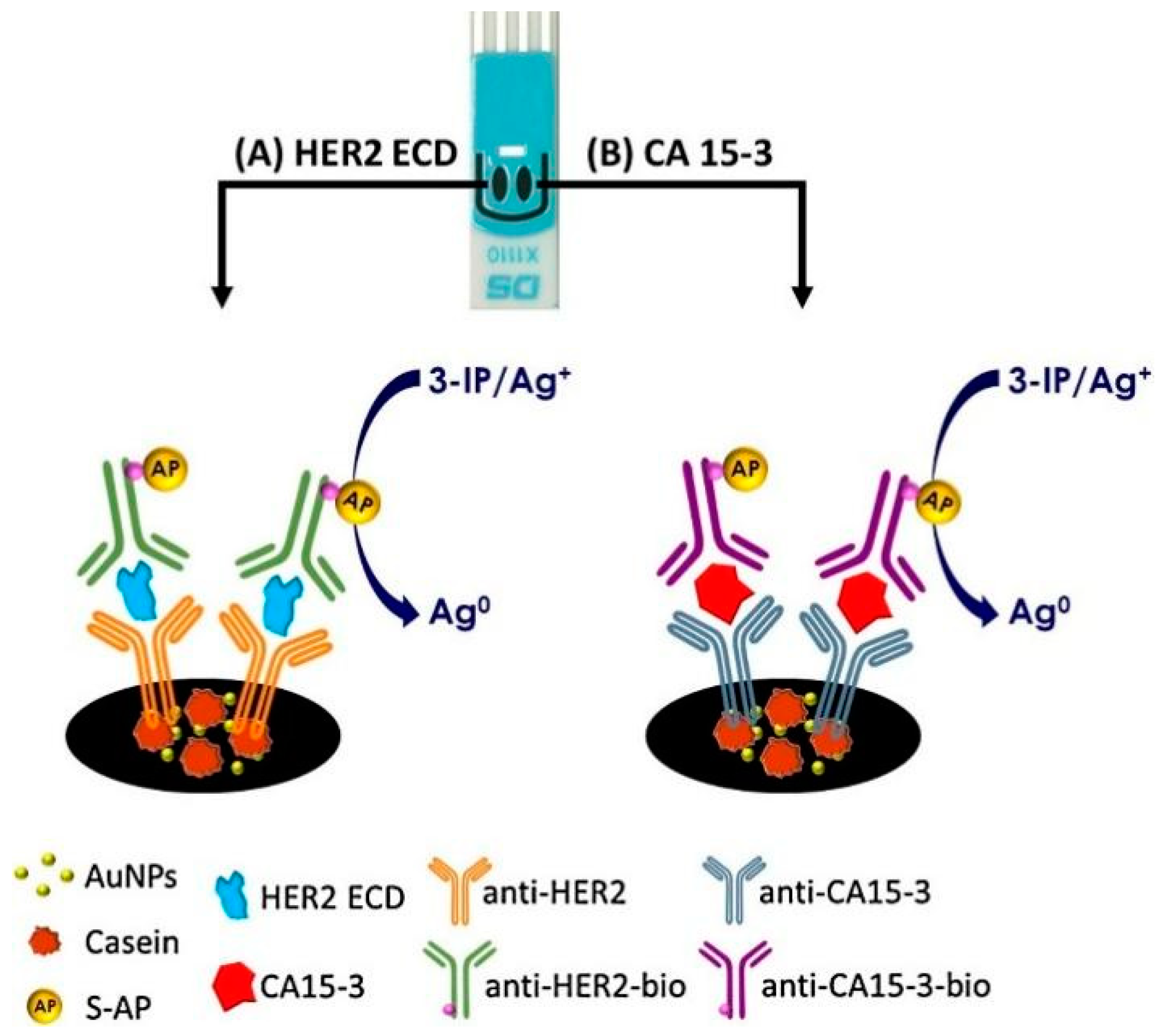
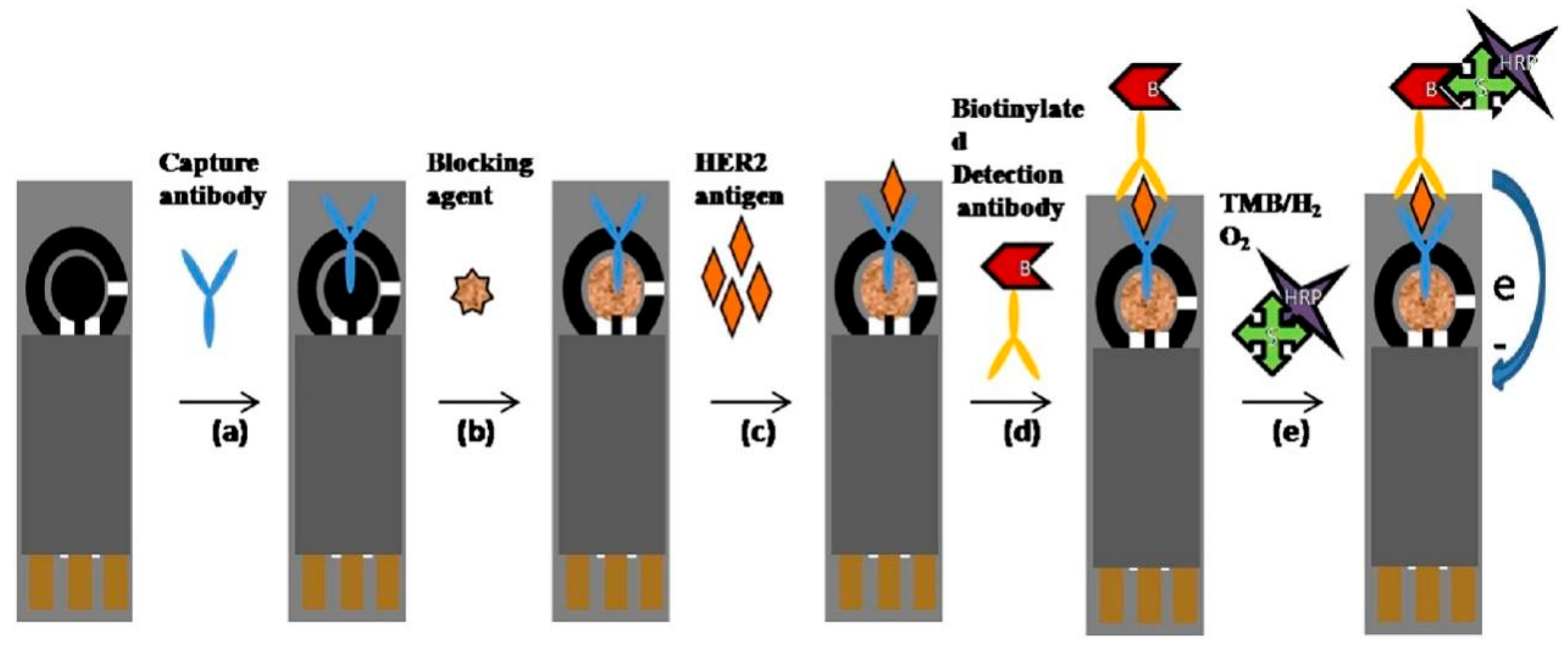

| Biomarker Name | Size | Utility | Level in Serum (Normal/Pathological) |
|---|---|---|---|
| BRCA1 | 5592 nt | Diagnostic for BC | N/A (genetic test) |
| BRCA2 | 11,385 nt | Diagnostic for BC | N/A (genetic test) |
| HER2 | 2200 nt | Diagnostic and prognostic for BC | <15 ng/mL/≥15 ng/mL [5] |
| CA15-3 | 300–450 kDa | Prognostic for BC | <30 U/mL/>30 U/mL [6] |
| CEA | 180–200 kDa | Important for BC treatment strategy | <5 ng/mL/>5 ng/mL [7] |
| CA125 | 200–2000 kDa | Prognostic for BC | <35 U/mL/>35 U/mL [8] |
| MUC1 | 2000 nt | Diagnostic and prognostic for BC | <30 U/mL/≥30 U/mL [9] |
| VEGF | 14,000 nt | Important for BC treatment strategy | <291 pg/mL/≥321.4 pg/mL [10] |
| CTC | N/A | Prognostic and important in therapy monitoring of BC | 0–1 CTC/7.5 mL/>2 CTC/7.5 mL [11] |
| miRNA-21 | 20–24 nt | Diagnostic for BC | N/A (expression levels compared to controls) |
| miRNA-16 | 20–24 nt | Diagnostic for BC | N/A (expression levels compared to controls) |
| miRNA-155 | 21–24 nt | Diagnostic for BC | N/A (expression levels compared to controls) |
| miRNA-652 | 22 nt | Diagnostic for BC | N/A (expression levels compared to controls) |
| Modification Components | Linear Detection Range | Limit of Detection | Total Assay Time | Automation/Manual Steps | Real Sample | Reference |
|---|---|---|---|---|---|---|
| MWCNTs+ MUC1 aptamers | 100 to 2000 ng/mL | 20 ng/mL | Not explicitly stated |
| Human blood serum | [45] |
| SPCE/CBAmN3/anti-HER2 | - | 0.005 ng/mL | Not explicitly stated |
| Human serum | [52] |
| DNA aptamer/MCH | 0.001 to 100 ng/mL | 0.179 ng/mL | Not explicitly stated |
| Human serum | [54] |
| bi-SPCE-AuNP-Ab | 9.8 to 50 ng/mL | 2.9 ng/mL | Not explicitly stated |
| - | [55] |
| MB-Ab1/HER2/Ab2-biotin-SA-AP/SPE | 0 to 15 ng/mL | 6 ng/mL | Not explicitly stated |
| Serum sample | [56] |
| AuNPs/Aptamer/MCH | 0.001 to 100 ng/mL | 0.001 ng/mL | Not explicitly stated |
| - | [24] |
| MPA/Cys/AuNP@HER2 aptamer | 0.01 to 15.0 ng/mL; 15.0 to 100.0 ng/mL | 1.52 ng/mL | Not explicitly stated |
| - | [58] |
| PLLF-RNA aptamer | 10 to 60 ng/mL. | 3 ng/mL | 4 min for detection |
| Human serum | [59] |
| ELISA-HER2 | 5–20 ng/mL; 20–200 ng/mL | 4 ng/mL | Not explicitly stated |
| Human serum | [57] |
| MPA/anti-HER-2/biotin-anti-HER-2/poly-HRP | - | 0.012 ng/mL | 15 min |
| Pooled human serum | [60] |
| AuNP-anti-HER2 | 15 to 100 ng/mL | 4.4 ng/mL | 2 h and 50 min |
| Human serum | [61] |
| MWCNT/AuNP | 7.5–50 ng/mL | 0.16 ng/mL | 2 h and 20 min |
| Human serum | [62] |
| Affibody/AuNPs | 0 to 40,000 ng/L | 6000 ng/L | Not explicitly stated |
| Human serum | [63] |
| AuNP-Ab-HRP | 0.25 to 50 ng/mL | 0.03 ng/mL | Not explicitly stated |
| Human serum | [64] |
| Strept-MB/Biot-Af | 0 to 20 ng/mL | 1.8 ng/mL | Not explicitly stated |
| Human serum | [65] |
| ABA/anti-HER2 | 0.005 to 0.04 ng/mL | 0.005 ng/mL | Not explicitly stated |
| Human saliva | [66] |
| Modification Components | Linear Detection Range | Limit of Detection | Total Assay Time | Automation/Manual Steps | Real Sample | Reference |
|---|---|---|---|---|---|---|
| Ab2-HRP/CA15-3/BSA/Ab1/MPA/Au-rGO | 1 × 10−9 to 1 × 104 U/mL | 8 × 10−10 U/mL | Not explicitly stated |
| Artificial saliva | [25] |
| HRP-MBs-Ab2/CA 15-3/Ab1-P(1,5DAN)/PPy NWs | 0.05 to 20 U/mL | 0.02 U/mL | Not explicitly stated |
| - | [50] |
| MIP | 5 to 50 U/mL | 1.5 U/mL | 15 min |
| Human serum | [51] |
| bi-SPCE-AuNP-Ab | 17 to 70 U/mL | 5.0 U/mL | Not explicitly stated |
| - | [55] |
| AuNP/Ab | 2 to 16 U/mL | 0.56 U/mL | 1 h |
| Human saliva and serum | [67] |
| BSA/Ab/MWCNT-dP2ClAn | 5 to 100 U/mL | 0.66 U/mL | Not explicitly stated |
| Human serum | [68] |
| CNE/AuNP/MIP | 5 to 35 U/mL | 1.16 U/mL | Not explicitly stated |
| Human serum | [69] |
| C-PDDA-AuNPs-Ab1-BSA | 0.01 to 1 U/mL | 0.006 U/mL | Not explicitly stated |
| Human serum | [70] |
| PoPDA/Au | 0.25 to 10.00 U/m | 0.05 U/mL | Less than 30 min |
| Human serum | [71] |
| MIP | 0.10 to 100 U/mL | 0.10 U/mL | Not explicitly stated |
| Human serum | [72] |
| AMPTMA-MIP | 0.001 to 100 U/mL | 0.000909 U/mL | Less than 20 min |
| Human serum | [73] |
| CoS2-GR-AuNPs | 0.1 to 150 U/mL | 0.03 U/mL | Not explicitly stated |
| Human serum | [74] |
| Modification Components | Linear Detection Range | Limit of Detection | Total Assay Time | Automation/Manual Steps | Real Sample | Reference |
|---|---|---|---|---|---|---|
| POct-Ab-CEA | 1.5 × 10−5 to 1.5 ng/mL | 1.36 × 10−4 ng/mL | Not explicitly stated |
| human serum | [77] |
| POct-Aff-CEA | 1.5 × 10−5 to 1.5 × 10−3 ng/mL | 1.76 × 10−4 ng/mL | Not explicitly stated | Same as above | Human serum | [77] |
| Ab1/AuNRs-PWE/Au/BSA-metal ion-Ab2 | 1 × 10−4 to 50 ng/mL | 8 × 10−5 ng/mL | Not explicitly stated |
| Human serum | [75] |
| HRP-Ab2/CEA/Ab1/AuNPs/rGO | 0.5 to 25 ng/mL; 250 to 2000 ng/mL | 0.28 ng/mL | Not explicitly stated |
| - | [46] |
| HRP-Ab2/CEA/Ab1/AgNPs-rGO | 50 to 500 ng/mL | 35 ng/mL | Not explicitly stated |
| - | [47] |
| GCE/Au NPs/Ab1/BSA/CEA/Ab2-Ag NPs@CS-Hemin/rGO | 2 × 10−5 to 200 ng/mL | 6.7 × 10−6 ng/mL | Not explicitly stated |
| Human serum | [49] |
| PdPt@PCN-224-P-dsDNA/Au | 0.001 to 100 ng/mL | 9.8 × 10−4 ng/mL | Not explicitly stated |
| Human serum | [76] |
| CEA-MWCNT-PEI | 0.005 to 500 ng/mL | 0.001 ng/mL | Not explicitly stated |
| Human serum | [78] |
| P-SPGE/R1/anti-CEA | 1.0 to 100.0 ng/mL | 0.33 ng/mL | Not explicitly stated |
| Human serum | [79] |
| NH2-G/Thi/AuNPs-SPWE/Anti-CEA | 0.05 to 500 ng/mL | 0.01 ng/mL | Not explicitly stated |
| Human serum | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Sun, Z.; Zhao, S.; Chen, F.; Shi, P.; Zhao, N.; Sun, K.; Ye, C.; Lin, C.; Fu, L. Screen-Printed Electrodes as Low-Cost Sensors for Breast Cancer Biomarker Detection. Sensors 2024, 24, 5679. https://doi.org/10.3390/s24175679
Shen Y, Sun Z, Zhao S, Chen F, Shi P, Zhao N, Sun K, Ye C, Lin C, Fu L. Screen-Printed Electrodes as Low-Cost Sensors for Breast Cancer Biomarker Detection. Sensors. 2024; 24(17):5679. https://doi.org/10.3390/s24175679
Chicago/Turabian StyleShen, Yin, Zhuang Sun, Shichao Zhao, Fei Chen, Peizheng Shi, Ningbin Zhao, Kaiqiang Sun, Chen Ye, Chengte Lin, and Li Fu. 2024. "Screen-Printed Electrodes as Low-Cost Sensors for Breast Cancer Biomarker Detection" Sensors 24, no. 17: 5679. https://doi.org/10.3390/s24175679






