Evaluation of Smartphone Technology on Spatiotemporal Gait in Older and Diseased Adult Populations
Abstract
:1. Introduction
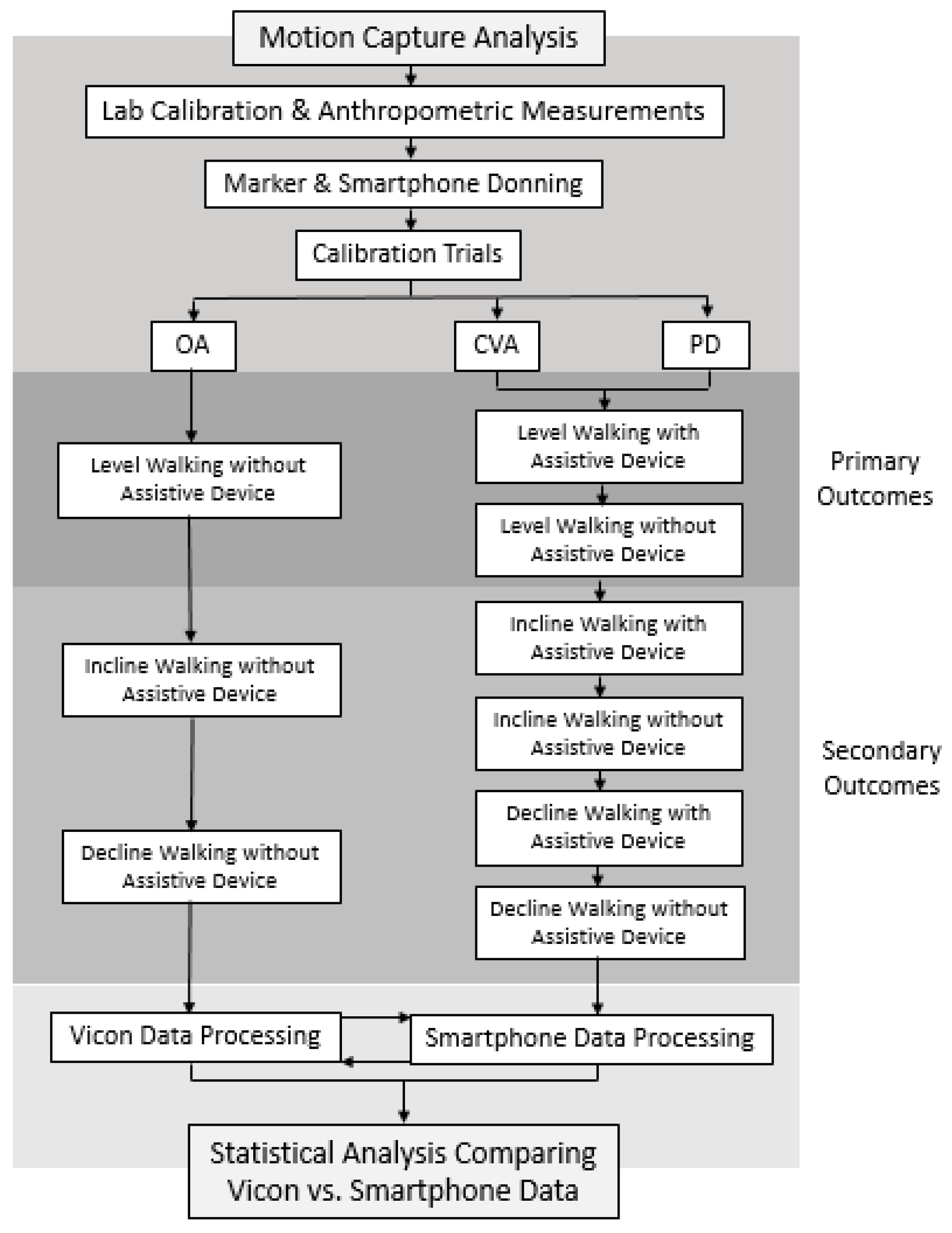
2. Methods
2.1. Participants
2.2. Experimental Procedures
- System Initialization: Gait analysis was performed using a 10-camera motion analysis system sampling at 200 Hz (Vicon Motion Systems; Oxford, UK). Kinetic data were obtained using a dual-belt instrumented treadmill (Bertec; Columbus, OH, USA) with sampling at 1000 Hz. Spatiotemporal data were recorded and synchronized using Nexus v2.15.0 software (Vicon, Oxford Metrics Ltd., Oxford, UK). The treadmill was equipped with a harness system to ensure the safety of each participant during the walking trials. Two lateral handrails that were not instrumented were also installed for further safety support. The motion-capture cameras and the instrumented treadmill were initialized as recommended by the manufacturer [25]. The initialization protocol consisted of calibration of the cameras within the capture volume, leveling the treadmill, setting the volume origin and zeroing the force plates of the treadmill [25].
- Participant Preparation: Each participant was fitted with compressive clothing, and retroreflective markers were affixed to bony landmarks throughout the pelvis and bilateral lower limbs, including the iliac crests, anterior/posterior superior iliac spine, greater trochanters, lateral femoral condyles, lateral malleoli, head of the 5th metatarsals and upper and lower aspects of the heels (Figure 2). Two non-rigid clusters with 4 non-collinear markers were placed at the lateral side of each thigh and shank segment. A single research physical therapist affixed all markers to each participant in the study. This was implemented to reduce the risk of marker placement error across the cohort. The modified Plug-In-Gait marker set (Vicon, Oxford Metrics Ltd., Oxford, UK) defined 1 HAT segment (combined head, arms and trunk), 1 pelvis segment, 2 thigh segments, 2 shank segments and 2 foot segments. Marker locations were used for attributing coordinate systems for each segment and were positioned as previously described [15]. Two smartphones (iPhone SE, Apple Inc., Cupertino, CA, USA) were also affixed to the right and left anterolateral thighs of each participant. The OneStep smartphone application was downloaded and activated on each device prior to formal data collection. The OneStep smartphone application uses data collected by the smartphone’s built-in sensors to measure gait parameters. Acceleration and angular data were collected by the smartphone’s sensors at a sampling rate of 100 Hz, and those data were analyzed by proprietary algorithms to measure the spatiotemporal variables for each stride. An upper-body harness was securely fastened to the participants. The participants were then directed onto the treadmill, and the harness was connected to an overhead support system.
- Calibration Trials: Two calibration trials were collected with each participant on the treadmill. Static Calibration: The participants were asked to stand stationary for five seconds with legs shoulder-width apart and arms out in front with elbows slightly bent [26]. Static calibration markers were removed after static calibration was completed. Joint Center Calibration: The participants were asked to swing each leg, individually, in a clock pattern from twelve to six or six to twelve, depending on the side. Then, the participants were asked to flex and extend each knee, individually, to the full available range of motion 5 times. Lastly, the participants were asked to circumduct each ankle, individually, within the full available range of motion 5 times [26].
- Walking Trials: The participants walked at a self-selected pace on the instrumented treadmill within three environments: (1) 0-degree level slope, (2) 5-degree uphill slope and (3) 5-degree downhill slope. The self-selected level gait speed was acquired using the 5 m walk test [27]. Participants were instructed to walk at a normal speed over the course of a standard 10 m measured walkway and two marked areas 5 m apart. Three separate trials of the 5 m walk test were conducted and their average was used for the self-selected level gait speed trials during formal data collection. The 5-degree slope angle was selected to replicate common adults’ experience during activities of daily living and is an environment that could be safely tested, while requiring greater mechanical demand to the lower limbs compared to level walking [28]. All participants completed a 1–2 min warm-up period during each gait environment before formal testing. Once participants confirmed they felt comfortable with the gait environment, they were instructed to walk as naturally as possible as data was collected. Participants walked continuously at a self-selected speed for 2–3 min. Participants were then provided a 5 to 10 min rest period prior to beginning the next gait environment to minimize the risk of fatigue. The OA participants performed all gait environments without a single-point cane. The PD and CVA participants performed all gait environments with and without a single-point cane. Rating of perceived exertion and numeric pain rating scale scores were recorded following the completion of each session. Trials in which participants lost their balance were excluded. For each walking environment, participants were brought up to the desired self-selected gait speed and were provided 30 s to acclimate before formal data collection was conducted. The first 30 successful steps on each limb, following the acclimate period, were averaged and used for statistical analysis.
2.3. Data Processing
2.4. Spatiotemporal Outcomes
- Double-limb stance (%): The time that both feet are in contact with the ground simultaneously, summed as the time elapsed during two periods of double-limb support in the gait cycle and calculated as a percentage of the gait cycle.
- Single-limb stance (%): The time only one foot was in contact with the ground, summed as the time elapsed between initial contact and toe off on the same foot and calculated as a percentage of the gait cycle.
- Swing (%): The time only one foot was off the ground, summed as the time elapsed between the last contact of the current toe off to initial contact of the next heel strike of the same foot and calculated as a percentage of the gait cycle.
- Step length (m): Anterior–posterior distance from the heel of one footprint to the heel of the opposite footprint.
- Stride length (m): Anterior–posterior distance between heels of two consecutive footprints of the same foot (left to left, right to right); two steps (e.g., a right step followed by a left step) comprise one stride or one gait cycle.
- Cadence (steps/min): Number of steps per minute, sometimes referred to as step rate.
- Gait speed (m/s): Calculated by dividing the distance walked by the ambulation time.
2.5. Statistical Analysis
3. Results
3.1. Participants
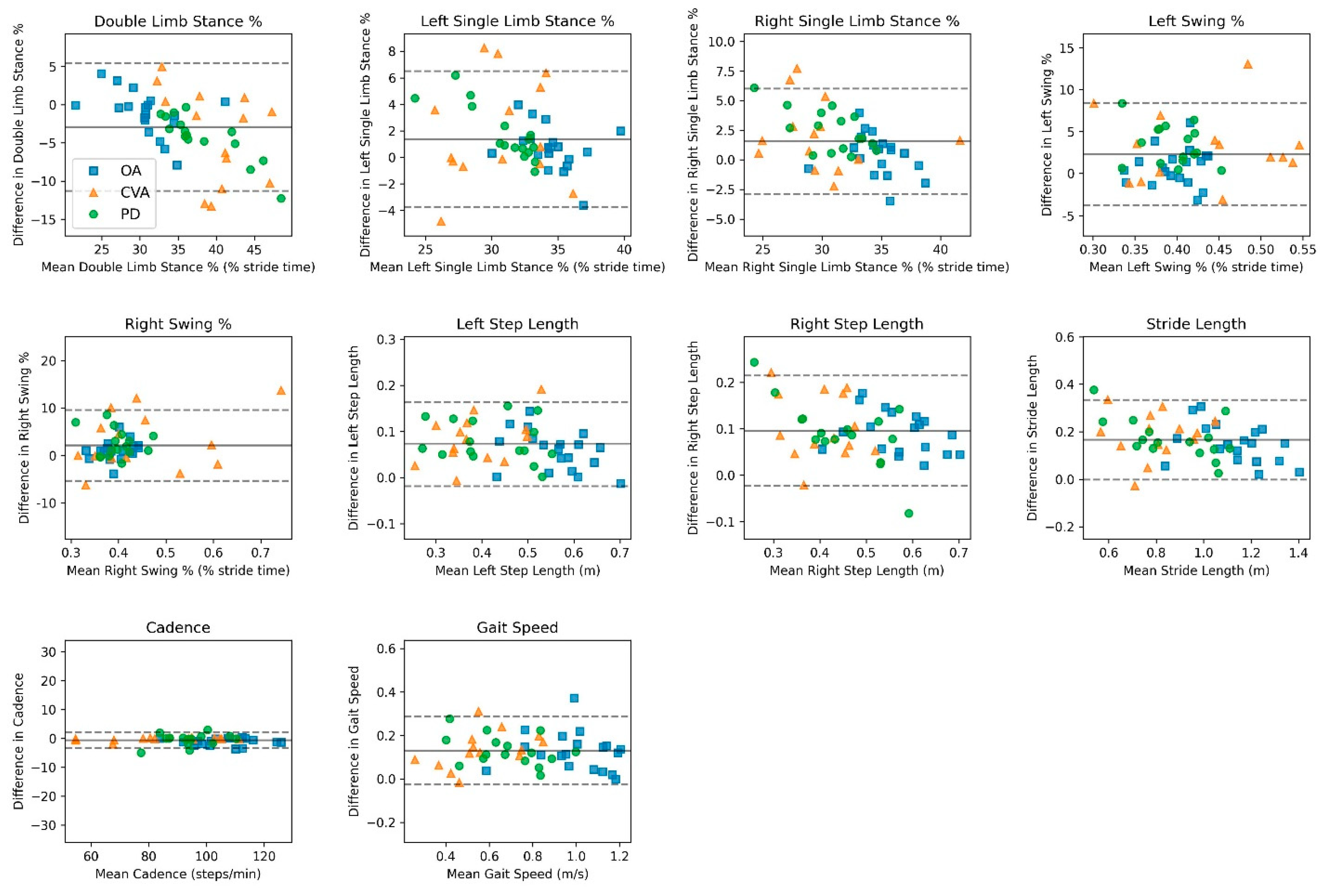
3.2. Validity
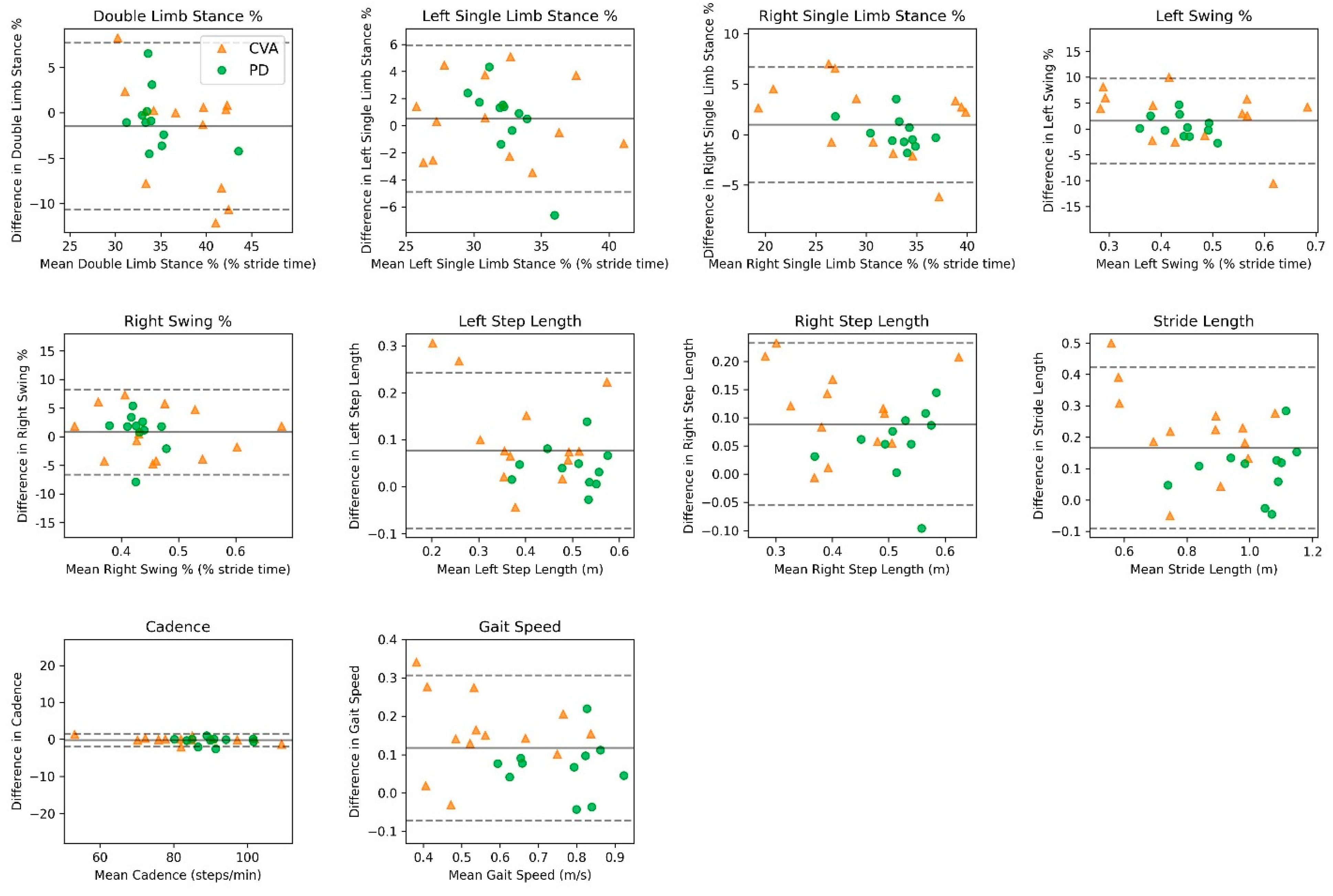
3.3. Reliability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Buekers, J.; Delgado-Ortiz, L.; Megaritis, D.; Polhemus, A.; Breuls, S.; Buttery, S.C.; Chynkiamis, N.; Demeyer, H.; Gimeno-Santos, E.; Hume, E.; et al. Gait differences between COPD and healthy controls: Systematic review and meta-analysis. Eur. Respir. Rev. 2024, 33, 230253. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.H.F.; Ferreira-Peruzzo, S.A.; Ribas, D.I.R.; Franklin, G.L.; Teive, H.A.G. Imbalance and gait impairment in Parkinson’s disease: Discussing postural instability and ataxia. Neurol. Sci. 2024, 45, 1377–1388. [Google Scholar] [CrossRef]
- Lee, P.Y.; Chen, C.H.; Tseng, H.Y.; Lin, S.I. Ipsilateral lower limb motor performance and its association with gait after stroke. PLoS ONE 2024, 19, e0297074. [Google Scholar] [CrossRef]
- Dai, S.; Piscicelli, C.; Clarac, E.; Baciu, M.; Hommel, M.; Pérennou, D. Balance, Lateropulsion, and Gait Disorders in Subacute Stroke. Neurology 2021, 96, e2147–e2159. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Talarska, D.; Strugała, M.; Szewczyczak, M.; Tobis, S.; Michalak, M.; Wróblewska, I.; Wieczorowska-Tobis, K. Is independence of older adults safe considering the risk of falls? BMC Geriatr. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, J.S.; Okorokov, D.Y.; Okita, N.; Stern, K.A. Walking strategies during the transition between level and hill surfaces. J. Appl. Biomech. 2011, 27, 355–361. [Google Scholar] [CrossRef]
- Sheehan, R.C.; Gottschall, J.S. Older Adult Hill Transition Strategies are Determined by Task Demands and Cautious Gait Patterns. J. Appl. Biomech. 2015, 31, 133–141. [Google Scholar] [CrossRef]
- Moreland, B.; Kakara, R.; Henry, A. Trends in Nonfatal Falls and Fall-Related Injuries Among Adults Aged ≥65 Years—United States, 2012–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 875–881. [Google Scholar] [CrossRef]
- Florence, C.S.; Bergen, G.; Atherly, A.; Burns, E.; Stevens, J.; Drake, C. Medical Costs of Fatal and Nonfatal Falls in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 693–698. [Google Scholar] [CrossRef]
- Winter, D.A. Overall principle of lower limb support during stance phase of gait. J. Biomech. 1980, 13, 923–927. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Perring, J.; Raj, S.M.; Maharaj, M.; Yoong, N.K.M.; Sy, L.W.; Fonseka, R.D.; Natarajan, P.; Choy, W.J. Gait metrics analysis utilizing single-point inertial measurement units: A systematic review. Mhealth 2022, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef] [PubMed]
- Matey-Sanz, M.; Casteleyn, S.; Granell, C. Dataset of inertial measurements of smartphones and smartwatches for human activity recognition. Data Brief 2023, 51, 109809. [Google Scholar] [CrossRef]
- Christensen, J.C.; Stanley, E.C.; Oro, E.G.; Carlson, H.B.; Naveh, Y.Y.; Shalita, R.; Teitz, L.S. The validity and reliability of the OneStep smartphone application under various gait conditions in healthy adults with feasibility in clinical practice. J. Orthop. Surg. Res. 2022, 17, 417. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.R.; Chiu, Y.L.; Chiang, S.L.; Chen, H.Y.; Sung, W.H. Development of a Smartphone-Based Balance Assessment System for Subjects with Stroke. Sensors 2019, 20, 88. [Google Scholar] [CrossRef]
- Manor, B.; Yu, W.; Zhu, H.; Harrison, R.; Lo, O.Y.; Lipsitz, L.; Travison, T.; Pascual-Leone, A.; Zhou, J. Smartphone App-Based Assessment of Gait During Normal and Dual-Task Walking: Demonstration of Validity and Reliability. JMIR mHealth uHealth 2018, 6, e36. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.S.; Salazar, A.P.; Hennig, E.M.; Spessato, B.C.; Domingo, A.; Pagnussat, A.S. Can We Rely on Mobile Devices and Other Gadgets to Assess the Postural Balance of Healthy Individuals? A Systematic Review. Sensors 2019, 19, 2972. [Google Scholar] [CrossRef]
- Silsupadol, P.; Prupetkaew, P.; Kamnardsiri, T.; Lugade, V. Smartphone-Based Assessment of Gait During Straight Walking, Turning, and Walking Speed Modulation in Laboratory and Free-Living Environments. IEEE J. Biomed. Health Inform. 2020, 24, 1188–1195. [Google Scholar] [CrossRef]
- Silsupadol, P.; Teja, K.; Lugade, V. Reliability and validity of a smartphone-based assessment of gait parameters across walking speed and smartphone locations: Body, bag, belt, hand, and pocket. Gait Posture 2017, 58, 516–522. [Google Scholar] [CrossRef]
- Zhong, R.; Rau, P.P. A Mobile Phone-Based Gait Assessment App for the Elderly: Development and Evaluation. JMIR mHealth uHealth 2020, 8, e14453. [Google Scholar] [CrossRef]
- Bristol, D.R. Sample sizes for constructing confidence intervals and testing hypotheses. Stat. Med. 1989, 8, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.-C.; Shao, J.; Wang, H. Sample Size Calculations in Clinical Research, 2nd ed.; Chapman & Hall/CRC: New York, NY, USA, 2008. [Google Scholar]
- Bonett, D.G. Sample size requirements for estimating intraclass correlations with desired precision. Stat. Med. 2002, 21, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Vicon Motion Systems Limited. Vicon Nexus User Guide; Vicon Documentation; Vicon Motion Systems Limited: Centennial, CO, USA, 2020. [Google Scholar]
- Vicon Motion Systems Limited. Creating Labeling Skeleton Templates (VSTs); Vicon Documentation; Vicon Motion Systems Limited: Centennial, CO, USA, 2016. [Google Scholar]
- Wilson, C.M.; Kostsuca, S.R.; Boura, J.A. Utilization of a 5-Meter Walk Test in Evaluating Self-selected Gait Speed during Preoperative Screening of Patients Scheduled for Cardiac Surgery. Cardiopulm. Phys. Ther. J. 2013, 24, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Leu, T.H.; Li, J.D.; Wang, T.M.; Ho, W.P.; Lu, T.W. Influence of inclination angles on intra- and inter-limb load-sharing during uphill walking. Gait Posture 2014, 39, 29–34. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological, 2nd ed.; Waterloo Biomechanics Press: Waterloo, ON, Canada, 1991. [Google Scholar]
- Hollman, J.H.; McDade, E.M.; Petersen, R.C. Normative spatiotemporal gait parameters in older adults. Gait Posture 2011, 34, 111–118. [Google Scholar] [CrossRef]
- Altman, D.; Bland, J. Measurement in medicine: The analysis of method comparison studies. Statistician 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Nelson, M. The validation of dietary assessment. In Design Concepts in Nutritional Epidemiology, 2nd ed.; Margetts, B., Nelson, M., Eds.; Oxford University Press: Oxford, UK, 1997; p. 241. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Vahtrik, D.; Gapeyeva, H.; Ereline, J.; Pääsuke, M. Relationship between leg extensor muscle strength and knee joint loading during gait before and after total knee arthroplasty. Knee 2014, 21, 216–220. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Rochester, L. Validation of an Accelerometer to Quantify a Comprehensive Battery of Gait Characteristics in Healthy Older Adults and Parkinson’s Disease: Toward Clinical and at Home Use. IEEE J. Biomed. Health Inform. 2016, 20, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Stuart, S.; McBarron, G.; Fino, P.C.; Mancini, M.; Curtze, C. Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol. Meas. 2019, 40, 095003. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Hübsch, T.; Brandt, A.U.; Pfueller, C.; Zange, L.; Seidel, A.; Kühn, A.A.; Paul, F.; Minnerop, M.; Doss, S. Accuracy and repeatability of two methods of gait analysis—GaitRite™ und Mobility Lab™—In subjects with cerebellar ataxia. Gait Posture 2016, 48, 194–201. [Google Scholar] [CrossRef]
- Hsu, A.L.; Tang, P.F.; Jan, M.H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch. Phys. Med. Rehabil. 2003, 84, 1185–1193. [Google Scholar] [CrossRef]
- Lin, P.Y.; Yang, Y.R.; Cheng, S.J.; Wang, R.Y. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch. Phys. Med. Rehabil. 2006, 87, 562–568. [Google Scholar] [CrossRef]
- Wutzke, C.J.; Faldowski, R.A.; Lewek, M.D. Individuals Poststroke Do Not Perceive Their Spatiotemporal Gait Asymmetries as Abnormal. Phys. Ther. 2015, 95, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Song, C.H.; Lee, K.J.; Jung, S.W.; Shin, D.C.; Shin, S.H. Concurrent Validity and Test-retest Reliability of the OPTOGait Photoelectric Cell System for the Assessment of Spatio-temporal Parameters of the Gait of Young Adults. J. Phys. Ther. Sci. 2014, 26, 81–85. [Google Scholar] [CrossRef]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef]
- Bilney, B.; Morris, M.; Webster, K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture 2003, 17, 68–74. [Google Scholar] [CrossRef]
- Lee, M.; Song, C.; Lee, K.; Shin, D.; Shin, S. Agreement between the spatio-temporal gait parameters from treadmill-based photoelectric cell and the instrumented treadmill system in healthy young adults and stroke patients. Med. Sci. Monit. 2014, 20, 1210–1219. [Google Scholar] [CrossRef]
- Lienhard, K.; Schneider, D.; Maffiuletti, N.A. Validity of the Optogait photoelectric system for the assessment of spatiotemporal gait parameters. Med. Eng. Phys. 2013, 35, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Robles-García, V.; Corral-Bergantiños, Y.; Espinosa, N.; Jácome, M.A.; García-Sancho, C.; Cudeiro, J.; Arias, P. Spatiotemporal Gait Patterns During Overt and Covert Evaluation in Patients With Parkinson’s Disease and Healthy Subjects: Is There a Hawthorne Effect? J. Appl. Biomech. 2015, 31, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Zeni, J.A., Jr.; Higginson, J.S. Gait parameters and stride-to-stride variability during familiarization to walking on a split-belt treadmill. Clin. Biomech. 2010, 25, 383–386. [Google Scholar] [CrossRef] [PubMed]

| Variable | OA (n = 20) | PD (n = 15) | CVA (n = 15) |
|---|---|---|---|
| Age, y | 74.2 (4.2) | 72.6 (9.1) | 52.3 (26.7) |
| Sex, % male | 60 | 60 | 87 |
| Weight, kg | 75.9 (16.5) | 84.1 (11.7) | 91.5 (17.5) |
| Height, m | 1.71 (0.13) | 1.71 (0.14) | 1.77 (0.08) |
| BMI, kg/m2 | 25.8 (4.4) | 28.9 (4.8) | 28.9 (4.8) |
| PROMIS Global Health—Physical | 55.6 (4.5) | 42.0 (7.1) | 44.2 (8.2) |
| PROMIS Global Health—Mental | 56.3 (6.3) | 48.7 (9.3) | 49.6 (10.8) |
| UCLA Activity Scale | 6 (3–9) | 4.8 (2–7) | 4.2 (1–8) |
| CCI | 3.5 (1.1) | 2.9 (1.0) | 3.5 (1.5) |
| Hoehn and Yahr | -- | 2.5 (0.7) | -- |
| Population/Variable | Vicon Mean (SD) | OneStep Mean (SD) | Mean Bias (95% CI) | LoA (95% CI) | r ꞙ | ICC ¥ (95% CI) |
|---|---|---|---|---|---|---|
| Older Adults | ||||||
| Double-Limb Stance, % | 31.3 | 30.3 | −1.03 | ±5.80 | 0.80 | 0.77 |
| (4.90) | (3.65) | (−2.39, 0.32) | (3.44, 8.15) | (0.51, 0.90) | ||
| Left Single-Limb Stance, % | 34.1 | 34.7 | 0.60 | ±3.41 | 0.72 | 0.71 |
| (2.47) | (2.01) | (−0.20, 1.39) | (2.03, 4.80) | (0.40, 0.87) | ||
| Right Single-Limb Stance, % | 34.5 | 34.9 | 0.43 | ±3.45 | 0.73 | 0.71 |
| (2.51) | (2.01) | (−0.38, 1.23) | (2.05, 4.85) | (0.40, 0.87) | ||
| Left Swing, % | 34.5 | 34.9 | 0.41 | ±3.48 | 0.72 | 0.71 |
| (2.52) | (2.01) | (−0.40, 1.22) | (2.07, 4.89) | (0.40, 0.87) | ||
| Right Swing, % | 34.1 | 34.7 | 0.61 | ±3.39 | 0.72 | 0.71 |
| (2.45) | (2.01) | (−0.18, 1.40) | (2.02, 4.76) | (0.40, 0.87) | ||
| Left Step Length, m | 0.53 | 0.58 | 0.06 | ±0.09 | 0.85 | 0.83 |
| (0.08) | (0.06) | (0.04, 0.08) | (0.05, 0.12) | (0.63, 0.93) | ||
| Right Step Length, m | 0.52 | 0.61 | 0.09 | ±0.09 | 0.86 | 0.85 |
| (0.08) | (0.07) | (0.07, 0.11) | (0.05, 0.12) | (0.66, 0.93) | ||
| Stride Length, m | 1.05 | 1.2 | 0.15 | ±0.16 | 0.88 | 0.86 |
| (0.16) | (0.13) | (0.11, 0.18) | (0.09, 0.22) | (0.69, 0.94) | ||
| Cadence, steps/min | 105.8 | 104.8 | −0.97 | ±2.42 | 0.99 | 0.99 |
| (10.7) | (10.6) | (−1.54, −0.41) | (1.43, 3.40) | (0.99, 0.99) | ||
| Gait Speed, m/s | 0.93 | 1.05 | 0.12 | ±0.19 | 0.87 | 0.86 |
| (0.19) | (0.16) | (0.07, 0.16) | (0.11, 0.26) | (0.69, 0.94) | ||
| Parkinson’s Disease | ||||||
| Double-Limb Stance, % | 40.5 | 36.2 | −4.23 | ±6.32 | 0.95 | 0.81 |
| (6.4) | (3.7) | (−5.98, −2.47) | (3.29, 9.35) | (0.54, 0.93) | ||
| Left Single-Limb Stance, % | 30.1 | 31.8 | 1.69 | ±4.20 | 0.90 | 0.73 |
| (3.6) | (1.8) | (0.54, 2.86) | (2.19, 6.21) | (0.38, 0.90) | ||
| Right Single-Limb Stance, % | 29.3 | 31.9 | 2.53 | ±3.63 | 0.87 | 0.81 |
| (3.4) | (2.4) | (1.52, 3.53) | (1.89, 5.37) | (0.53, 0.93) | ||
| Left Swing, % | 29.3 | 31.9 | 2.52 | ±3.62 | 0.87 | 0.81 |
| (3.5) | (2.4) | (1.51, 3.52) | (1.88, 5.36) | (0.54, 0.93) | ||
| Right Swing, % | 30.1 | 31.8 | 1.71 | ±4.18 | 0.90 | 0.74 |
| (3.6) | (1.8) | (0.55, 2.86) | (2.18, 6.19) | (0.38, 0.90) | ||
| Left Step Length, m | 0.38 | 0.45 | 0.07 | ±0.09 | 0.91 | 0.91 |
| (0.10) | (0.09) | (0.05, 0.10) | (0.05, 0.13) | (0.75, 0.96) | ||
| Right Step Length, m | 0.38 | 0.48 | 0.10 | ±0.11 | 0.89 | 0.84 |
| (0.11) | (0.07) | (0.07, 0.13) | (0.06, 0.16) | (0.59, 0.94) | ||
| Stride Length, m | 0.77 | 0.94 | 0.18 | ±0.17 | 0.93 | 0.90 |
| (0.21) | (0.16) | (0.13, 0.22) | (0.09, 0.25) | (0.74, 0.96) | ||
| Cadence, steps/min | 94.0 | 93.6 | −0.42 | ±4.10 | 0.97 | 0.97 |
| (8.7) | (9.3) | (−1.55, 0.71) | (2.14, 6.07) | (0.92, 0.99) | ||
| Gait Speed, m/s | 0.60 | 0.74 | 0.14 | ±0.13 | 0.94 | 0.93 |
| (0.18) | (0.16) | (0.10, 0.17) | (0.07, 0.19) | (0.82, 0.97) | ||
| Cerebrovascular Accident | ||||||
| Double-Limb Stance, % | 30.4 | 30.5 | 0.15 | ±6.96 | 0.71 | 0.68 |
| (4.96) | (3.64) | (−1.47, 1.78) | (4.14, 9.78) | (0.35, 0.85) | ||
| Left Single-Limb Stance, % | 34.6 | 33.8 | −0.78 | ±3.36 | 0.72 | 0.67 |
| (2.43) | (1.66) | (−1.57, 0.01) | (2.00, 4.73) | (0.34, 0.85) | ||
| Right Single-Limb Stance, % | 34.9 | 35.5 | 0.64 | ±5.10 | 0.57 | 0.56 |
| (2.68) | (2.81) | (−0.55, 1.83) | (3.04, 7.17) | (0.18, 0.80) | ||
| Left Swing, % | 34.9 | 35.5 | 0.63 | ±5.11 | 0.56 | 0.56 |
| (2.68) | (2.81) | (−0.57, 1.82) | (3.04, 7.18) | (0.17, 0.80) | ||
| Right Swing, % | 34.6 | 33.8 | −0.77 | ±3.36 | 0.72 | 0.67 |
| (2.43) | (1.66) | (−1.56, 0.01) | (1.99, 4.72) | (0.33, 0.85) | ||
| Left Step Length, m | 0.48 | 0.55 | 0.06 | ±0.08 | 0.92 | 0.89 |
| (0.09) | (0.07) | (0.05, 0.08) | (0.05, 0.11) | (0.76, 0.95) | ||
| Right Step Length, m | 0.47 | 0.57 | 0.10 | ±0.09 | 0.88 | 0.88 |
| (0.09) | (0.09) | (0.08, 0.12) | (0.05, 0.13) | (0.72, 0.95) | ||
| Stride Length, m | 0.96 | 1.12 | 0.17 | ±0.14 | 0.92 | 0.92 |
| (0.18) | (0.16) | (0.13, 0.20) | (0.08, 0.20) | (0.81, 0.96) | ||
| Cadence, steps/min | 105.54 | 105.42 | −0.13 | ±1.89 | 0.99 | 0.99 |
| (11.5) | (11.5) | (−0.57, 0.32) | (1.13, 2.67) | (0.99, 0.99) | ||
| Gait Speed, m/s | 0.86 | 0.98 | 0.13 | ±0.15 | 0.92 | 0.91 |
| (0.17) | (0.16) | (0.09, 0.16) | (0.09, 0.21) | (0.78, 0.96) | ||
| Population/Variable | Vicon Mean (SD) | OneStep Mean (SD) | Mean Bias (95% CI) | LoA (95% CI) | r ꞙ | ICC ¥ (95% CI) |
|---|---|---|---|---|---|---|
| Parkinson’s Disease | ||||||
| Double-Limb Stance, % | 35.1 | 34.3 | −0.79 | ±6.83 | 0.60 | 0.57 |
| (4.2) | (3.0) | (−3.24, 1.65) | (2.67, 11.07) | (−0.04, 0.87) | ||
| Left Single-Limb Stance, % | 32.1 | 32.5 | 0.44 | ±5.85 | 0.35 | 0.22 |
| (3.1) | (1.1) | (−1.65, 2.52) | (2.23, 9.48) | (−0.43, 0.72) | ||
| Right Single-Limb Stance, % | 32.7 | 33.1 | 0.36 | ±3.06 | 0.86 | 0.84 |
| (3.0) | (2.4) | (−0.74, 1.45) | (1.16, 4.95) | (0.50, 0.96) | ||
| Left Swing, % | 32.7 | 33.1 | 0.36 | ±3.06 | 0.86 | 0.84 |
| (3.0) | (2.4) | (−0.73, 1.46) | (1.15, 4.96) | (0.50, 0.96) | ||
| Right Swing, % | 32.1 | 32.5 | 0.43 | ±5.83 | 0.35 | 0.22 |
| (3.1) | (1.1) | (−1.65, 2.51) | (2.22, 9.45) | (−0.43, 0.72) | ||
| Left Step Length, m | 0.47 | 0.51 | 0.04 | ±0.09 | 0.81 | 0.81 |
| (0.07) | (0.07) | (0.01, 0.07) | (0.04, 0.15) | (0.41, 0.95) | ||
| Right Step Length, m | 0.47 | 0.54 | 0.07 | ±0.08 | 0.88 | 0.82 |
| (0.05) | (0.07) | (0.04, 0.10) | (0.03, 0.13) | (0.44, 0.95) | ||
| Stride Length, m | 0.95 | 1.06 | 0.11 | ±0.16 | 0.84 | 0.83 |
| (0.12) | (0.15) | (0.05, 0.17) | (0.06, 0.26) | (0.46, 0.95) | ||
| Cadence, steps/min | 90.1 | 89.7 | −0.42 | ±2.08 | 0.98 | 0.98 |
| (7.0) | (7.1) | (−1.16, 0.33) | (0.79, 3.37) | (0.95, 0.99) | ||
| Gait Speed, m/s | 0.71 | 0.80 | 0.09 | ±0.14 | 0.83 | 0.83 |
| (0.12) | (0.12) | (0.03, 0.13) | (0.05, 0.23) | (0.47, 0.95) | ||
| Cerebrovascular Accident | ||||||
| Double-Limb Stance, % | 38.1 | 36.1 | −1.97 | ±11.2 | 0.50 | 0.44 |
| (6.4) | (3.8) | (−5.22, 1.27) | (5.69, 16.85) | (0.08, 0.78) | ||
| Left Single-Limb Stance, % | 31.3 | 31.8 | 0.56 | ±5.73 | 0.82 | 0.82 |
| (4.8) | (4.8) | (−1.09, 2.21) | (2.86, 8.60) | (0.53, 0.93) | ||
| Right Single-Limb Stance, % | 30.5 | 31.9 | 1.40 | ±7.37 | 0.87 | 0.85 |
| (7.6) | (6.1) | (−0.71, 3.53) | (3.74, 11.05) | (0.61, 0.95) | ||
| Left Swing, % | 30.4 | 31.9 | 1.47 | ±7.25 | 0.88 | 0.86 |
| (7.7) | (6.1) | (−0.62, 3.56) | (3.67, 10.88) | (0.63, 0.95) | ||
| Right Swing, % | 31.3 | 31.8 | 0.56 | ±5.71 | 0.82 | 0.82 |
| (4.8) | (4.8) | (−1.09, 2.20) | (2.86, 8.57) | (0.54, 0.94) | ||
| Left Step Length, m | 0.35 | 0.45 | 0.10 | ±0.20 | 0.68 | 0.64 |
| (0.13) | (0.09) | (0.05, 0.16) | (0.10, 0.30) | (0.20, 0.87) | ||
| Right Step Length, m | 0.37 | 0.47 | 0.10 | ±0.18 | 0.67 | 0.65 |
| (0.12) | (0.09) | (0.05, 0.15) | (0.09, 0.28) | (0.20, 0.87) | ||
| Stride Length, m | 0.73 | 0.93 | 0.20 | ±0.31 | 0.74 | 0.69 |
| (0.23) | (0.15) | (0.11, 0.29) | (0.16, 0.46) | (0.28, 0.89) | ||
| Cadence, steps/min | 84 | 83.9 | −0.09 | ±1.56 | 0.99 | 0.99 |
| (14.5) | (14.1) | (−0.55, 0.36) | (0.77, 2.34) | (0.99, 0.99) | ||
| Gait Speed, m/s | 0.49 | 0.65 | 0.16 | ±0.23 | 0.78 | 0.76 |
| (0.18) | (0.15) | (0.09, 0.22) | (0.12, 0.35) | (0.41, 0.91) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, C.; Stanley, E.C.; Deschamps-Prescott, C.; Burnap, S.; Hopkins, M.; Browning, B.; Christensen, J.C. Evaluation of Smartphone Technology on Spatiotemporal Gait in Older and Diseased Adult Populations. Sensors 2024, 24, 5839. https://doi.org/10.3390/s24175839
Contreras C, Stanley EC, Deschamps-Prescott C, Burnap S, Hopkins M, Browning B, Christensen JC. Evaluation of Smartphone Technology on Spatiotemporal Gait in Older and Diseased Adult Populations. Sensors. 2024; 24(17):5839. https://doi.org/10.3390/s24175839
Chicago/Turabian StyleContreras, Coby, Ethan C. Stanley, Chanc Deschamps-Prescott, Susan Burnap, Madison Hopkins, Bennett Browning, and Jesse C. Christensen. 2024. "Evaluation of Smartphone Technology on Spatiotemporal Gait in Older and Diseased Adult Populations" Sensors 24, no. 17: 5839. https://doi.org/10.3390/s24175839






