Differences in Trunk Acceleration-Derived Gait Indexes in Stroke Subjects with and without Stroke-Induced Immunosuppression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Gait Data Acquisition
2.2.1. Harmonic Ratio (HR) Calculation
2.2.2. Short-Term Largest Lyapunov Exponent (sLLE) Calculation
2.2.3. Log-Dimensionless Jerk of Accelerations (LDLJ) Calculation
2.2.4. Symmetry Indexes Calculation
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Modification of Clinical Scales after Rehabilitation (T1)
3.3. Baseline (T0) Trunk Acceleration-Derived Gait Indexes
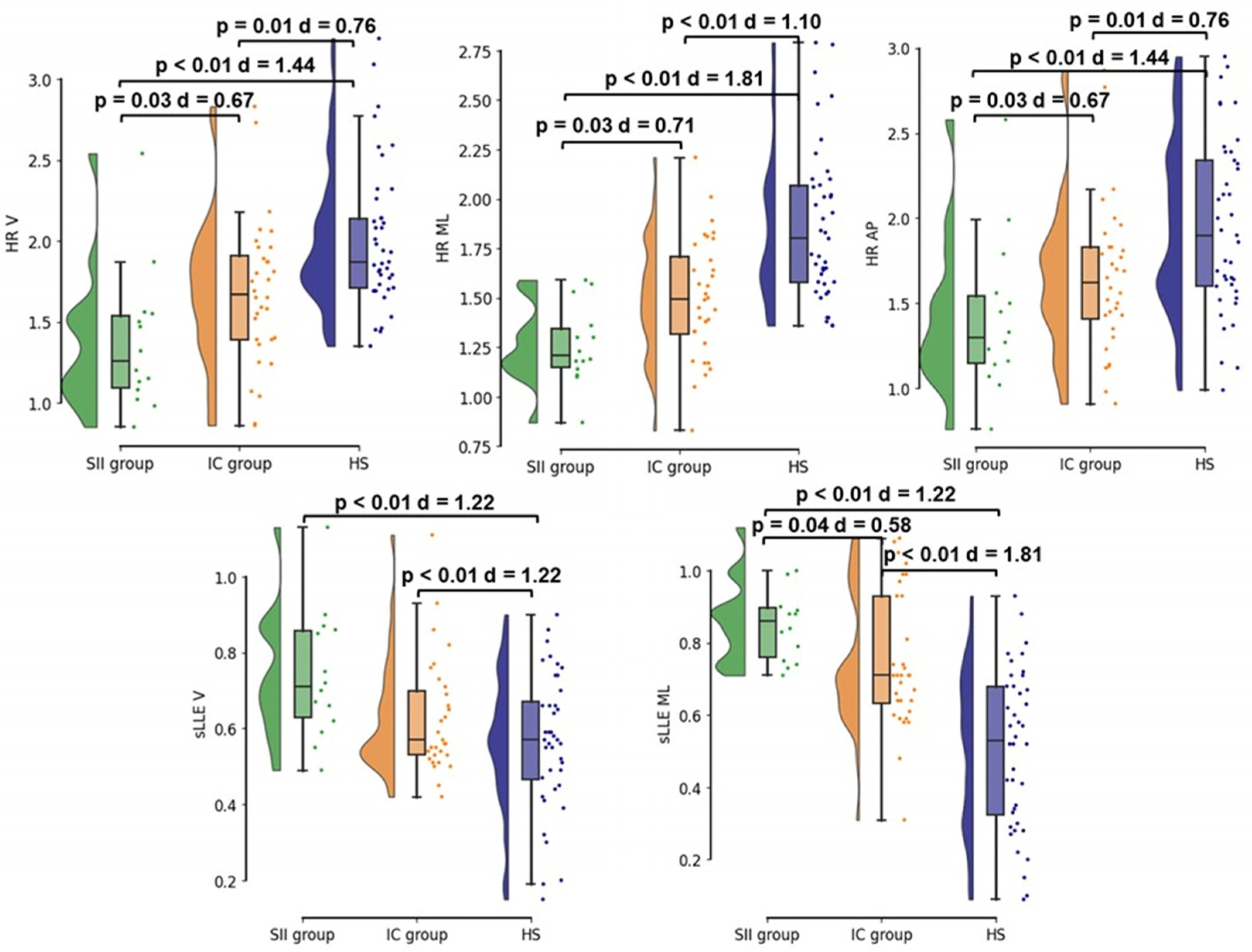
- Legend: SII group, stroke-induced immunosuppression group (green); IC group, immunocompetent group (orange); HS, age and gait speed-matched healthy subjects (blue); HR, harmonic ratio; sLLE, short–term Lyapunov’s exponent; V, vertical direction of the acceleration signal; ML, medio-lateral direction of the acceleration signal; AP, antero-posterior direction of the acceleration signal.
3.4. Modification of Trunk-Acceleration-Derived Gait Indexes after Rehabilitation (T1)
4. Discussion
- -
- At NRB admission, SII subjects were characterized by a more severe gait pattern when compared to the IC and HS groups. Specifically, SII participants showed lower HR values, which are consistent with a more asymmetric gait pattern, and higher sLLE values, suggesting a more impaired gait stability.
- -
- The rehabilitative intervention ameliorated the gait behavior in both IC and SII stroke subjects with improvements observed in the HRs in the three spatial directions, sLLE in the vertical and medio-lateral panels, and in gait speed. These findings are consistent with an improvement in trunk symmetry and dynamic stability during gait, as well as overall gait performance.
- -
- Although the rehabilitative intervention improved gait pattern to a similar extent in both groups, gait of SII patients was more compromised at both NRB admission and discharge when compared with IC group. Thus, SII qualifies as a negative prognostic factor for gait rehabilitation, although its presence did not hamper a certain degree of improvement.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259, Erratum in Lancet 2017, 390, e38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krishnamurthi, R.V.; Feigin, V.L.; Forouzanfar, M.H.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.M.; Truelsen, T.; et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 2013, 1, e259–e281. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Shi, K.; Wood, K.; Shi, F.-D.; Wang, X.; Liu, Q. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc. Neurol. 2018, 3, 34–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meisel, C.; Schwab, J.M.; Prass, K.; Meisel, A.; Dirnagl, U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005, 6, 775–786. [Google Scholar] [CrossRef] [PubMed]
- van Gemmeren, T.; Schuppner, R.; Grosse, G.M.; Fering, J.; Gabriel, M.M.; Huber, R.; Worthmann, H.; Lichtinghagen, R.; Weissenborn, K. Early Post-Stroke Infections Are Associated with an Impaired Function of Neutrophil Granulocytes. J. Clin. Med. 2020, 9, 872. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Med. J. 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Celikbilek, A.; Ismailogullari, S.; Zararsiz, G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J. Clin. Lab. Anal. 2014, 28, 27–31. [Google Scholar] [CrossRef]
- Li, W.; Hou, M.; Ding, Z.; Liu, X.; Shao, Y.; Li, X. Prognostic value of neutrophil-to-lymphocyte ratio in stroke: A systematic review and meta-analysis. Front. Neurol. 2021, 12, 686983. [Google Scholar] [CrossRef]
- Wu, B.; Liu, F.; Sun, G.; Wang, S. Prognostic role of dynamic neutrophil-to-lymphocyte ratio in acute ischemic stroke after reperfusion therapy: A meta-analysis. Front. Neurol. 2023, 14, 1118563. [Google Scholar] [CrossRef]
- Wang, L.; Song, Q.; Wang, C.; Wu, S.; Deng, L.; Li, Y.; Zheng, L.; Liu, M. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: A cohort study and systematic review. J. Neurol. Sci. 2019, 406, 116445. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.D.; Spears, C.; Cummings, C.; VanGilder, R.L.; Stinehart, K.R.; Gutmann, L.; Domico, J.; Culp, S.; Carpenter, J.; Rai, A.; et al. Admission neutrophil–lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J. NeuroInterventional Surg. 2014, 6, 578–583. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Wang, F.; Zhang, L.; Zhang, L.; Zhao, W. Increased neutrophil-to-lymphocyte ratio predicts the development of post-stroke infections in patients with acute ischemic stroke. BMC Neurol. 2020, 20, 328. [Google Scholar] [CrossRef]
- Gokhan, S.; Ozhasenekler, A.; Durgun, H.M.; Akil, E.; Ustündag, M.; Orak, M. Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attack. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 653–657. [Google Scholar] [PubMed]
- Guo, Z.; Yu, S.; Xiao, L.; Chen, X.; Ye, R.; Zheng, P.; Dai, Q.; Sun, W.; Zhou, C.; Wang, S.; et al. Dynamic change of neutrophil to lymphocyte ratio and hemorrhagic transformation after thrombolysis in stroke. J. Neuroinflamm. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Pektezel, M.Y.; Yilmaz, E.; Arsava, E.M.; Topcuoglu, M.A. Neutrophil-to-lymphocyte ratio and response to intravenous thrombolysis in patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 1853–1859. [Google Scholar] [CrossRef]
- Vaghi, G.; Morotti, A.; Piella, E.M.; Avenali, M.; Martinelli, D.; Cristina, S.; Allena, M.; Grillo, V.; Corrado, M.; Bighiani, F.; et al. The role of stroke-induced immunosuppression as a predictor of functional outcome in the neurorehabilitation setting. Sci. Rep. 2024, 14, 8320. [Google Scholar] [CrossRef]
- Lord, S.E.; McPherson, K.; McNaughton, H.K.; Rochester, L.; Weatherall, M. Community ambulation after stroke: How important and obtainable is it and what measures appear predictive? Arch. Phys. Med. Rehabil. 2004, 85, 234–239. [Google Scholar] [CrossRef]
- Jette, D.U.; Latham, N.K.; Smout, R.J.; Gassaway, J.; Slavin, M.D.; Horn, S.D. Physical therapy interventions for patients with stroke in inpatient rehabilitation facilities. Phys. Ther. 2005, 85, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Platz, T.; Kugler, J.; Pohl, M. Electromechanical and robot-assisted arm training for improving arm function and activities of daily living after stroke. Cochrane Database Syst. Rev. 2008, 4, CD006876, Erratum in Cochrane Database Syst. Rev. 2012, 6, CD006876. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Zorowitz, R.; Bates, B.; Choi, J.Y.; Glasberg, J.J.; Graham, G.D.; Katz, R.C.; Lamberty, K.; Reker, D. Management of Adult Stroke Rehabilitation Care: A clinical practice guideline. Stroke 2005, 36, e100–e143. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Stott, D.J.; Robertson, L.; MacDonald, J.; Jones, L.; McAlpine, C.; Dick, F.; Taylor, G.S.; Murray, G. Medical complications after stroke. Stroke 2000, 31, 1223–1229. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Heremans, C.; Burridge, J.; Deutsch, J.E.; Hammerbeck, U.; Hollands, K.; Karthikbabu, S.; Mehrholz, J.; Moore, J.L.; Salbach, N.M.; et al. Standardized measurement of balance and mobility post-stroke: Consensus-based core recommendations from the third Stroke Recovery and Rehabilitation Roundtable. Neurorehabilit. Neural Repair 2024, 38, 41–51. [Google Scholar] [CrossRef]
- Little, V.L.; Perry, L.A.; Mercado, M.W.; Kautz, S.A.; Patten, C. Gait asymmetry pattern following stroke determines acute response to locomotor task. Gait Posture 2020, 77, 300–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Gait asymmetry in community-ambulating stroke survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Punt, M.; Bruijn, S.M.; van Schooten, K.S.; Pijnappels, M.; van de Port, I.G.; Wittink, H.; van Dieën, J.H. Characteristics of daily life gait in fall and non fall-prone stroke survivors and controls. J. Neuroeng. Rehabil. 2016, 13, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Felius, R.A.W.; Wouda, N.C.; Geerars, M.; Bruijn, S.M.; van Dieën, J.H.; Punt, M. Beyond gait speed: Exploring the added value of Inertial Measurement Unit-based measurements of gait in the estimation of the walking ability in daily life. BMC Neurol. 2024, 24, 129. [Google Scholar] [CrossRef]
- Zhang, W.; Smuck, M.; Legault, C.; Ith, M.A.; Muaremi, A.; Aminian, K. Gait Symmetry Assessment with a Low Back 3D Accelerometer in Post-Stroke Patients. Sensors 2018, 18, 3322. [Google Scholar] [CrossRef]
- Wonsetler, E.C.; Bowden, M.G. A systematic review of mechanisms of gait speed change post-stroke. Part 1: Spatiotemporal parameters and asymmetry ratios. Top. Stroke Rehabil. 2017, 24, 435–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dasgupta, P.; VanSwearingen, J.; Godfrey, A.; Redfern, M.; Montero-Odasso, M.; Sejdic, E. Acceleration Gait Measures as Proxies for Motor Skill of Walking: A Narrative Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 249–261. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Lee, Y.; Shin, H.; Lee, G. Gait velocity and walking distance to predict community walking after stroke. Nurs. Health Sci. 2015, 17, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, C.K.; Clark, D.J.; Fox, E.J. Walking adaptability after a stroke and its assessment in clinical settings. Stroke Res. Treat. 2014, 2014, 591013. [Google Scholar] [CrossRef] [PubMed]
- Van Criekinge, T.; Saeys, W.; Hallemans, A.; Velghe, S.; Viskens, P.-J.; Vereeck, L.; De Hertogh, W.; Truijen, S. Trunk biomechanics during hemiplegic gait after stroke: A systematic review. Gait Posture 2017, 54, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Bower, K.; Thilarajah, S.; Pua, Y.-H.; Williams, G.; Tan, D.; Mentiplay, B.; Denehy, L.; Clark, R. Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. J. Neuroeng. Rehabil. 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hacmon, R.R.B.; Krasovsky, T.M.; Lamontagne, A.; Levin, M.F. Deficits in intersegmental trunk coordination during walking are related to clinical balance and gait function in chronic stroke. J. Neurol. Phys. Ther. 2012, 36, 173–181. [Google Scholar] [CrossRef]
- Selves, C.; Stoquart, G.; Lejeune, T. Gait rehabilitation after stroke: Review of the evidence of predictors, clinical outcomes and timing for interventions. Acta Neurol. Belg. 2020, 120, 783–790. [Google Scholar] [CrossRef]
- Alomari, R.A.; BinMulayh, E.A.; Alqarni, A.M.; Alsobhi, M.; Chevidikunnan, M.F.; Basuodan, R.; Khan, F. Trunk control and acute-phase multifactorial predictors of community mobility after stroke: A longitudinal observational study. Front. Neurol. 2024, 15, 1376444. [Google Scholar] [CrossRef]
- Buckley, C.; Micó-Amigo, M.E.; Dunne-Willows, M.; Godfrey, A.; Hickey, A.; Lord, S.; Rochester, L.; Del Din, S.; Moore, S.A. Gait Asymmetry Post-Stroke: Determining Valid and Reliable Methods Using a Single Accelerometer Located on the Trunk. Sensors 2019, 20, 37. [Google Scholar] [CrossRef]
- Picerno, P.; Iosa, M.; D’souza, C.; Benedetti, M.G.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis: A five-year update. Expert Rev. Med. Devices 2021, 18, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Oyake, K.; Yamaguchi, T.; Sugasawa, M.; Oda, C.; Tanabe, S.; Kondo, K.; Otaka, Y.; Momose, K. Validity of gait asymmetry estimation by using an accelerometer in individuals with hemiparetic stroke. J. Phys. Ther. Sci. 2017, 29, 307–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, S.A.; Hickey, A.; Lord, S.; Del Din, S.; Godfrey, A.; Rochester, L. Comprehensive measurement of stroke gait characteristics with a single accelerometer in the laboratory and community: A feasibility, validity and reliability study. J. Neuroeng. Rehabil. 2017, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Pavic, A.; Goodwin, V.A. Wearable inertial sensors to measure gait and posture characteristic differences in older adult fallers and non-fallers: A scoping review. Gait Posture 2020, 76, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Gough, C.; Gordon, S.J. Inertial Sensor Reliability and Validity for Static and Dynamic Balance in Healthy Adults: A Systematic Review. Sensors 2021, 21, 5167. [Google Scholar] [CrossRef]
- Rehman, R.Z.U.; Zhou, Y.; Del Din, S.; Alcock, L.; Hansen, C.; Guan, Y.; Hortobágyi, T.; Maetzler, W.; Rochester, L.; Lamoth, C.J.C. Gait analysis with wearables can accurately classify fallers from non-fallers: A step toward better management of neurological disorders. Sensors 2020, 20, 6992. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Trabassi, D.; Tatarelli, A.; Ranavolo, A.; Varrecchia, T.; Fiori, L.; Di Lenola, D.; Cioffi, E.; Raju, M.; Coppola, G.; et al. Identification of Gait Unbalance and Fallers Among Subjects with Cerebellar Ataxia by a Set of Trunk Acceleration-Derived Indices of Gait. Cerebellum 2023, 22, 46–58. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Trabassi, D.; De Icco, R.; Tatarelli, A.; Avenali, M.; Corrado, M.; Grillo, V.; Coppola, G.; Denaro, A.; Tassorelli, C.; et al. Harmonic ratio is the most responsive trunk-acceleration derived gait index to rehabilitation in people with Parkinson’s disease at moderate disease stages. Gait Posture 2022, 97, 152–158. [Google Scholar] [CrossRef]
- Castiglia, S.F.; Tatarelli, A.; Trabassi, D.; De Icco, R.; Grillo, V.; Ranavolo, A.; Varrecchia, T.; Magnifica, F.; Di Lenola, D.; Coppola, G.; et al. Ability of a Set of Trunk Inertial Indexes of Gait to Identify Gait Instability and Recurrent Fallers in Parkinson’s Disease. Sensors 2021, 21, 3449. [Google Scholar] [CrossRef] [PubMed]
- Castiglia, S.F.; Trabassi, D.; Conte, C.; Gioiosa, V.; Sebastianelli, G.; Abagnale, C.; Ranavolo, A.; Di Lorenzo, C.; Coppola, G.; Casali, C.; et al. Local Dynamic Stability of Trunk During Gait is Responsive to Rehabilitation in Subjects with Primary Degenerative Cerebellar Ataxia. Cerebellum 2024, 23, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Bini, F.; Marinozzi, F.; Fusco, A.; Morone, G.; Koch, G.; Cinnera, A.M.; Bonnì, S.; Paolucci, S. Stability and Harmony of Gait in Patients with Subacute Stroke. J. Med. Biol. Eng. 2016, 36, 635–643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasciuto, I.; Bergamini, E.; Iosa, M.; Vannozzi, G.; Cappozzo, A. Overcoming the limitations of the Harmonic Ratio for the reliable assessment of gait symmetry. J. Biomech. 2017, 53, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kempski, K.; Awad, L.N.; Buchanan, T.S.; Higginson, J.S.; Knarr, B.A. Dynamic structure of lower limb joint angles during walking post-stroke. J. Biomech. 2018, 68, 1–5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kao, P.-C.; Dingwell, J.B.; Higginson, J.S.; Binder-Macleod, S. Dynamic instability during post-stroke hemiparetic walking. Gait Posture 2014, 40, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Germanotta, M.; Iacovelli, C.; Aprile, I. Evaluation of Gait Smoothness in Patients with Stroke Undergoing Rehabilitation: Comparison between Two Metrics. Int. J. Environ. Res. Public Health 2022, 19, 13440. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Bustos, A.S.O.; Montemurro, R.; Vasta, S.; Marangon, G.; Belluscio, V.; Morone, G.; Modugno, N.; Buzzi, M.G.; Formisano, R.; et al. Dynamic Stability, Symmetry, and Smoothness of Gait in People with Neurological Health Conditions. Sensors 2024, 24, 2451. [Google Scholar] [CrossRef]
- Iosa, M.; Fusco, A.; Morone, G.; Paolucci, S. Development and decline of upright gait stability. Front. Aging Neurosci. 2014, 6, 14. [Google Scholar] [CrossRef]
- Riva, F.; Bisi, M.; Stagni, R. Gait Variability and Stability Measures: Minimum Number of Strides and within-Session Reliability. Comput. Biol. Med. 2014, 50, 9–13. [Google Scholar] [CrossRef]
- Kroneberg, D.; Elshehabi, M.; Meyer, A.C.; Otte, K.; Doss, S.; Paul, F.; Nussbaum, S.; Berg, D.; Kühn, A.A.; Maetzler, W.; et al. Less Is More—Estimation of the Number of Strides Required to Assess Gait Variability in Spatially Confined Settings. Front. Aging Neurosci. 2019, 10, 435. [Google Scholar]
- Micó-Amigo, M.E.; Bonci, T.; Paraschiv-Ionescu, A.; Ullrich, M.; Kirk, C.; Soltani, A.; Küderle, A.; Gazit, E.; Salis, F.; Alcock, L.; et al. Assessing real-world gait with digital technology? Validation, insights and recommendations from the Mobilise-D consortium. J. Neuroeng. Rehabil. 2023, 20, 78. [Google Scholar] [CrossRef]
- McCamley, J.; Donati, M.; Grimpampi, E.; Mazzà, C. An enhanced estimate of initial contact and final contact instants of time using lower trunk inertial sensor data. Gait Posture 2012, 36, 316–318. [Google Scholar] [CrossRef]
- Paraschiv-Ionescu, A.; Newman, C.J.; Carcreff, L.; Gerber, C.N.; Armand, S.; Aminian, K. Locomotion and cadence detection using a single trunk-fixed accelerometer: Validity for children with cerebral palsy in daily life-like conditions. J. Neuroeng. Rehabil. 2019, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv-Ionescu, A.; Soltani, A.; Aminian, K. (Eds.) Real-world speed estimation using single trunk IMU: Methodological challenges for impaired gait patterns. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar]
- Bellanca, J.; Lowry, K.; VanSwearingen, J.; Brach, J.; Redfern, M. Harmonic ratios: A quantification of step to step symmetry. J. Biomech. 2013, 46, 828–831. [Google Scholar] [CrossRef]
- Ghamari, N.; Ghaderpanah, R.; Sadrian, S.H.; Fallah, N. Effect of a visual dual task on postural stability—A comparative study using linear and nonlinear methods. Health Sci. Rep. 2023, 6, e1437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chini, G.; Ranavolo, A.; Draicchio, F.; Casali, C.; Conte, C.; Martino, G.; Leonardi, L.; Padua, L.; Coppola, G.; Pierelli, F.; et al. Local Stability of the Trunk in Patients with Degenerative Cerebellar Ataxia During Walking. Cerebellum 2017, 16, 26–33. [Google Scholar] [CrossRef]
- Melendez-Calderon, A.; Shirota, C.; Balasubramanian, S. Estimating Movement Smoothness From Inertial Measurement Units. Front. Bioeng. Biotechnol. 2021, 8, 558771. [Google Scholar] [CrossRef]
- Igarashi, T.; Tani, Y.; Takeda, R.; Asakura, T. Relationship between gait regularity and harmony, and gait speed at discharge in inpatients with subacute stroke. J. Phys. Ther. Sci. 2023, 35, 40–45. [Google Scholar] [CrossRef]
- Bisi, M.C.; Riva, F.; Stagni, R. Measures of gait stability: Performance on adults and toddlers at the beginning of independent walking. J. Neuroeng. Rehabil. 2014, 11, 131. [Google Scholar] [CrossRef]
- Brach, J.S.; McGurl, D.; Wert, D.; VanSwearingen, J.M.; Perera, S.; Cham, R.; Studenski, S. Validation of a measure of smoothness of walking. J. Gerontol. Ser. A 2011, 66, 136–141. [Google Scholar] [CrossRef]
- Iosa, M.; Picerno, P.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis. Expert Rev. Med. Devices 2016, 13, 641–659. [Google Scholar]
- Raffalt, P.C.; Kent, J.A.; Wurdeman, S.R.; Stergiou, N. Selection Procedures for the Largest Lyapunov Exponent in Gait Biomechanics. Ann. Biomed. Eng. 2019, 47, 913–923. [Google Scholar] [CrossRef]
- Raffalt, P.C.; Senderling, B.; Stergiou, N. Filtering affects the calculation of the largest Lyapunov exponent. Comput. Biol. Med. 2020, 122, 103786. [Google Scholar] [CrossRef]
- Pedraza, S.; Puig, J.; Blasco, G.; Daunis-I-Estadella, J.; Boada, I.; Bardera, A.; Castellanos, M.; Serena, J. Reliability of the ABC/2 method in determining acute infarct volume. J. Neuroimaging 2012, 22, 155–159. [Google Scholar] [CrossRef]
- Igarashi, T.; Tani, Y.; Takeda, R.; Asakura, T. Accelerometer-based gait characteristics and their discrimination of gait independence in inpatients with subacute stroke. Gait Posture 2024, 110, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Tani, Y.; Takeda, R.; Asakura, T. Minimal detectable change in inertial measurement unit-based trunk acceleration indices during gait in inpatients with subacute stroke. Sci. Rep. 2023, 13, 19262. [Google Scholar] [CrossRef]
- Bergamini, E.; Cereatti, A.; Pavei, G. Just a matter of indexes: Symmetry in walking. Gait Posture 2022, 97 (Suppl. 2), 11–12. [Google Scholar] [CrossRef]
- Meyer, S.; Strittmatter, M.; Fischer, C.; Georg, T.; Schmitz, B. Lateralization in autononic dysfunction in ischemic stroke involving the insular cortex. NeuroReport 2004, 15, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Clark, D.J.; Fox, E.J.; Rose, D.K. Sympathetic nervous system responses during complex walking tasks and community ambulation post-stroke. Sci. Rep. 2023, 13, 20068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dal Lin, C.; Tona, F.; Osto, E. The crosstalk between the cardiovascular and the immune system. Vasc. Biol. 2019, 1, H83–H88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Total (n = 46) | IC Group (n = 32) | SII Group (n = 14) | p-Value | ||
|---|---|---|---|---|---|
| Sex (n (%)) | F | 18 (39.13%) | 12 (37.50%) | 6 (42.86%) | 0.73 |
| M | 28 (60.87%) | 20 (62.50%) | 8 (57.14%) | ||
| Stroke type (n (%)) | Ischemic | 43 (93.48%) | 31 (96.86%) | 12 (85.71%) | 0.16 |
| Hemorrhagic | 3 (6.52%) | 1 (3.12%) | 2 (15.38%) | ||
| Side of lesion (n (%)) | Left | 18 (39.13%) | 13 (28.26%) | 5 (10.87%) | 0.75 |
| Right | 28 (60.87%) | 19 (41.30%) | 9 (19.56%) | ||
| Hemorrhagic transformation in ischemic stroke (n (%)) | Yes | 4 (9.30%) | 3 (9.67%) | 1 (8.33%) | 1.00 |
| No | 39 (90.70%) | 28 (90.32%) | 11 (91.67%) | ||
| Stroke volume (cm3/mL) | 16.10 (35.85) | 15.76 (32.94) | 16.95 (42.22) | 0.32 | |
| Number of lesions (n (%)) | Single | 30 (65.22%) | 22 (68.75%) | 8 (57.14%) | 0.45 |
| Multiple | 16 (34.78%) | 10 (31.25%) | 6 (42.86%) | ||
| Aphasia (n (%)) | Yes | 12 (26.09%) | 6 (18.75%) | 6 (42.86%) | 0.09 |
| No | 34 (79.13%) | 26 (81.25%) | 8 (57.14%) | ||
| Dysphagia (n (%)) | Yes | 9 (19.57%) | 3 (9.38%) | 6 (42.86%) | 0.01 |
| No | 37 (80.43%) | 29 (90.63%) | 8 (57.14%) | ||
| Cognitive impairment (n (%)) | Yes | 10 (21.74%) | 8 (25%) | 2 (14.29%) | 0.42 |
| No | 36 (78.26%) | 24 (75%) | 12 (85.71%) | ||
| Hypertension (n (%)) | Yes | 38 (82.61%) | 25 (78.12%) | 13 (92.86%) | 0.22 |
| No | 8 (17.39%) | 7 (21.87%) | 1 (7.14%) | ||
| Hypercholesterolemia (n (%)) | Yes | 27 (58.70%) | 14 (43.75%) | 9 (64.29%) | 0.61 |
| No | 19 (42.30%) | 18 (56.25%) | 5 (64.29%) | ||
| Alcohol abuse (n (%)) | Yes | 16 (34.78%) | 12 (37.50%) | 4 (28.57%) | 0.84 |
| No | 29 (65.21%) | 20 (65.5%) | 10 (71.42%) | ||
| Diabetes (n (%)) | Yes | 13 (28.26%) | 11 (34.37%) | 2 (14.29%) | 0.16 |
| No | 33 (71.74%) | 21 (65.62%) | 12 (85.71%) | ||
| Smoking (n (%)) | Active | 16 (34.78%) | 12 (37.50%) | 4 (28.57%) | 0.69 |
| Former | 10 (21.74%) | 7 (21.87%) | 3 (21.43%) | ||
| Never | 20 (43.48%) | 13 (40.62%) | 7 (50.00%) | ||
| NLR | 3.31 (1.90) | 2.70 (0.98) | 6.68 (2.28) | 0.01 | |
| Time from stroke onset to NRB admission (days) | 6.89 (3.85) | 7.53 (4.28) | 5.43 (2.06) | 0.09 | |
| Length of NRB hospitalization (days) | 44.65 (14.39) | 45.44 (13.90) | 45.86 (18.51) | 0.97 | |
| NIHSS | 5.56 (3.29) | 5.15 (2.47) | 7.86 (5.96) | 0.04 | |
| BI | 54.56 (20.43) | 57.05 (19.92) | 40.71 (18.80) | 0.02 | |
| FIM | 85.41 (19.69) | 86.61 (19.39) | 77.33 (23.23) | 0.33 | |
| Tinetti | 17.40 (8.03) | 18.0 (7.57) | 15.90 (9.12) | 0.54 | |
| IC group (n = 32) | SII group (n = 14) | HS (n = 42) | H | p-Value | η² | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Age | 62.9 (15.9) | 71.1 (14.2) | 62.1 (8.7) | 6.31 | 0.07 | 0.06 | |
| Sex | F M | 12 (37.50%) 20 (62.50%) | 6 (42.86%) 8 (57.14%) | 16 (39.02%) 24 (60.98%) | 0.12 * | 0.94 | |
| Gait speed | 0.72 (0.21) | 0.66 (0.26) | 0.78 (0.19) | 4.48 | 0.11 | 0.05 | |
| HR V | 1.68 (0.45) | 1.36 (0.45) | 2.01 (0.47) | 21.58 | <0.01 | 0.23 | |
| HR ML | 1.50 (0.30) | 1.24 (0.19) | 1.87 (0.39) | 32.04 | <0.01 | 0.34 | |
| HR AP | 1.64 (0.44) | 1.41 (0.48) | 1.95 (0.51) | 14.40 | <0.01 | 0.16 | |
| sLLE V | 0.63 (0.15) | 0.75 (0.17) | 0.56 (0.18) | 8.69 | 0.01 | 0.12 | |
| sLLE ML | 0.75 (0.19) | 0.87 (0.12) | 0.51 (0.22) | 31.41 | <0.01 | 0.35 | |
| sLLE AP | 0.60 (0.14) | 0.71 (0.23) | 0.54 (0.23) | 3.92 | 0.14 | 0.07 | |
| LDLJa V | −4.95 (0.31) | −5.03 (0.37) | −4.95 (0.31) | 0.98 | 0.61 | 0.06 | |
| LDLJa ML | −5.44 (0.33) | −5.40 (0.39) | −5.54 (0.27) | 3.70 | 0.16 | 0.03 | |
| LDLJa AP | −5.05 (0.45) | −4.94 (0.24) | −4.98 (0.34) | 1.20 | 0.55 | 0.01 | |
| SI_stance (%) | 7.40 (9.08) | 7.82 (8.79) | 3.48 (2.90) | 3.22 | 0.20 | 0.08 | |
| SI_swing (%) | 9.44 (11.16) | 12.22 (13.13) | 5.82 (4.75) | 3.29 | 0.19 | 0.07 | |
| SI_double support (%) | 16.96 (19.50) | 24.53 (18.18) | 17.94 (15.06) | 2.78 | 0.25 | 0.03 | |
| SI_single support (%) | 10.60 (13.18) | 12.10 (12.98) | 5.60 (4.91) | 2.95 | 0.23 | 0.07 |
| T0 Mean (SD) | T1 Mean (SD) | IC group | SII group | Factor (p-Values) | |||||
|---|---|---|---|---|---|---|---|---|---|
| T0 Mean (SD) | T1 Mean (SD) | T0 (Mean SD) | T1 Mean (SD) | Time (T0 vs. T1) | Group (IC vs. SII) | TimeXGroup Interaction | |||
| HRV | 1.58 (0.46) | 1.93 (0.62) | 1.68 (0.45) | 2.04 (0.64) | 1.36 (0.45) | 1.67 (0.49) | 0.02 | <0.01 | 0.59 |
| HRML | 1.43 (0.30) | 1.70 (0.49) | 1.50 (0.30) | 1.79 (0.51) | 1.24 (0.19) | 1.47 (0.38) | <0.01 | <0.01 | 0.88 |
| HRAP | 1.58 (0.46) | 1.86 (0.52) | 1.64 (0.44) | 1.91 (0.53) | 1.41 (0.48) | 1.75 (0.50) | 0.03 | <0.01 | 0.35 |
| sLLEV | 0.66 (0.16) | 0.59 (0.18) | 0.63 (0.15) | 0.57 (0.17) | 0.75 (0.17) | 0.62 (0.19) | 0.02 | 0.02 | 0.55 |
| sLLEML | 0.78 (0.18) | 0.71 (0.17) | 0.75 (0.19) | 0.68 (0.16) | 0.87 (0.12) | 0.78 (0.18) | <0.01 | 0.02 | 0.37 |
| Gait speed (m/s) | 0.70 (0.23) | 0.90 (0.27) | 0.72 (0.21) | 0.90 (0.26) | 0.66 (0.26) | 0.88 (0.29) | <0.01 | <0.01 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinis, L.; Castiglia, S.F.; Vaghi, G.; Morotti, A.; Grillo, V.; Corrado, M.; Bighiani, F.; Cammarota, F.; Antoniazzi, A.; Correale, L.; et al. Differences in Trunk Acceleration-Derived Gait Indexes in Stroke Subjects with and without Stroke-Induced Immunosuppression. Sensors 2024, 24, 6012. https://doi.org/10.3390/s24186012
Martinis L, Castiglia SF, Vaghi G, Morotti A, Grillo V, Corrado M, Bighiani F, Cammarota F, Antoniazzi A, Correale L, et al. Differences in Trunk Acceleration-Derived Gait Indexes in Stroke Subjects with and without Stroke-Induced Immunosuppression. Sensors. 2024; 24(18):6012. https://doi.org/10.3390/s24186012
Chicago/Turabian StyleMartinis, Luca, Stefano Filippo Castiglia, Gloria Vaghi, Andrea Morotti, Valentina Grillo, Michele Corrado, Federico Bighiani, Francescantonio Cammarota, Alessandro Antoniazzi, Luca Correale, and et al. 2024. "Differences in Trunk Acceleration-Derived Gait Indexes in Stroke Subjects with and without Stroke-Induced Immunosuppression" Sensors 24, no. 18: 6012. https://doi.org/10.3390/s24186012
APA StyleMartinis, L., Castiglia, S. F., Vaghi, G., Morotti, A., Grillo, V., Corrado, M., Bighiani, F., Cammarota, F., Antoniazzi, A., Correale, L., Liberali, G., Piella, E. M., Trabassi, D., Serrao, M., Tassorelli, C., & De Icco, R. (2024). Differences in Trunk Acceleration-Derived Gait Indexes in Stroke Subjects with and without Stroke-Induced Immunosuppression. Sensors, 24(18), 6012. https://doi.org/10.3390/s24186012









