Assessing the Quality of Solvent-Assisted Lipid Bilayers Formed at Different Phases and Aqueous Buffer Media: A QCM-D Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipid Sample Preparation
2.3. Experimental Setup
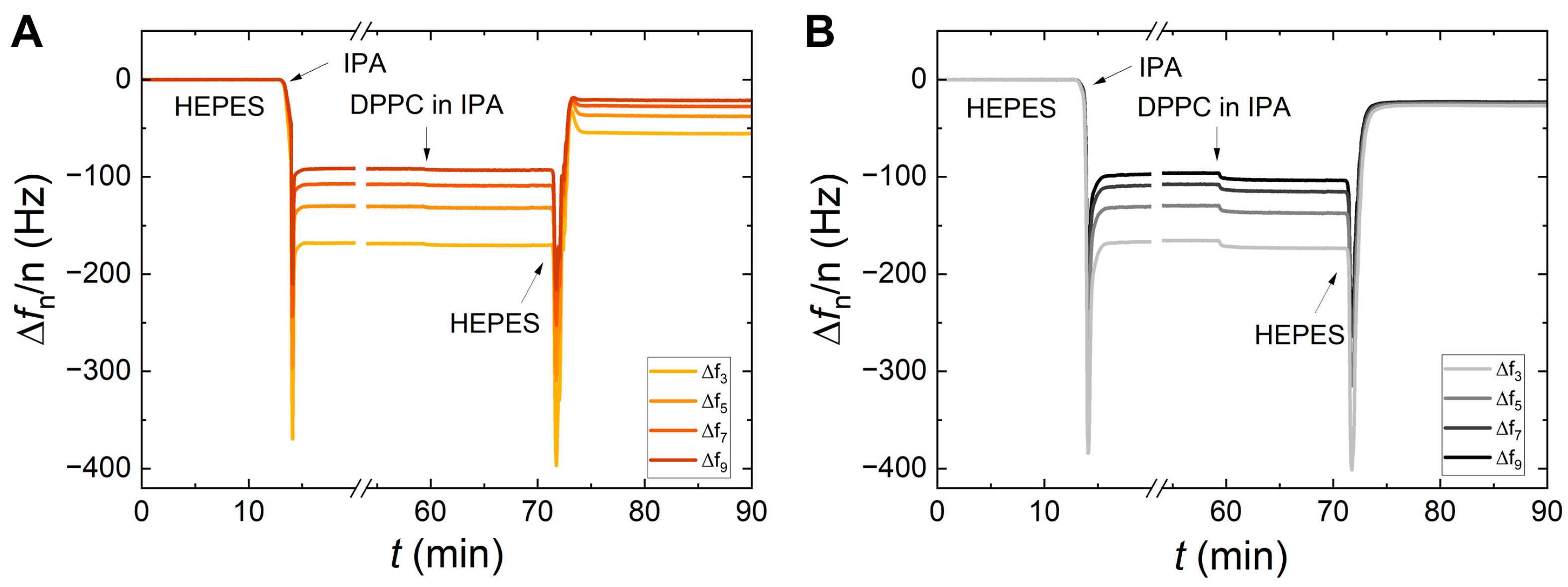
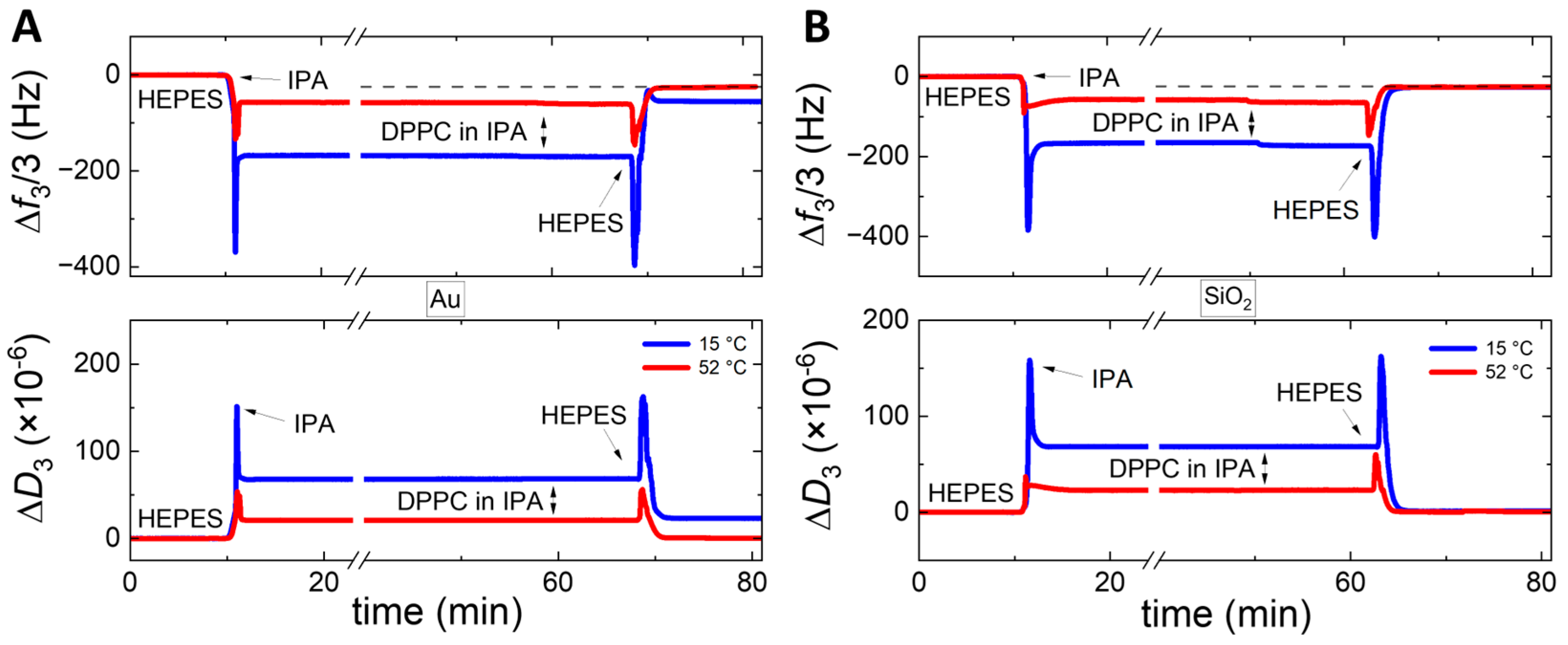
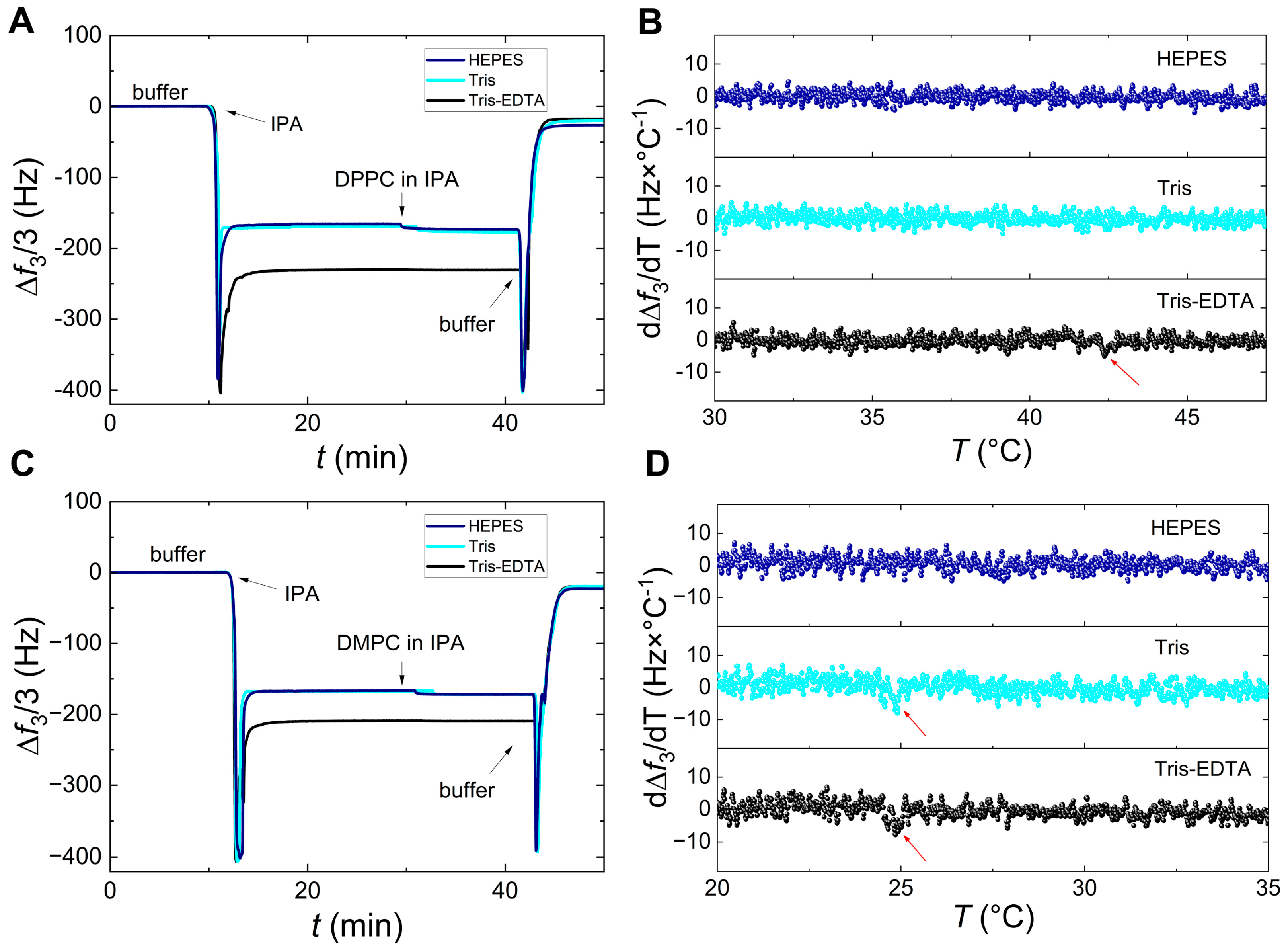
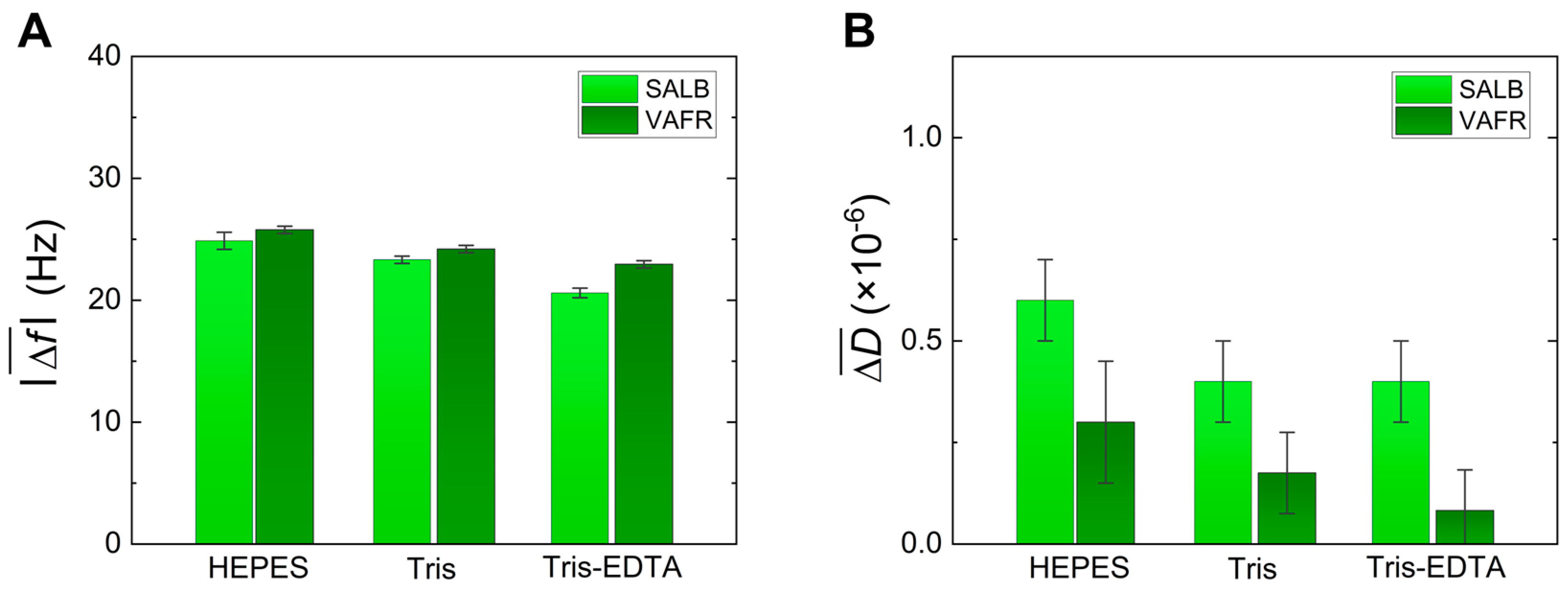
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes: Cell Membranes Are Viewed as Two-Dimensional Solutions of Oriented Globular Proteins and Lipids. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Iglič, A.; Hägerstrand, H.; Veranič, P.; Plemenitaš, A.; Kralj-Iglič, V. Curvature-Induced Accumulation of Anisotropic Membrane Components and Raft Formation in Cylindrical Membrane Protrusions. J. Theor. Biol. 2006, 240, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N.; Marčelja, S.; Horn, R.G. Physical Principles of Membrane Organization. Q. Rev. Biophys. 1980, 13, 121–200. [Google Scholar] [CrossRef]
- Varshney, P.; Yadav, V.; Saini, N. Lipid Rafts in Immune Signalling: Current Progress and Future Perspective. Immunology 2016, 149, 13–24. [Google Scholar] [CrossRef]
- Baumgart, T.; Capraro, B.R.; Zhu, C.; Das, S.L. Thermodynamics and Mechanics of Membrane Curvature Generation and Sensing by Proteins and Lipids. Annu. Rev. Phys. Chem. 2011, 62, 483–506. [Google Scholar] [CrossRef]
- Kralj-Iglič, V. Stability of Membranous Nanostructures: A Possible Key Mechanism in Cancer Progression. Int. J. Nanomed. 2012, 7, 3579. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, A.; Uysalel, C.; Rangamani, P. The Mechanics and Thermodynamics of Tubule Formation in Biological Membranes. J. Membr. Biol. 2021, 254, 273–291. [Google Scholar] [CrossRef]
- Kralj-Iglič, V.; Pocsfalvi, G.; Mesarec, L.; Šuštar, V.; Hägerstrand, H.; Iglič, A. Minimizing Isotropic and Deviatoric Membrane Energy—An Unifying Formation Mechanism of Different Cellular Membrane Nanovesicle Types. PLoS ONE 2020, 15, e0244796. [Google Scholar] [CrossRef]
- Kumar, G.; Duggisetty, S.C.; Srivastava, A. A Review of Mechanics-Based Mesoscopic Membrane Remodeling Methods: Capturing Both the Physics and the Chemical Diversity. J. Membr. Biol. 2022, 255, 757–777. [Google Scholar] [CrossRef]
- Schamberger, B.; Ziege, R.; Anselme, K.; Ben Amar, M.; Bykowski, M.; Castro, A.P.G.; Cipitria, A.; Coles, R.A.; Dimova, R.; Eder, M.; et al. Curvature in Biological Systems: Its Quantification, Emergence, and Implications across the Scales. Adv. Mater. 2023, 35, 2206110. [Google Scholar] [CrossRef] [PubMed]
- Tero, R. Substrate Effects on the Formation Process, Structure and Physicochemical Properties of Supported Lipid Bilayers. Materials 2012, 5, 2658–2680. [Google Scholar] [CrossRef]
- Bange, L.; Mukhina, T.; Fragneto, G.; Rondelli, V.; Schneck, E. Influence of Adhesion-Promoting Glycolipids on the Structure and Stability of Solid-Supported Lipid Double-Bilayers. Soft Matter 2024, 20, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Miyagi, A.; Redondo-Morata, L.; Scheuring, S. Temperature-Controlled High-Speed AFM: Real-Time Observation of Ripple Phase Transitions. Small 2016, 12, 6106–6113. [Google Scholar] [CrossRef]
- Reviakine, I.; Johannsmann, D.; Richter, R.P. Hearing What You Cannot See and Visualizing What You Hear: Interpreting Quartz Crystal Microbalance Data from Solvated Interfaces. Anal. Chem. 2011, 83, 8838–8848. [Google Scholar] [CrossRef]
- Peschel, A.; Langhoff, A.; Uhl, E.; Dathathreyan, A.; Haindl, S.; Johannsmann, D.; Reviakine, I. Lipid Phase Behavior Studied with a Quartz Crystal Microbalance: A Technique for Biophysical Studies with Applications in Screening. J. Chem. Phys. 2016, 145, 204904. [Google Scholar] [CrossRef] [PubMed]
- Bar, L.; Villanueva, M.E.; Neupane, S.; Cordoyiannis, G.; Losada-Pérez, P. QCM-D Study of the Formation of Solid-Supported Artificial Lipid Membranes: State-of-the-Art, Recent Advances, and Perspectives. Phys. Status Solidi A 2023, 220, 2200625. [Google Scholar] [CrossRef]
- Ohlsson, G.; Langhammer, C.; Zorić, I.; Kasemo, B. A Nanocell for Quartz Crystal Microbalance and Quartz Crystal Microbalance with Dissipation-Monitoring Sensing. Rev. Sci. Instrum. 2009, 80, 083905. [Google Scholar] [CrossRef]
- Tsortos, A.; Papadakis, G.; Gizeli, E. Shear Acoustic Wave Biosensor for Detecting DNA Intrinsic Viscosity and Conformation: A Study with QCM-D. Biosens. Bioelectron. 2008, 24, 836–841. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wei, X.; Xue, Y.; Wan, H.; Wang, P. Recent Advances in Acoustic Wave Biosensors for the Detection of Disease-Related Biomarkers: A Review. Anal. Chim. Acta 2021, 1164, 338321. [Google Scholar] [CrossRef]
- Xie, M.; Lv, X.; Wang, K.; Zhou, Y.; Lin, X. Advancements in Chemical and Biosensors for Point-of-Care Detection of Acrylamide. Sensors 2024, 24, 3501. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, G.; Tigerström, A.; Höök, F.; Kasemo, B. Phase Transitions in Adsorbed Lipid Vesicles Measured Using a Quartz Crystal Microbalance with Dissipation Monitoring. Soft Matter 2011, 7, 10749. [Google Scholar] [CrossRef]
- Neupane, S.; De Smet, Y.; Renner, F.U.; Losada-Pérez, P. Quartz Crystal Microbalance With Dissipation Monitoring: A Versatile Tool to Monitor Phase Transitions in Biomimetic Membranes. Front. Mater. 2018, 5, 46. [Google Scholar] [CrossRef]
- Yousefi, N.; Tufenkji, N. Probing the Interaction between Nanoparticles and Lipid Membranes by Quartz Crystal Microbalance with Dissipation Monitoring. Front. Chem. 2016, 4, 46. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Gillissen, J.J.J.; Vafaei, S.; Groves, J.T.; Cho, N.-J. Size-Dependent, Stochastic Nature of Lipid Exchange between Nano-Vesicles and Model Membranes. Nanoscale 2016, 8, 13513–13520. [Google Scholar] [CrossRef]
- Sheridan, A.J.; Thompson, K.C.; Slater, J.M. Interaction of Negatively and Positively Capped Gold Nanoparticle with Different Lipid Model Membranes. Biophys. Chem. 2022, 290, 106896. [Google Scholar] [CrossRef]
- Bar, L.; Perissinotto, F.; Redondo-Morata, L.; Giannotti, M.I.; Goole, J.; Losada-Pérez, P. Interactions of Hydrophilic Quantum Dots with Defect-Free and Defect Containing Supported Lipid Membranes. Colloids Surf. B Biointerfaces 2022, 210, 112239. [Google Scholar] [CrossRef]
- Voinova, M.V.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic Acoustic Response of Layered Polymer Films at Fluid-Solid Interfaces: Continuum Mechanics Approach. Phys. Scr. 1999, 59, 391–396. [Google Scholar] [CrossRef]
- Johannsmann, D.; Langhoff, A.; Leppin, C.; Reviakine, I.; Maan, A.M.C. Effect of Noise on Determining Ultrathin-Film Parameters from QCM-D Data with the Viscoelastic Model. Sensors 2023, 23, 1348. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Mertens, N.; De Medio-Vasconcelos, B.; Slenders, E.; Leys, J.; Peeters, M.; Van Grinsven, B.; Gruber, J.; Glorieux, C.; Pfeiffer, H.; et al. Phase Transitions of Binary Lipid Mixtures: A Combined Study by Adiabatic Scanning Calorimetry and Quartz Crystal Microbalance with Dissipation Monitoring. Adv. Condens. Matter Phys. 2015, 2015, 479318. [Google Scholar] [CrossRef]
- Paiva, J.G.; Paradiso, P.; Serro, A.P.; Fernandes, A.; Saramago, B. Interaction of Local and General Anaesthetics with Liposomal Membrane Models: A QCM-D and DSC Study. Colloids Surf. B Biointerfaces 2012, 95, 65–74. [Google Scholar] [CrossRef]
- Redondo-Morata, L.; Losada-Pérez, P.; Giannotti, M.I. Lipid Bilayers: Phase Behavior and Nanomechanics. In Current Topics in Membranes; Elsevier: Amsterdam, The Netherlands, 2020; Volume 86, pp. 1–55. ISBN 978-0-12-821021-5. [Google Scholar]
- Reimhult, E.; Höök, F.; Kasemo, B. Vesicle Adsorption on SiO2 and TiO2: Dependence on Vesicle Size. J. Chem. Phys. 2002, 117, 7401–7404. [Google Scholar] [CrossRef]
- Hochrein, M.B.; Reich, C.; Krause, B.; Rädler, J.O.; Nickel, B. Structure and Mobility of Lipid Membranes on a Thermoplastic Substrate. Langmuir 2006, 22, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Hohner, A.O.; David, M.P.C.; Rädler, J.O. Controlled Solvent-Exchange Deposition of Phospholipid Membranes onto Solid Surfaces. Biointerphases 2010, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, S.R.; Choi, J.-H.; Haw Zan, G.; Zhdanov, V.P.; Cho, N.-J. Solvent-Assisted Lipid Bilayer Formation on Silicon Dioxide and Gold. Langmuir 2014, 30, 10363–10373. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Vafaei, S.; Cho, N.-J. Fabrication of Charged Membranes by the Solvent-Assisted Lipid Bilayer (SALB) Formation Method on SiO 2 and Al2O3. Phys. Chem. Chem. Phys. 2015, 17, 11546–11552. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Yoon, B.K.; Park, S.; Sut, T.N.; Chin, H.; Park, J.H.; Jackman, J.A.; Cho, N.-J. Solvent-Assisted Preparation of Supported Lipid Bilayers. Nat. Protoc. 2019, 14, 2091–2118. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Jackman, J.A.; Kim, S.-O.; Zhdanov, V.P.; Cho, N.-J. Solvent-Assisted Lipid Self-Assembly at Hydrophilic Surfaces: Factors Influencing the Formation of Supported Membranes. Langmuir 2015, 31, 3125–3134. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Ng, W.B.; Cho, S.-J.; Cho, N.-J. Controlling the Formation of Phospholipid Monolayer, Bilayer, and Intact Vesicle Layer on Graphene. ACS Appl. Mater. Interfaces 2016, 8, 11875–11880. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Polat, O.; Parikh, A.N.; Seker, E.; Renner, F.U. Engineering the Interface between Lipid Membranes and Nanoporous Gold: A Study by Quartz Crystal Microbalance with Dissipation Monitoring. Biointerphases 2018, 13, 011002. [Google Scholar] [CrossRef]
- Betlem, K.; Cordoyiannis, G.; Losada-Pérez, P. Supported Lipid Membranes at the Au-Buffer Interface by Solvent Exchange: The Effect of Initial Solvent Concentration. Phys. Status Solidi A 2020, 217, 1900837. [Google Scholar] [CrossRef]
- Neupane, S.; Betlem, K.; Renner, F.U.; Losada-Pérez, P. Solvent-Assisted Lipid Bilayer Formation on Au Surfaces: Effect of Lipid Concentration on Solid-Supported Membrane Formation. Phys. Status Solidi A 2021, 218, 2000662. [Google Scholar] [CrossRef]
- Di Leone, S.; Kyropoulou, M.; Köchlin, J.; Wehr, R.; Meier, W.P.; Palivan, C.G. Tailoring a Solvent-Assisted Method for Solid-Supported Hybrid Lipid–Polymer Membranes. Langmuir 2022, 38, 6561–6570. [Google Scholar] [CrossRef] [PubMed]
- Di Leone, S.; Vallapurackal, J.; Yorulmaz Avsar, S.; Kyropolou, M.; Ward, T.R.; Palivan, C.G.; Meier, W. Expanding the Potential of the Solvent-Assisted Method to Create Bio-Interfaces from Amphiphilic Block Copolymers. Biomacromolecules 2021, 22, 3005–3016. [Google Scholar] [CrossRef] [PubMed]
- Cawley, J.L.; Santa, D.E.; Singh, A.N.; Odudimu, A.T.; Berger, B.A.; Wittenberg, N.J. Chaotropic Agent-Assisted Supported Lipid Bilayer Formation. Langmuir 2024. [Google Scholar] [CrossRef]
- Méléard, P.; Gerbeaud, C.; Pott, T.; Fernandez-Puente, L.; Bivas, I.; Mitov, M.D.; Dufourcq, J.; Bothorel, P. Bending Elasticities of Model Membranes: Influences of Temperature and Sterol Content. Biophys. J. 1997, 72, 2616–2629. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Isarankura-Na-Ayudhya, C.; Tantimongcolwat, T.; Nantasenamat, C.; Galla, H.-J. EDTA-Induced Membrane Fluidization and Destabilization: Biophysical Studies on Artificial Lipid Membranes. Acta Biochim. Biophys. Sin. 2007, 39, 901–913. [Google Scholar] [CrossRef]
- Vazdar, K.; Tempra, C.; Olżyńska, A.; Biriukov, D.; Cwiklik, L.; Vazdar, M. Stealthy Player in Lipid Experiments? EDTA Binding to Phosphatidylcholine Membranes Probed by Simulations and Monolayer Experiments. J. Phys. Chem. B 2023, 127, 5462–5469. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Jackman, J.A.; Cho, N.-J. Investigating How Vesicle Size Influences Vesicle Adsorption on Titanium Oxide: A Competition between Steric Packing and Shape Deformation. Phys. Chem. Chem. Phys. 2017, 19, 2131–2139. [Google Scholar] [CrossRef]
- Demetzos, C. Differential Scanning Calorimetry (DSC): A Tool to Study the Thermal Behavior of Lipid Bilayers and Liposomal Stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef]
- Di Foggia, M.; Bonora, S.; Tinti, A.; Tugnoli, V. DSC and Raman Study of DMPC Liposomes in Presence of Ibuprofen at Different pH. J. Therm. Anal. Calorim. 2017, 127, 1407–1417. [Google Scholar] [CrossRef]
- Cho, N.-J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz Crystal Microbalance with Dissipation Monitoring of Supported Lipid Bilayers on Various Substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef]
- Keller, C.A.; Kasemo, B. Surface Specific Kinetics of Lipid Vesicle Adsorption Measured with a Quartz Crystal Microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of Lipid Vesicle Deposition on Solid Surfaces: A Combined QCM-D and AFM Study. Biophys. J. 2003, 85, 3035–3047. [Google Scholar] [CrossRef]
- Bibissidis, N.; Betlem, K.; Cordoyiannis, G.; Bonhorst, F.P.; Goole, J.; Raval, J.; Daniel, M.; Góźdź, W.; Iglič, A.; Losada-Pérez, P. Correlation between Adhesion Strength and Phase Behaviour in Solid-Supported Lipid Membranes. J. Mol. Liq. 2020, 320, 114492. [Google Scholar] [CrossRef]
- Yarrow, F.; Kuipers, B.W.M. AFM Study of the Thermotropic Behaviour of Supported DPPC Bilayers with and without the Model Peptide WALP23. Chem. Phys. Lipids 2011, 164, 9–15. [Google Scholar] [CrossRef]
- Gerelli, Y. Phase Transitions in a Single Supported Phospholipid Bilayer: Real-Time Determination by Neutron Reflectometry. Phys. Rev. Lett. 2019, 122, 248101. [Google Scholar] [CrossRef]
- Feng, Z.V.; Spurlin, T.A.; Gewirth, A.A. Direct Visualization of Asymmetric Behavior in Supported Lipid Bilayers at the Gel-Fluid Phase Transition. Biophys. J. 2005, 88, 2154–2164. [Google Scholar] [CrossRef]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Structural Impact of Cations on Lipid Bilayer Models: Nanomechanical Properties by AFM-Force Spectroscopy. Mol. Membr. Biol. 2014, 31, 17–28. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrič, M.; Bar, L.; Villanueva, M.E.; Losada-Pérez, P.; Iglič, A.; Novak, N.; Cordoyiannis, G. Assessing the Quality of Solvent-Assisted Lipid Bilayers Formed at Different Phases and Aqueous Buffer Media: A QCM-D Study. Sensors 2024, 24, 6093. https://doi.org/10.3390/s24186093
Lavrič M, Bar L, Villanueva ME, Losada-Pérez P, Iglič A, Novak N, Cordoyiannis G. Assessing the Quality of Solvent-Assisted Lipid Bilayers Formed at Different Phases and Aqueous Buffer Media: A QCM-D Study. Sensors. 2024; 24(18):6093. https://doi.org/10.3390/s24186093
Chicago/Turabian StyleLavrič, Marta, Laure Bar, Martin E. Villanueva, Patricia Losada-Pérez, Aleš Iglič, Nikola Novak, and George Cordoyiannis. 2024. "Assessing the Quality of Solvent-Assisted Lipid Bilayers Formed at Different Phases and Aqueous Buffer Media: A QCM-D Study" Sensors 24, no. 18: 6093. https://doi.org/10.3390/s24186093
APA StyleLavrič, M., Bar, L., Villanueva, M. E., Losada-Pérez, P., Iglič, A., Novak, N., & Cordoyiannis, G. (2024). Assessing the Quality of Solvent-Assisted Lipid Bilayers Formed at Different Phases and Aqueous Buffer Media: A QCM-D Study. Sensors, 24(18), 6093. https://doi.org/10.3390/s24186093







