Thyroid Screening Techniques via Bioelectromagnetic Sensing: Imaging Models and Analytical and Computational Methods
Abstract
:1. Introduction
2. Models and Techniques
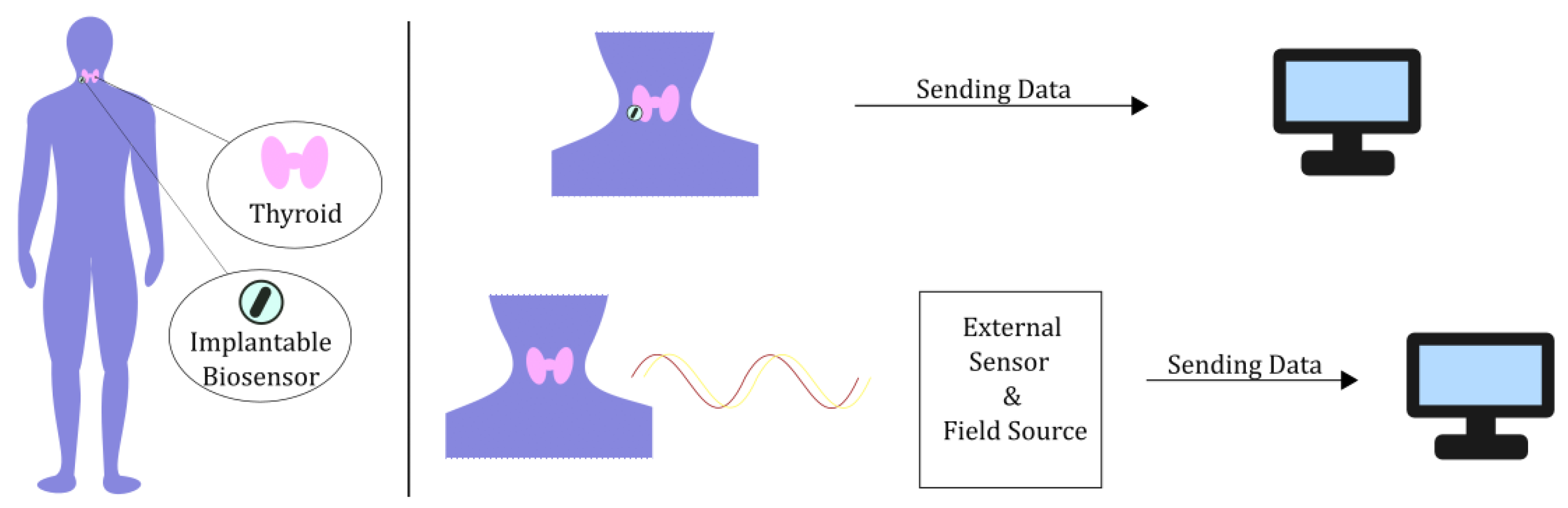
2.1. Bioelectromagnetic Models for Medical Imaging Applied to the Thyroid
2.1.1. Volumetric Models
- -
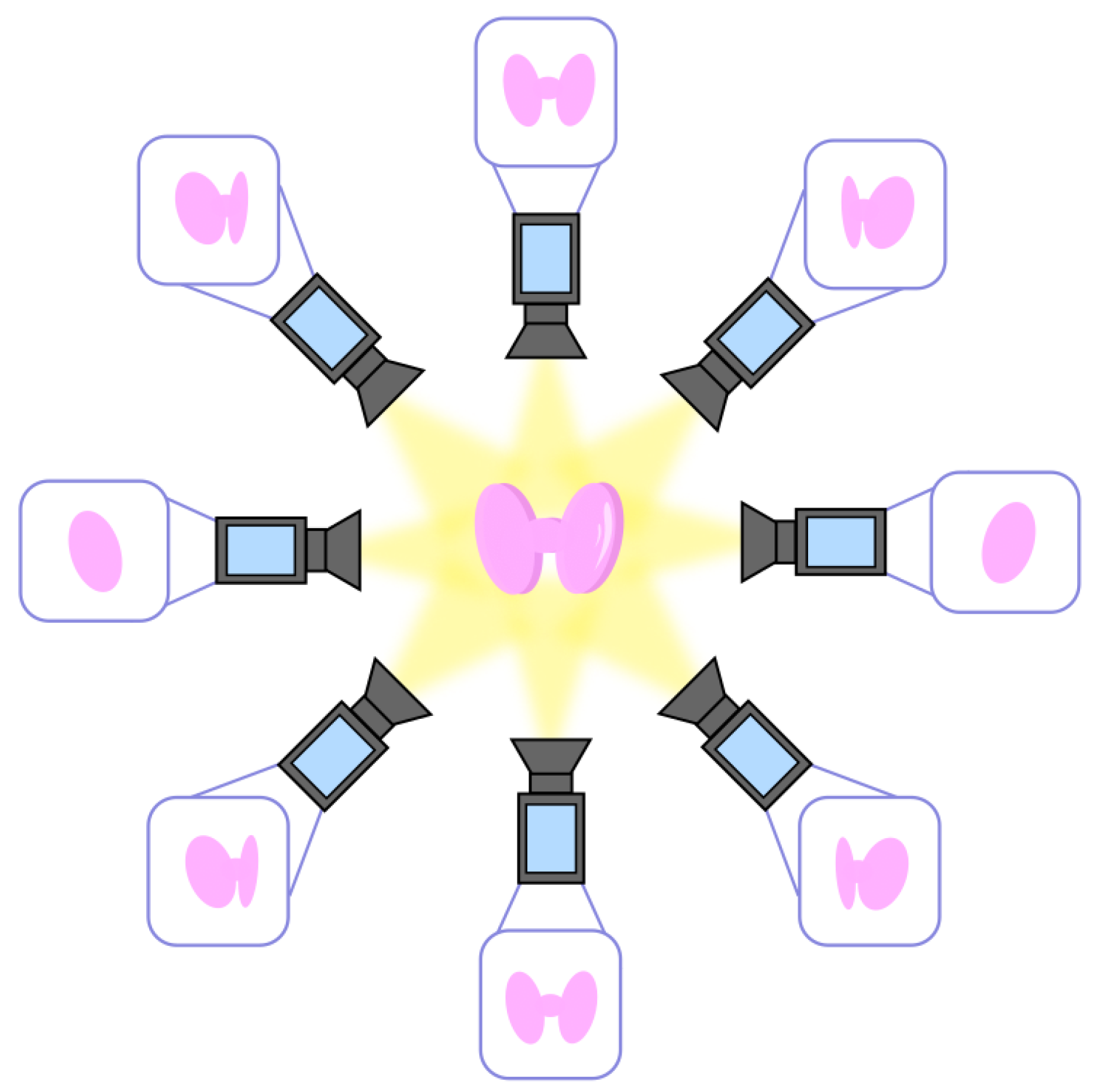
- Step 1: Multiple 2D images. Extraction of 2D images of the thyroid from multiple angles, as qualitatively depicted in Figure 3;
- -
- Step 2: Point correspondences. The point correspondences from multiple images are called tracks and are associated with 3D points of the thyroid. The computation of the tracks can be performed pairwise for two consecutive camera angles until all are fully described;
- -
- Step 3: Construction of the 3D model. The data are combined to create a 3D coordinate system, which leads to the complete 3D model of the thyroid.
2.1.2. Thermographic Models
2.1.3. Radiation Detection/Ablation Models
2.1.4. Model Attributes and Application Scope
2.1.5. Statistical Classification of the Related Bibliography
2.2. Models for the Analysis of Bioelectromagnetic Data
2.2.1. Analytical Methods
2.2.2. Computational Techniques
- -
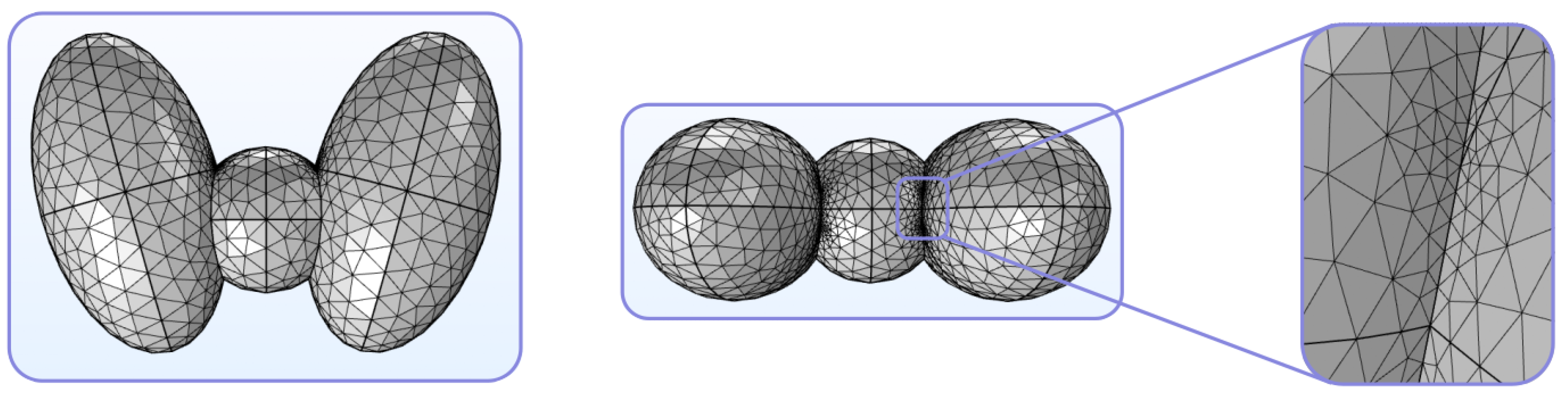
- Step 1: Discretization. The mesh of the thyroid is constructed, as depicted in Figure 8;
- -
- Step 2: Selection of shape functions and formulation of the finite element equations. A pattern is selected for the distribution of the unknown variables, and the suitable governing laws are applied to the general equation of every finite element, i.e.,where k is a square matrix of the characteristics of the homogeneous medium, q is a column vector of nodal values (output), and Q is a column vector that serves as the excitation (e.g., the field from an illuminating source);
- -
- Step 3: Assembly of the system of equations. With the equations of every finite element in the mesh appropriately derived, the global system of equations (modeling the entire problem) is constructed in the general form ofwhere K is the assembly (stiffness) matrix, r is the assembly vector of nodal degrees of freedom, and R is the assembly vector of nodal forcing parameters;
- -
- -
- Step 5: Interpretation of the results. The solution of the system leads to an output, which can then be post-processed accordingly in order to calculate several quantities, gauges, or metrics of the model.
2.2.3. Artificial Intelligence and Machine Learning
- -
- Step 1: Data generation. The core mesh is generated through the use of widely accepted parameters, accompanied by the necessary statistical bounds, limits, or ranges, and its overall biomechanical behavior is then investigated with the FEM. In this manner and via the appropriate changes and adaptations, instead of the application of the regular computational method to the electromagnetic data, ML schemes can be used to study the behavior of demanding biomedical systems or even suggest means for the improvement of their statistical performance;
- -
- Step 2: Splitting the data. The data are split into three sets for training, validation, and testing. This step may be repeated to increase the robustness of the overall system and serve as a trustworthy tool for safe cross-validation;
- -
- Step 3: Model training. Using the datasets obtained in step 2, the model is trained according to the initial assumptions or physical conventions;
- -
- Step 4: Performance evaluation. The performance metrics primarily utilized in ML approaches are the mean square error and the mean absolute error, which refer to the difference between the calculated and reference quantities of the bioelectromagnetic problem.
2.2.4. Model Characteristics and Limitations
- -
- Complex system modeling: They can manipulate systems that are too complicated for any analytical solution—a fact that is very frequently encountered in biomedical problems.
- -
- Parametric studies: The parameters and conditions of the problem can easily be varied in order to investigate a broad range of cases and applications, which is basically impossible in the majority of biomedical problems.
- -
- Incorporation of theoretical frameworks: They can be employed to verify theoretical formulations by offering numerical solutions to various demanding cases.
- -
- Computational overhead: High-fidelity simulations can require considerable computational power, RAM, and CPU time, which may not always be available.
- -
- Dependence on models: They are frequently based on certain conventions and approximations, which can reduce their universality and yield errors.
- -
- Algorithmic limitations: Their efficiency can be restrained by the methods available, whose inherent artifacts (e.g., numerical dispersion, dissipation, and anisotropy errors or lack of convergence) can deteriorate the levels of accuracy.
3. Summary and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiners, C.; Drozd, V.; Yamashita, S. Hypothyroidism after radiation exposure: Brief narrative review. J. Neural Transm. 2020, 127, 1455–1466. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of thyroid cancer. Cancer Epidemiol. Biomarkers Preven. 2022, 31, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Alkayyali, T.; Ochuba, O.; Srivastava, K.; Sandhu, J.K.; Joseph, C.; Ruo, S.W.; Jain, A.; Waqar, A.; Poudel, S. An exploration of the effects of radiofrequency radiation emitted by mobile phones and extremely low frequency radiation on thyroid hormones and thyroid gland histopathology. Cureus 2021, 13, e17329. [Google Scholar] [CrossRef] [PubMed]
- Zufry, H.; Rudijanto, A.; Soeatmadji, D.; Sakti, S.; Munadi, K.; Sujuti, H.; Mintaroem, K. Do electromagnetic fields significantly affect thyroid cells and their functions? A systematic review. F1000Research 2024, 13, 12. [Google Scholar] [CrossRef]
- Tuncal, S.; Önalan, A.; Aydoğan, F.; Unsal, V.; Yumuşak, N.; Büyükatalay, E.; Öztürk, G.; Seyhan, N.; Celepli, S.; Devrim, E.; et al. The effects of 2100 MHz radio frequency radiation on thyroid tissues. West Indian Med. J. 2021, 69, 51–55. [Google Scholar] [CrossRef]
- Muhammad, H.; Santhanam, P.; Russell, J.O. Radiofrequency ablation and thyroid nodules: Updated systematic review. Endocrine 2021, 72, 619–632. [Google Scholar] [CrossRef]
- Rahimnia, R.; Shabestari, A.N.; Khoshchehreh, M.; Aghaii, M.; Mesbah, G.; Zahmatkesh, P.; Zareian Baghdadabad, L.; Noori, N.; Khajavi, A.; Yarandi, V.A. Comprehensive study about mobile phone radiation effects on body weight and body composition. Transl. Res. Urol. 2023, 5, 33–40. [Google Scholar] [CrossRef]
- Kruger, E.; Toraih, E.A.; Hussein, M.H.; Shehata, S.A.; Waheed, A.; Fawzy, M.S.; Kandil, E. Thyroid carcinoma: A review for 25 years of environmental risk factors studies. Cancers 2022, 14, 6172. [Google Scholar] [CrossRef]
- Rai, G.; Kumar, A.; Mahobiya, P. The effect of radiation on thyroid gland. Int. J. Biol. Res. 2018, 3, 217–222. Available online: https://www.biologyjournal.in/archives/2018/vol3/issue1 (accessed on 17 August 2024).
- Héroux, P.; Belyaev, I.; Chamberlin, K.; Dasdag, S.; De Salles, A.A.A.; Rodriguez, C.E.F.; Hardell, L.; Elizabeth Kelley, E.; Kesari, K.K.; Erica Mallery-Blythe, E.; et al. Cell phone radiation exposure limits and engineering solutions. Int. J. Environ. Res. Public Health 2023, 20, 5398. [Google Scholar] [CrossRef]
- Huang, S.; Cai, W.; Han, S.; Lin, Y.; Wang, Y.; Chen, F.; Shao, G.; Liu, Y.; Yu, X.; Cai, Z.; et al. Differences in the dielectric properties of various benign and malignant thyroid nodules. Med. Phys. 2021, 48, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Saenko, V.; Mitsutake, N. Radiation-related thyroid cancer. Endocrine Rev. 2024, 45, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid cancer. Lancet 2023, 401, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lee, S.M.; Lee, N.Y.; Ko, B.C.; Kim, H.; Jang, D.J.; Lee, J.H. High-resolution tactile-sensation diagnostic imaging system for thyroid cancer. Sensors 2023, 23, 3451. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, M.; Koppel, T.; Hedendahl, L.K.; Hardell, L. Is the increasing incidence of thyroid cancer in the Nordic countries caused by use of mobile phones? Int. J. Environ. Res. Public Health 2020, 17, 9129. [Google Scholar] [CrossRef]
- Zufry, H.; Rudijanto, A.; Soeatmadji, D.W.; Sakti, S.P.; Munadi, K.; Sujuti, H.; Mintaroem, K. A study protocol for investigating the effects of mobile phone-originated electromagnetic waves on thyroid gland and thyroid hormone activities in the brain. F1000Research 2023, 12, 132. [Google Scholar] [CrossRef]
- Hasbek, Z.; Taş, A.; Ertürk, S.A.; Sarıakçalı, B.; Babacan, Ö.U.; Duman, G.; Siliğ, Y. Evaluation of the relationship between mobile phone usage and miRNA-574-5p and miRNA-30C-5p levels in thyroid cancer patients. Mol. Imaging Radionucl. Ther. 2024, 33, 19–27. [Google Scholar] [CrossRef]
- Naglah, A.; Khalifa, F.; Khaled, R.; Abdel Razek, A.A.K.; Ghazal, M.; Giridharan, G.; El-Baz, A. Novel MRI-based CAD system for early detection of thyroid cancer using multi-input CNN. Sensors 2021, 21, 3878. [Google Scholar] [CrossRef]
- Traylor, K.S. Computed tomography and MR imaging of thyroid disease. Radiol. Clin. 2020, 58, 1059–1070. [Google Scholar] [CrossRef]
- Bonjoc, K.J.; Young, H.; Warner, S.; Gernon, T.; Maghami, E.; Chaudhry, A. Thyroid cancer diagnosis in the era of precision imaging. J. Thorac. Dis. 2020, 12, 5128. [Google Scholar] [CrossRef]
- Zampella, E.; Klain, M.; Pace, L.; Cuocolo, A. PET/CT in the management of differentiated thyroid cancer. Diagn. Interv. Imaging 2021, 102, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Bafaraj, S.M. Assessment of sensitivity, specificity, and accuracy of nuclear medicines, CT scan, and ultrasound in diagnosing thyroid disorders. Cur. Med. Imaging 2020, 16, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kiourti, A. RFID antennas for body-area applications: From wearables to implants. IEEE Antennas Propag. Mag. 2018, 60, 14–25. [Google Scholar] [CrossRef]
- Rathod, K.; Gandhi, T.; Harne, V.; Bhagywant, S.; Mulaparti, M.K.; Dalal, P. Design and optimization of non-woven polyester made U shaped flexible patch antenna for early detection of thyroid cancer. In Proceedings of the IEEE Wireless Antenna and Microwave Symposium (WAMS), Visakhapatnam, India, 29 February–3 March 2024; pp. 1–5. [Google Scholar] [CrossRef]
- Badawi, M.I.; Ismail, N.H.; Shams-Eldin, R.S.; Mohamed, E.I. Modeling and simulation of a microwave super-lens array for hyperthermia treatment of thyroid cancer. In Proceedings of the International Telecommunications Conference (ITC-Egypt), Alexandria, Egypt, 26–28 July 2022; pp. 1–7. [Google Scholar] [CrossRef]
- Patnaik, P.K.; Malijeddi, M.; Panda, D.C. Wearable microstrip patch antenna for disease detection and WiMAX application. In Proceedings of the 2nd International Conference on Range Technology (ICORT), Chandipur, Balasore, India, 5–6 August 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Abbas, A.M.; Kumar, P.D.; Raghulraj, D.; Suraj, K. Design and implementation of a flexible wearable antenna for the detection of cancer in the thyroid gland. In Proceedings of the 2nd International Conference on Smart Technologies and Systems for Next Generation Computing (ICSTSN), Villupuram, India, 21–22 April 2023; pp. 1–5. [Google Scholar] [CrossRef]
- Jenisha, J.; Kumar, K.M. Design of H-shape microstrip patch antenna for wearable applications to detect the thyroid gland cancer cells. ICTACT J. Microelectron. 2020, 29, 928–933. Available online: https://ictactjournals.in/paper/IJME_Vol_6_Iss_2_Paper_3_928_933.pdf (accessed on 15 July 2024).
- Varma, D.R.; Murali, M.; Krishna, M.V.; Raju, G. Miniaturized Novel Textile Antenna for Biomedical Applications. In Proceedings of the 2nd International Conference on Paradigm Shifts in Communications Embedded Systems, Machine Learning and Signal Processing (PCEMS), Nagpur, India, 5–6 April 2023; pp. 1–6. [Google Scholar] [CrossRef]
- De Vita, E.; De Tommasi, F.; Altomare, C.; Ialongo, S.; Massaroni, C.; Presti, D.L.; Faiella, E.; Andresciani, F.; Pacella, G.; Palermo, A.; et al. Fiber bragg gratings for temperature monitoring during thyroid microwave ablation: A preliminary analysis. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- De Vita, E.; De Tommasi, F.; Altomare, C.; Presti, D.L.; Pacella, G.; Iadicicco, A.; Carassiti, M.; Grasso, R.F.; Massaroni, C.; Campopiano, S.; et al. Thyroid microwave ablation study based on fiber Bragg gratings thermal mapping. IEEE J. Electromagn. RF Microw. Med. Biol. 2024, 8, 26–35. [Google Scholar] [CrossRef]
- Bahramian, F.; Mojra, A. Analysis of thyroid thermographic images for detection of thyroid tumor: An experimental-numerical study. Int. J. Numer. Method. Biomed. Eng. 2019, 35, e3192. [Google Scholar] [CrossRef]
- Salaam, A.; Danjem, S.; Salaam, A.; Angba, H.; Ibinaiye, P. Determination of relationship between thyroid gland volume and anthropometric indices. J. Adv. Med. Med. Res. 2019, 31, 1–12. [Google Scholar] [CrossRef]
- Abreu de Souza, M.; Alka Cordeiro, D.C.; de Oliveira, J.; de Oliveira, M.F.A.; Bonafini, B.L. 3D multi-modality medical imaging: Combining anatomical and infrared thermal images for 3D reconstruction. Sensors 2023, 23, 1610. [Google Scholar] [CrossRef]
- Wang, H.; Yu, D.; Tan, Z.; Hu, R.; Zhang, B.; Yu, J. Estimation of thyroid volume from scintigraphy through 2D/3D registration of a statistical shape model. Phys. Med. Biol. 2019, 64, 095015. [Google Scholar] [CrossRef]
- Yeom, Y.S.; Choi, C.; Shin, B.; Kim, S.; Han, H.; Moon, S.; Son, G.; Kim, H.; Nguyen, T.T.; Chung, B.S.; et al. New thyroid models for ICRP pediatric mesh-type reference computational phantoms. Nucl. Eng. Technol. 2022, 54, 4698–4707. [Google Scholar] [CrossRef]
- Bahramian, F.; Mojra, A. Thermal imaging of the human neck for thyroid gland detection based on CT scan images. In Proceedings of the 24th National and 2nd International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 30 November–1 December 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Paz, A.A.C.; Fiorin, R.; de Oliveira, M.F.A.; de Souza, M.A. 3D thermal models: A case study of the neck. Int. J. Devel. Res. 2020, 10, 40705–40710. Available online: https://mail.journalijdr.com/3dthermal-models-case-study-neck (accessed on 20 May 2024).
- de Camargo, V.M.B.; Ulbricht, L.; Coninck, J.C.P.; Ripka, W.L.; Gamba, H.R. Thermography as an aid for the complementary diagnosis of nodules in the thyroid gland. BioMed. Eng. OnLine 2022, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Damião, C.; Montero, J.; Moran, M.; da Cruz Filho, R.; Fontes, C.; Lima, G.; Conci, A. On the possibility of using temperature to aid in thyroid nodule investigation. Sci. Rep. 2020, 10, 21010. [Google Scholar] [CrossRef] [PubMed]
- Faisal Abdulkareem, A.; Qusai Hashim, A. Infrared medical thermography, medical applications, and its basic principles: A review. In Proceedings of the BIO Web of Conferences: 5th International Scientific Conference of Alkafeel University (ISCKU), Al-Najaf, Iraq, 17–18 February 2024; Volume 97, p. 00140. [Google Scholar] [CrossRef]
- Helmy, A.; Holdmann, M.; Rizkalla, M. Application of thermography for non-invasive diagnosis of thyroid gland disease. IEEE Trans. Biomed. Eng. 2008, 55, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, J.; Tilbury, W.; Helmy, A.; Suryadevara, V.K.; Rizkalla, M.; Holdmann, M.M. Computer simulation/practical models for human thyroid thermographic imaging. J. Biomed. Sci. Eng. 2015, 8, 246–256. [Google Scholar] [CrossRef]
- Paz, A.A.C.; de Souza, M.A.; Brock, P.W.; Mercuri, E.G.F. Finite element analysis to predict temperature distribution in the human neck with abnormal thyroid: A proof of concept. Comput. Methods Programs Biomed. 2022, 227, 107234. [Google Scholar] [CrossRef]
- Yadav, A.; Ali, I.; Helmy, A.; Rizkalla, M. High sensitive graphene devices for non-invasive early diagnosis of hyperthyroidism: A feasibility study. J. Biomed. Sci. Eng. 2019, 12, 522–532. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, G. Electromagnetic-thermal analysis of the effect of microwave ablation of thyroid nodules. In Proceedings of the Photonics & Electromagnetics Research Symposium (PIERS), Hangzhou, China, 21–25 November 2021; pp. 2425–2432. [Google Scholar] [CrossRef]
- González, J.R.; Damião, C.; Moran, M.; Pantaleão, C.A.; Cruz, R.A.; Balarini, G.A.; Conci, A. A computational study on the role of parameters for identification of thyroid nodules by infrared images (and comparison with real data). Sensors 2021, 21, 4459. [Google Scholar] [CrossRef]
- Stauffer, P.R.; Rodrigues, D.B.; Maccarini, P.F. Utility of microwave radiometry for diagnostic and therapeutic applications of non-invasive temperature monitoring. In Proceedings of the IEEE Benjamin Franklin Symposium on Microwave and Antenna Sub-systems for Radar, Telecommunications, and Biomedical Applications (BenMAS), Philadelphia, PA, USA, 26 September 2014; pp. 1–3. [Google Scholar] [CrossRef]
- Ahmed, F.H. Propagation characteristics of electromagnetic waves in multilayered biological human tissue. Zanco J. Pure Appl. Sci. 2021, 33, 18–29. [Google Scholar] [CrossRef]
- Baskaran, D.; Arunachalam, K. Design of site-specific microwave phased array hyperthermia applicators using 434 MHz reduced cavity-backed patch antenna. Bioelectromagn. 2020, 41, 630–648. [Google Scholar] [CrossRef]
- Gavriloaia, G.; Serban, G.; Sofron, E.; Gavriloaia, M.R.; Ghemiogean, A.M. Evaluation of microwave electromagnetic field absorbed by human thyroid gland. In Proceedings of the IEEE 16th International Symposium for Design and Technology in Electronic Packaging (SIITME), Pitesti, Romania, 23–26 September 2010; pp. 43–46. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, F.; Giusti, A.; Leoncini, A.; Trimboli, P. Model-optimizing radiofrequency parameters of 3D finite element analysis for ablation of benign thyroid nodules. Bioengineering 2023, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; He, Z.; Liu, J. MRI-based finite element simulation on radiofrequency ablation of thyroid cancer. Comput. Methods Programs Biomed. 2014, 113, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wu, X.Y. Study of specific absorption rate (SAR) induced in human endocrine glands for using mobile phones. In Proceedings of the Asia-Pacific International Symposium on Electromagnetic Compatibility (APEMC), Shenzhen, China, 17–21 May 2016; Volume 1, pp. 1084–1086. [Google Scholar] [CrossRef]
- Sediq, H.T.; Nourinia, J.; Ghobadi, C.; Mohammadi, B. A novel shaped ultrawideband fractal antenna for medical purposes. Biomed. Signal Process. Control. 2023, 80, 104363. [Google Scholar] [CrossRef]
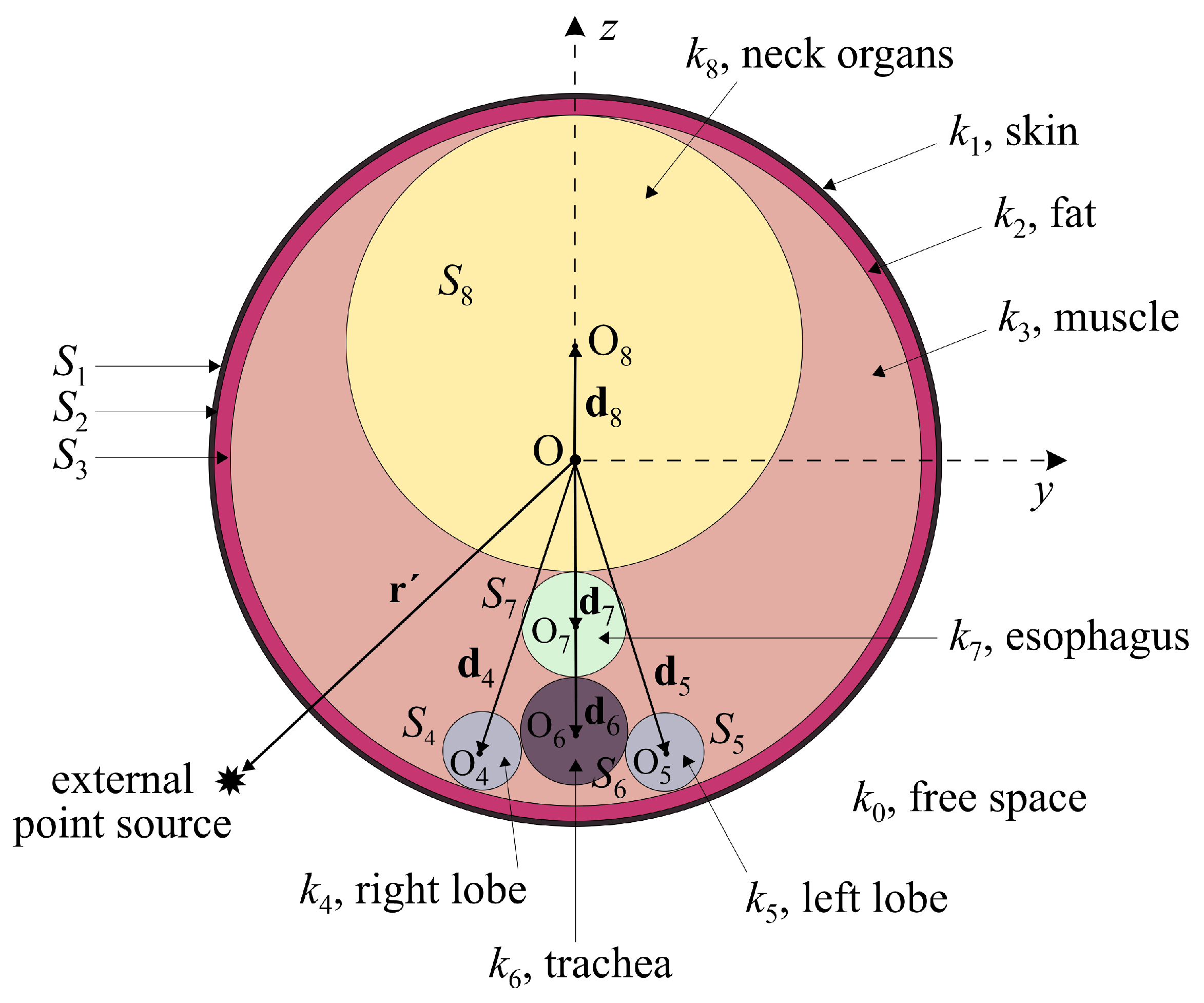
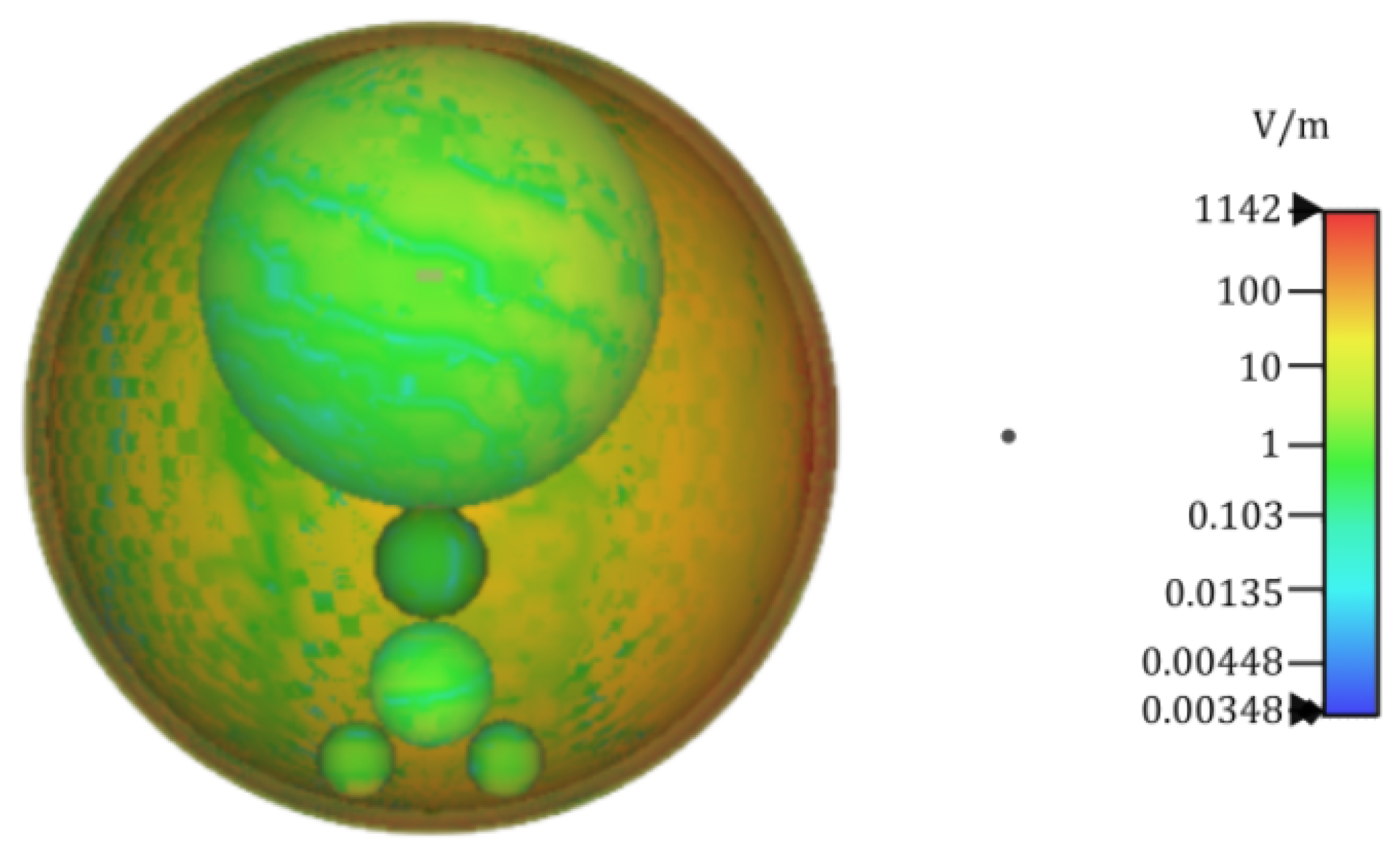
- Varvari, A.; Karatzidis, D.; Kantartzis, N.V. Electric field evaluation due to an external point source near a non-spherical human neck model. In Proceedings of the 26th International Workshop on Electromagnetic Nondestructive Evaluation (ENDE), Thessaloniki, Greece, 28–30 June 2023; pp. 1–4. Available online: https://www.ende2023.gr/ (accessed on 22 June 2024).
- Varvari, A.A.; Karatzidis, D.I.; Ohtani, T.; Kanai, Y.; Kantartzis, N.V. Impact evaluation of an external point source to a generalized model of the human neck. In Proceedings of the Applied Computational Electromagnetics Society Symposium (ACES), Monterey/Seaside, CA, USA, 26–30 March 2023. [Google Scholar] [CrossRef]
- Tai, C.T. Dyadic Green’s Functions in Electromagnetic Theory; IEEE Press: New York, NY, USA, 1993. [Google Scholar]
- Jin, J.M. The Finite Element Method in Electromagnetics, 3rd ed.; Wiley-IEEE Press: New York, NY, USA, 2014. [Google Scholar]
- Cardoso, J.R. Electromagnetics through the Finite Element Method: A Simplified Approach Using Maxwell’s Equations; CRC Press: New York, NY, USA, 2016. [Google Scholar]
- COMSOL Multiphysics®; COMSOL AB: Stockholm, Sweden, 2022.
- Perumal, L.; Mon, D.T.T. Finite elements for engineering analysis: A brief review. In Proceedings of the International Conference on Modeling, Simulation and Control (ICMSC), Singapore, 15–17 June 2011; Volume 10, pp. 60–68. Available online: https://www.semanticscholar.org/paper/Finite-Elements-for-Engineering-Analysis%3A-A-Brief-Perumal-Mon/216dd6de4e2dbacf050d0f6adf18747c9f9559c5 (accessed on 12 June 2024).
- Chang, C.Y.; Chung, P.C.; Hong, Y.C.; Tseng, C.h. A neural network for thyroid segmentation and volume estimation in CT images. IEEE Comput. Intell. Mag. 2011, 6, 43–55. [Google Scholar] [CrossRef]
- Sharafeldeen, A.; Elsharkawy, M.; Khaled, R.; Shaffie, A.; Khalifa, F.; Soliman, A.; Abdel Razek, A.A.k.; Hussein, M.M.; Taman, S.; Naglah, A.; et al. Texture and shape analysis of diffusion-weighted imaging for thyroid nodules classification using machine learning. Med. Phys. 2022, 49, 988–999. [Google Scholar] [CrossRef]
- Bahramian, F.; Mojra, A. Thyroid cancer estimation using infrared thermography data. Infrared Phys. Techn. 2020, 104, 103126. [Google Scholar] [CrossRef]
- Guo, R.; Huang, T.; Li, M.; Zhang, H.; Eldar, Y.C. Physics-embedded machine learning for electromagnetic data imaging: Examining three types of data-driven imaging methods. IEEE Signal Process. Mag. 2023, 40, 18–31. [Google Scholar] [CrossRef]
- Yao, H.M.; Jiang, L.; Zhang, H.H.; Wei, E. Machine learning methodology review for computational electromagnetics. In Proceedings of the 2019 International Applied Computational Electromagnetics Society Symposium-China (ACES), Nanjing, China, 8–11 August 2019; Volume 1, pp. 1–4. [Google Scholar] [CrossRef]
- Phellan, R.; Hachem, B.; Clin, J.; Mac-Thiong, J.M.; Duong, L. Real-time biomechanics using the finite element method and machine learning: Review and perspective. Med. Phys. 2021, 48, 7–18. [Google Scholar] [CrossRef]
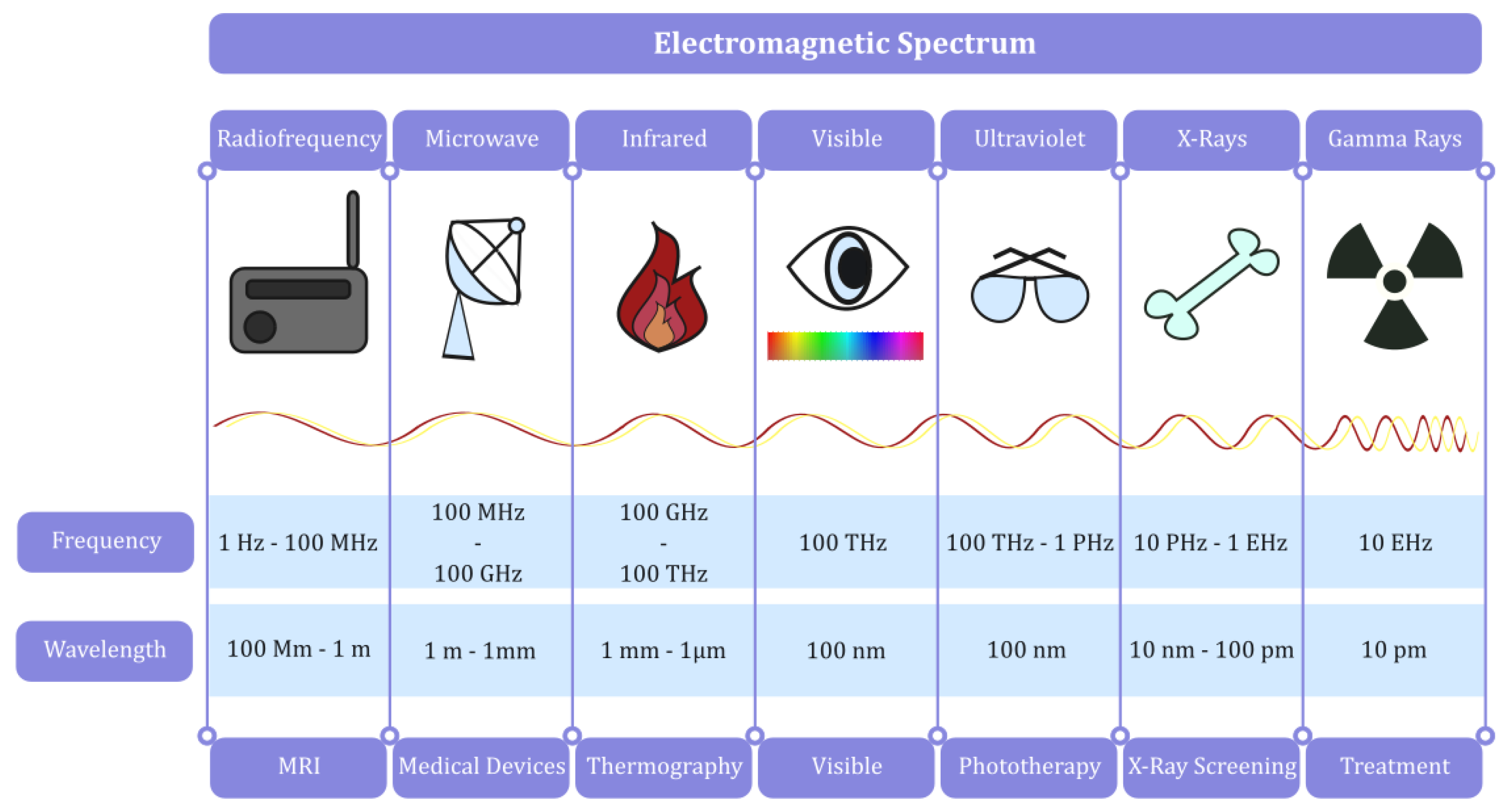


| Screening Technique | Operational Spectrum | Applications |
|---|---|---|
| Magnetic Resonance Imaging (MRI) | Radio frequency | Volumetric models and tissue property assessment |
| Computer Tomography (CT) | X-ray | Volumetric models and tissue property assessment |
| Positron Emission Tomography (PET) | Gamma rays | Volumetric models and tissue property assessment |
| X-Ray | X-ray | Volumetric models and tissue property assessment |
| Thermal Imaging | Infrared | Volumetric models and thermal mapping |
| Device | Measured Parameter | Advantages | Disadvantages |
|---|---|---|---|
| Thermocouple | Voltage | Simplicity, durability, affordability, diversity, self-powered, wide temperature range | Non linearity, low voltage signal, lowest sensitivity, lowest stability reference |
| Resistance temperature detector | Resistance | Accuracy, stability, linearity | Expensiveness, self heating, low output signal, low absolute resistance, current source required |
| Thermistor | Resistance | High sensitivity, fast response time, high output signal, 2-wire Ohms measurement | Self heating, non linearity, narrow temperature range, current source required, fragility |
| Liquid crystal sensor | Color change | Simplicity, durability, resistance sensitivity | Narrow temperature range, low response time, limited number of possible configurations |
| Method | Source | Frequency | Objective |
|---|---|---|---|
| Volumetry | [32,34,35,36] | Infrared and X-ray | Reference and diagnostic |
| Thermography | [32,37,42,43,44,45,46,47] | Infrared, microwave, and X-ray | Reference, diagnostic, and therapeutic |
| Radiation | [25,27,28,50,51,52,53,54,55] | RF and microwave | Reference, diagnostic, and therapeutic |
| Model | Applications | Advantages | Disadvantages |
|---|---|---|---|
| Analytical | Diagnostics and reference | Simplicity, speed, computational affordability, and generality | Not case-sensitive |
| Computational | Diagnostics and reference | Accuracy and generality | Computationally expensive and slow |
| Artificial Intelligence | Diagnostics | Case sensitivity, accuracy, and speed | Computational expensive and lack of generality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varvari, A.A.; Pitilakis, A.; Karatzidis, D.I.; Kantartzis, N.V. Thyroid Screening Techniques via Bioelectromagnetic Sensing: Imaging Models and Analytical and Computational Methods. Sensors 2024, 24, 6104. https://doi.org/10.3390/s24186104
Varvari AA, Pitilakis A, Karatzidis DI, Kantartzis NV. Thyroid Screening Techniques via Bioelectromagnetic Sensing: Imaging Models and Analytical and Computational Methods. Sensors. 2024; 24(18):6104. https://doi.org/10.3390/s24186104
Chicago/Turabian StyleVarvari, Anna A., Alexandros Pitilakis, Dimitrios I. Karatzidis, and Nikolaos V. Kantartzis. 2024. "Thyroid Screening Techniques via Bioelectromagnetic Sensing: Imaging Models and Analytical and Computational Methods" Sensors 24, no. 18: 6104. https://doi.org/10.3390/s24186104







