Validation of a 3D Markerless Motion Capture Tool Using Multiple Pose and Depth Estimations for Quantitative Gait Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup and Procedure
2.3. Data Recording and Processing
2.3.1. Marker-Based System (MOCAP)
2.3.2. Markerless System (3D MMC)
2.4. Data Analysis and Statistical Calculation
3. Results
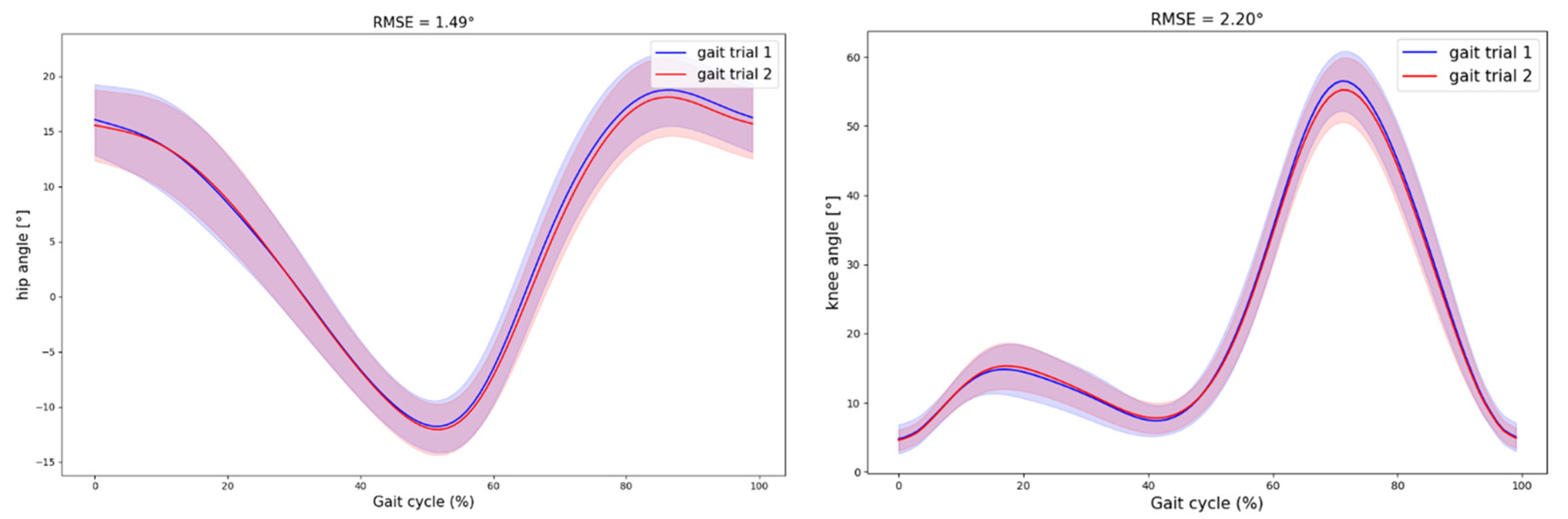
3.1. Inter-Session Reliability
3.2. Accuracy Against MOCAP
3.2.1. Non-Corrected Joint Angles
3.2.2. Corrected Joint Angles
| Joint (Sagittal Plan) | Speed [m/s] | Max | Min | ROM | RMSE [°] (SD) | LCC (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|
| B-A Bias [°] (LoA) | r | B-A Bias [°] (LoA) | r | B-A Bias [°] (LoA) | r | ||||
| Knee | 0.7 | 5.04 (−2.47 12.56) | 0.58 | −2.54 (−5.39 0.30) | 0.90 | 7.59 (−2.05 17.23) | 0.50 | 4.10 (1.58) | 0.96 (0.94 0.98) |
| 1 | 3.86 (−4.37 12.08) | 0.45 | −3.43 (−6.37 −0.48) | 0.91 | 7.28 (−2.83 17.39) | 0.54 | 4.14 (1.56) | 0.97 (0.95 0.98) | |
| 1.3 | 2.46 (−3.72 8.63) | 0.73 | −3.90 (−6.34 −1.47) | 0.94 | 6.36 (−0.95 13.67) | 0.65 | 4.85 (1.33) | 0.96 (0.95 0.97) | |
| Hip | 0.7 | 4.23 (2.34 6.12) | 0.96 | −5.19 (−7.67 −2.72) | 0.95 | 9.42 (5.61 13.23) | 0.81 | 4.14 (0.95) | 0.91 (0.88 0.95) |
| 1 | 4.49 (2.10 6.89) | 0.94 | −6.33 (−9.75 −2.92) | 0.89 | 10.82 (5.6 16.05) | 0.53 | 4.73 (0.92) | 0.91 (0.89 0.93) | |
| 1.3 | 5.64 (2.38 8.90) | 0.89 | −7.03 (−10.73 −3.33) | 0.86 | 12.67 (6.83 18.51) | 0.52 | 5.40 (0.84) | 0.91 (0.90 0.93) |
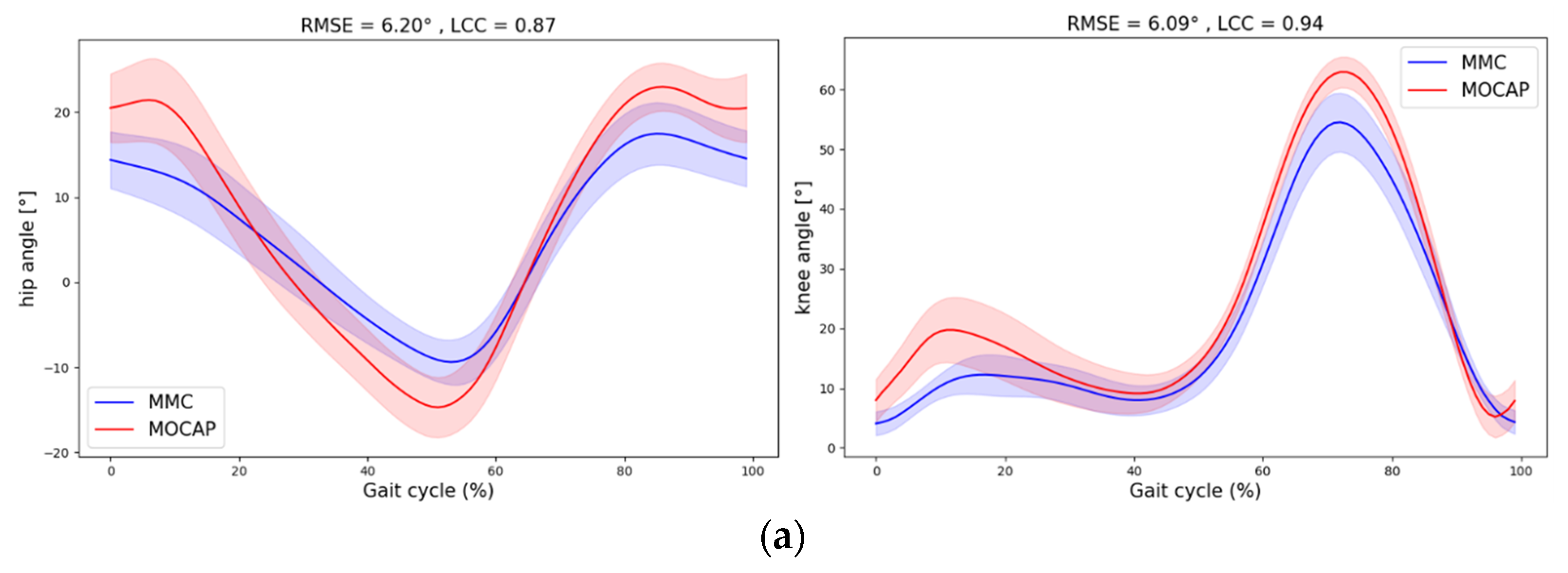

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christopher, A.; Kraft, E.; Olenick, H.; Kiesling, R.; Doty, A. The Reliability and Validity of the Timed up and Go as a Clinical Tool in Individuals with and without Disabilities across a Lifespan: A Systematic Review: Psychometric Properties of the Timed up and Go. Disabil. Rehabil. 2021, 43, 1799–1813. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, C.J. The Reliability and Validity of a 6-Minute Walk Test as a Measure of Physical Endurance in Older Adults. J. Aging Phys. Act. 1998, 6, 363–375. [Google Scholar] [CrossRef]
- Benedetti, M.; Agostini, V.; Knaflitz, M.; Gasparroni, V.; Boschi, M.; Piperno, R. Self-Reported Gait Unsteadiness in Mildly Impaired Neurological Patients: An Objective Assessment through Statistical Gait Analysis. J. Neuroeng. Rehabil. 2012, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Nebel, M.B.; Sims, E.L.; Keefe, F.J.; Kraus, V.B.; Guilak, F.; Caldwell, D.S.; Pells, J.J.; Queen, R.; Schmitt, D. The Relationship of Self-Reported Pain and Functional Impairment to Gait Mechanics in Overweight and Obese Persons With Knee Osteoarthritis. Arch. Phys. Med. Rehabil. 2009, 90, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Dierick, F.; Stoffel, P.-L.; Schütz, G.; Buisseret, F. High Specificity of Single Inertial Sensor-Supplemented Timed up and Go Test for Assessing Fall Risk in Elderly Nursing Home Residents. Sensors 2022, 22, 2339. [Google Scholar] [CrossRef]
- Kurosawa, C.; Shimadu, N.; Yamamoto, S. Where Do Healthy Older Adults Take More Time during the Timed up and Go Test? J. Phys. Ther. Sci. 2020, 32, 663–668. [Google Scholar] [CrossRef]
- Baker, R. Gait Analysis Methods in Rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 4. [Google Scholar] [CrossRef]
- Camomilla, V.; Bonci, T.; Cappozzo, A. Soft Tissue Displacement over Pelvic Anatomical Landmarks during 3-D Hip Movements. J. Biomech. 2017, 62, 14–20. [Google Scholar] [CrossRef]
- Leigh, R.J.; Pohl, M.B.; Ferber, R. Does Tester Experience Influence the Reliability with Which 3D Gait Kinematics Are Collected in Healthy Adults? Phys. Ther. Sport 2014, 15, 112–116. [Google Scholar] [CrossRef]
- Stagni, R.; Fantozzi, S.; Cappello, A.; Leardini, A. Quantification of Soft Tissue Artefact in Motion Analysis by Combining 3D Fluoroscopy and Stereophotogrammetry: A Study on Two Subjects. Clin. Biomech. 2005, 20, 320–329. [Google Scholar] [CrossRef]
- Toshev, A.; Szegedy, C. DeepPose: Human Pose Estimation via Deep Neural Networks. In Proceedings of the 2014 IEEE Conference on Computer Vision and Pattern Recognition, Columbus, OH, USA, 23–28 June 2014; pp. 1653–1660. [Google Scholar]
- Cao, Z.; Hidalgo, G.; Simon, T.; Wei, S.-E.; Sheikh, Y. OpenPose: Realtime Multi-Person 2D Pose Estimation Using Part Affinity Fields 2019. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Long Beach, CA, USA, 15–20 June 2019. [Google Scholar]
- Haberkamp, L.D.; Garcia, M.C.; Bazett-Jones, D.M. Validity of an Artificial Intelligence, Human Pose Estimation Model for Measuring Single-Leg Squat Kinematics. J. Biomech. 2022, 144, 111333. [Google Scholar] [CrossRef] [PubMed]
- Ino, T.; Samukawa, M.; Ishida, T.; Wada, N.; Koshino, Y.; Kasahara, S.; Tohyama, H. Validity of AI-Based Gait Analysis for Simultaneous Measurement of Bilateral Lower Limb Kinematics Using a Single Video Camera. Sensors 2023, 23, 9799. [Google Scholar] [CrossRef]
- Ota, M.; Tateuchi, H.; Hashiguchi, T.; Kato, T.; Ogino, Y.; Yamagata, M.; Ichihashi, N. Verification of Reliability and Validity of Motion Analysis Systems during Bilateral Squat Using Human Pose Tracking Algorithm. Gait Posture 2020, 80, 62–67. [Google Scholar] [CrossRef]
- Kanko, R.M.; Laende, E.K.; Davis, E.M.; Selbie, W.S.; Deluzio, K.J. Concurrent Assessment of Gait Kinematics Using Marker-Based and Markerless Motion Capture. J. Biomech. 2021, 127, 110665. [Google Scholar] [CrossRef]
- Needham, L.; Evans, M.; Wade, L.; Cosker, D.P.; McGuigan, M.P.; Bilzon, J.L.; Colyer, S.L. The Development and Evaluation of a Fully Automated Markerless Motion Capture Workflow. J. Biomech. 2022, 144, 111338. [Google Scholar] [CrossRef] [PubMed]
- Ripic, Z.; Nienhuis, M.; Signorile, J.F.; Best, T.M.; Jacobs, K.A.; Eltoukhy, M. A Comparison of Three-Dimensional Kinematics between Markerless and Marker-Based Motion Capture in Overground Gait. J. Biomech. 2023, 159, 111793. [Google Scholar] [CrossRef]
- Albert, J.A.; Owolabi, V.; Gebel, A.; Brahms, C.M.; Granacher, U.; Arnrich, B. Evaluation of the Pose Tracking Performance of the Azure Kinect and Kinect v2 for Gait Analysis in Comparison with a Gold Standard: A Pilot Study. Sensors 2020, 20, 5104. [Google Scholar] [CrossRef] [PubMed]
- Gabel, M.; Gilad-Bachrach, R.; Renshaw, E.; Schuster, A. Full Body Gait Analysis with Kinect. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1964–1967. [Google Scholar]
- Hatamzadeh, M.; Busé, L.; Chorin, F.; Alliez, P.; Favreau, J.-D.; Zory, R. A Kinematic-Geometric Model Based on Ankles’ Depth Trajectory in Frontal Plane for Gait Analysis Using a Single RGB-D Camera. J. Biomech. 2022, 145, 111358. [Google Scholar] [CrossRef]
- Thomas, J.; Hall, J.B.; Bliss, R.; Guess, T.M. Comparison of Azure Kinect and Optical Retroreflective Motion Capture for Kinematic and Spatiotemporal Evaluation of the Sit-to-Stand Test. Gait Posture 2022, 94, 153–159. [Google Scholar] [CrossRef]
- Nguyen, M.-H.; Hsiao, C.-C.; Cheng, W.-H.; Huang, C.-C. Practical 3D Human Skeleton Tracking Based on Multi-View and Multi-Kinect Fusion. Multimed. Syst. 2022, 28, 529–552. [Google Scholar] [CrossRef]
- Liu, P.-L.; Chang, C.-C. Simple Method Integrating OpenPose and RGB-D Camera for Identifying 3D Body Landmark Locations in Various Postures. Int. J. Ind. Ergon. 2022, 91, 103354. [Google Scholar] [CrossRef]
- Meyer, C.; Killeen, T.; Easthope, C.S.; Curt, A.; Bolliger, M.; Linnebank, M.; Zörner, B.; Filli, L. Familiarization with Treadmill Walking: How Much Is Enough? Sci. Rep. 2019, 9, 5232. [Google Scholar] [CrossRef] [PubMed]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef]
- Maji, D.; Nagori, S.; Mathew, M.; Poddar, D. YOLO-Pose: Enhancing YOLO for Multi Person Pose Estimation Using Object Keypoint Similarity Loss. In Proceedings of the 2022 IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), New Orleans, LA, USA, 19–20 June 2022; pp. 2636–2645. [Google Scholar]
- Robert-Lachaine, X.; Parent, G.; Fuentes, A.; Hagemeister, N.; Aissaoui, R. Inertial Motion Capture Validation of 3D Knee Kinematics at Various Gait Speed on the Treadmill with a Double-Pose Calibration. Gait Posture 2020, 77, 132–137. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- McBride, G.B. A Proposal for Strength-of-Agreement Criteria for Lin’s Concordance Correlation Coefficient. NIWA Client Rep. HAM2005-062 2005, 45, 307–310. [Google Scholar]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The Reliability of Three-Dimensional Kinematic Gait Measurements: A Systematic Review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Balta, D.; Figari, G.; Paolini, G.; Pantzar-Castilla, E.; Riad, J.; Croce, U.D.; Cereatti, A. A Model-Based Markerless Protocol for Clinical Gait Analysis Based on a Single RGB-Depth Camera: Concurrent Validation on Patients with Cerebral Palsy. IEEE Access 2023, 11, 144377–144393. [Google Scholar] [CrossRef]
- Kanko, R.M.; Laende, E.; Selbie, W.S.; Deluzio, K.J. Inter-Session Repeatability of Markerless Motion Capture Gait Kinematics. J. Biomech. 2021, 121, 110422. [Google Scholar] [CrossRef]
- Needham, L.; Evans, M.; Cosker, D.P.; Wade, L.; McGuigan, P.M.; Bilzon, J.L.; Colyer, S.L. The Accuracy of Several Pose Estimation Methods for 3D Joint Centre Localisation. Sci. Rep. 2021, 11, 20673. [Google Scholar] [CrossRef] [PubMed]
- Nüesch, C.; Roos, E.; Pagenstert, G.; Mündermann, A. Measuring Joint Kinematics of Treadmill Walking and Running: Comparison between an Inertial Sensor Based System and a Camera-Based System. J. Biomech. 2017, 57, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Piche, E.; Guilbot, M.; Chorin, F.; Guerin, O.; Zory, R.; Gerus, P. Validity and Repeatability of a New Inertial Measurement Unit System for Gait Analysis on Kinematic Parameters: Comparison with an Optoelectronic System. Measurement 2022, 198, 111442. [Google Scholar] [CrossRef]
- Nakano, N.; Sakura, T.; Ueda, K.; Omura, L.; Kimura, A.; Iino, Y.; Fukashiro, S.; Yoshioka, S. Evaluation of 3D Markerless Motion Capture Accuracy Using OpenPose With Multiple Video Cameras. Front. Sports Act. Living 2020, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Collings, T.J.; Devaprakash, D.; Pizzolato, C.; Lloyd, D.G.; Barrett, R.S.; Lenton, G.K.; Thomeer, L.T.; Bourne, M.N. Inclusion of a Skeletal Model Partly Improves the Reliability of Lower Limb Joint Angles Derived from a Markerless Depth Camera. J. Biomech. 2024, 170, 112160. [Google Scholar] [CrossRef]
- Wishaupt, K.; Schallig, W.; Van Dorst, M.H.; Buizer, A.I.; Van Der Krogt, M.M. The Applicability of Markerless Motion Capture for Clinical Gait Analysis in Children with Cerebral Palsy. Sci. Rep. 2024, 14, 11910. [Google Scholar] [CrossRef]
- Busch, T.D.A.; Duarte, Y.A.; Pires Nunes, D.; Lebrão, M.L.; Satya Naslavsky, M.; Dos Santos Rodrigues, A.; Amaro, E. Factors Associated with Lower Gait Speed among the Elderly Living in a Developing Country: A Cross-Sectional Population-Based Study. BMC Geriatr. 2015, 15, 35. [Google Scholar] [CrossRef]

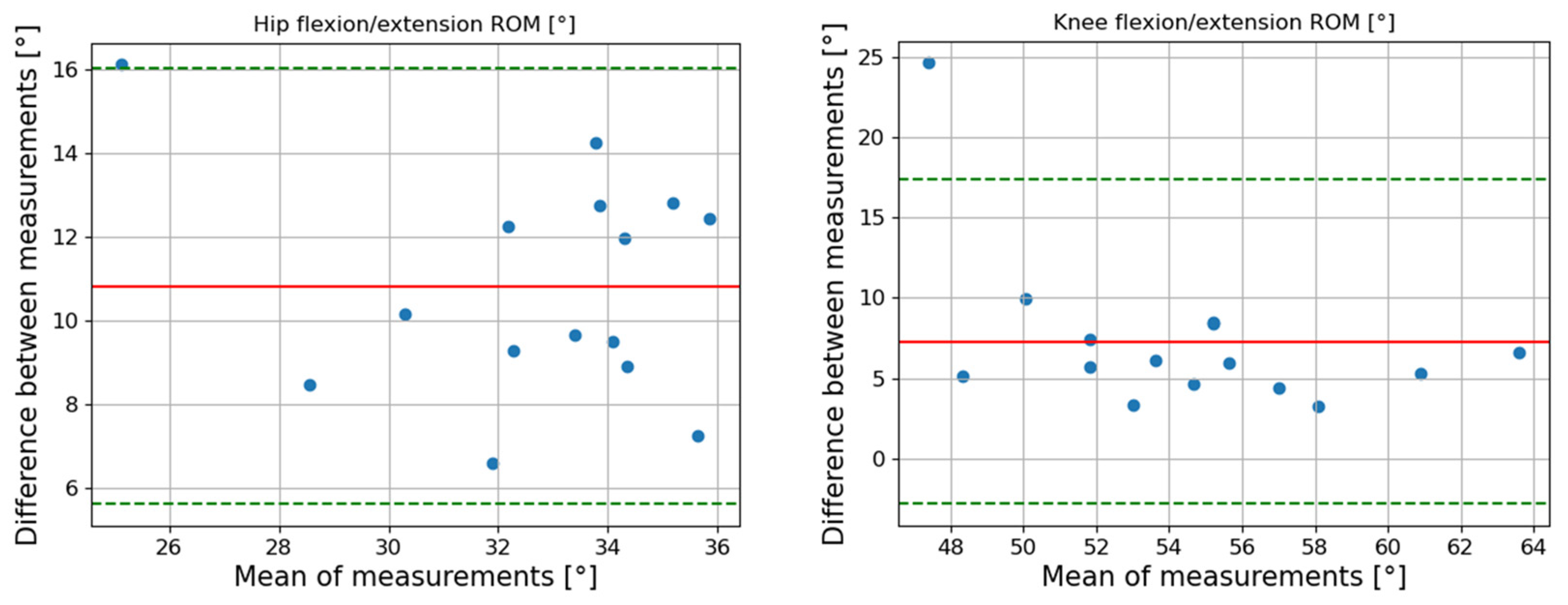
| Joint (Sagittal Plan) | Speed [m/s] | Max | Min | ROM | RMSE [°] (SD) | LCC (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICC2,1 (95% CI) | SEM [°] | MDC [°] | ICC2,1 (95% CI) | SEM [°] | MDC [°] | ICC2,1 (95% CI) | SEM [°] | MDC [°] | ||||
| Knee | 0.7 | 0.85 (0.63 0.95) | 1.78 | 2.46 | 0.85 (0.61 0.95) | 0.66 | 0.92 | 0.85 (0.63 0.95) | 1.85 | 2.56 | 2.34 (0.90) | 0.96 (0.93 0.99) |
| 1 | 0.82 (0.49 0.94) | 1.85 | 2.56 | 0.86 (0.64 0.95) | 0.80 | 1.11 | 0.81 (0.48 0.93) | 2.20 | 3.00 | 2.20 (0.97) | 0.99 (0.98 0.99) | |
| 1.3 | 0.86 (0.59 0.96) | 1.60 | 2.22 | 0.61 (0.14 0.85) | 1.19 | 1.64 | 0.82 (0.53 0.93) | 2.00 | 2.77 | 2.42 (1.44) | 0.98 (0.97 1.00) | |
| Hip | 0.7 | 0.90 (0.74 0.97) | 1.01 | 1.40 | 0.84 (0.57 0.94) | 1.37 | 1.9 | 0.80 (0.52 0.93) | 1.26 | 1.74 | 1.80 (0.82) | 0.98 (0.98 0.99) |
| 1 | 0.94 (0.83 0.98) | 0.83 | 1.15 | 0.77 (0.44 0.92) | 1.22 | 1.69 | 0.80 (0.50 0.93) | 1.47 | 2.04 | 1.49 (0.60) | 0.98 (0.97 0.99) | |
| 1.3 | 0.90 (0.71 0.97) | 1.05 | 1.45 | 0.78 (0.46 0.92) | 1.05 | 1.45 | 0.84 (0.59 0.94) | 1.02 | 1.41 | 1.80 (1.18) | 0.98 (0.96 0.99) |
| Joint (Sagittal Plan) | Speed [m/s] | Max | Min | ROM | RMSE [°] (SD) | LCC (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|
| B-A Bias [°] (LoA) | r | B-A Bias [°] (LoA) | r | B-A Bias [°] (LoA) | r | ||||
| Knee | 0.7 | 9.2 (0.3 18.0) | 0.41 | 1.6 (−3.2 6.5) | 0.7 | 7.6 (−2.0 17.2) | 0.5 | 6.00 (2.05) | 0.92 (0.88 0.93) |
| 1 | 8.2 (−1.6 18.0) | 0.46 | 0.9 (−2.5 4.3) | 0.93 | 7.2 (−2.8 17.4) | 0.54 | 6.09 (2.10) | 0.93 (0.90 0.96) | |
| 1.3 | 7.0 (0.1 13.9) | 0.63 | 0.6 (−3.9 5.2) | 0.71 | 6.4 (−1.0 13.7) | 0.65 | 6.80 (1.60) | 0.92 (0.90 0.94) | |
| Hip | 0.7 | 6.1 (−2.0 14.2) | 0.14 | −3.3 (−12.1 5.4) | 0.29 | 9.4 (5.6 13.2) | 0.81 | 5.86 (1.70) | 0.83 (0.80 0.87) |
| 1 | 5.7 (−2.8 14.2) | 0.19 | −5.1 (−13.4 3.1) | 0.13 | 10.8 (5.6 16.0) | 0.53 | 6.20 (1.50) | 0.86 (0.82 0.89) | |
| 1.3 | 6.9 (−1.5 15.4) | 0.28 | −5.7 (−13.7 2.2) | 0.14 | 12.7 (6.8 18.5) | 0.52 | 6.62 (1.60) | 0.88 (0.85 0.90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Haene, M.; Chorin, F.; Colson, S.S.; Guérin, O.; Zory, R.; Piche, E. Validation of a 3D Markerless Motion Capture Tool Using Multiple Pose and Depth Estimations for Quantitative Gait Analysis. Sensors 2024, 24, 7105. https://doi.org/10.3390/s24227105
D’Haene M, Chorin F, Colson SS, Guérin O, Zory R, Piche E. Validation of a 3D Markerless Motion Capture Tool Using Multiple Pose and Depth Estimations for Quantitative Gait Analysis. Sensors. 2024; 24(22):7105. https://doi.org/10.3390/s24227105
Chicago/Turabian StyleD’Haene, Mathis, Frédéric Chorin, Serge S. Colson, Olivier Guérin, Raphaël Zory, and Elodie Piche. 2024. "Validation of a 3D Markerless Motion Capture Tool Using Multiple Pose and Depth Estimations for Quantitative Gait Analysis" Sensors 24, no. 22: 7105. https://doi.org/10.3390/s24227105
APA StyleD’Haene, M., Chorin, F., Colson, S. S., Guérin, O., Zory, R., & Piche, E. (2024). Validation of a 3D Markerless Motion Capture Tool Using Multiple Pose and Depth Estimations for Quantitative Gait Analysis. Sensors, 24(22), 7105. https://doi.org/10.3390/s24227105






