Monitoring of Ammonium and Nitrate Ions in Soil Using Ion-Sensitive Potentiometric Microsensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Microdevice Fabrication
2.2. Adaptation of FET-Based Sensors to Ion Detection
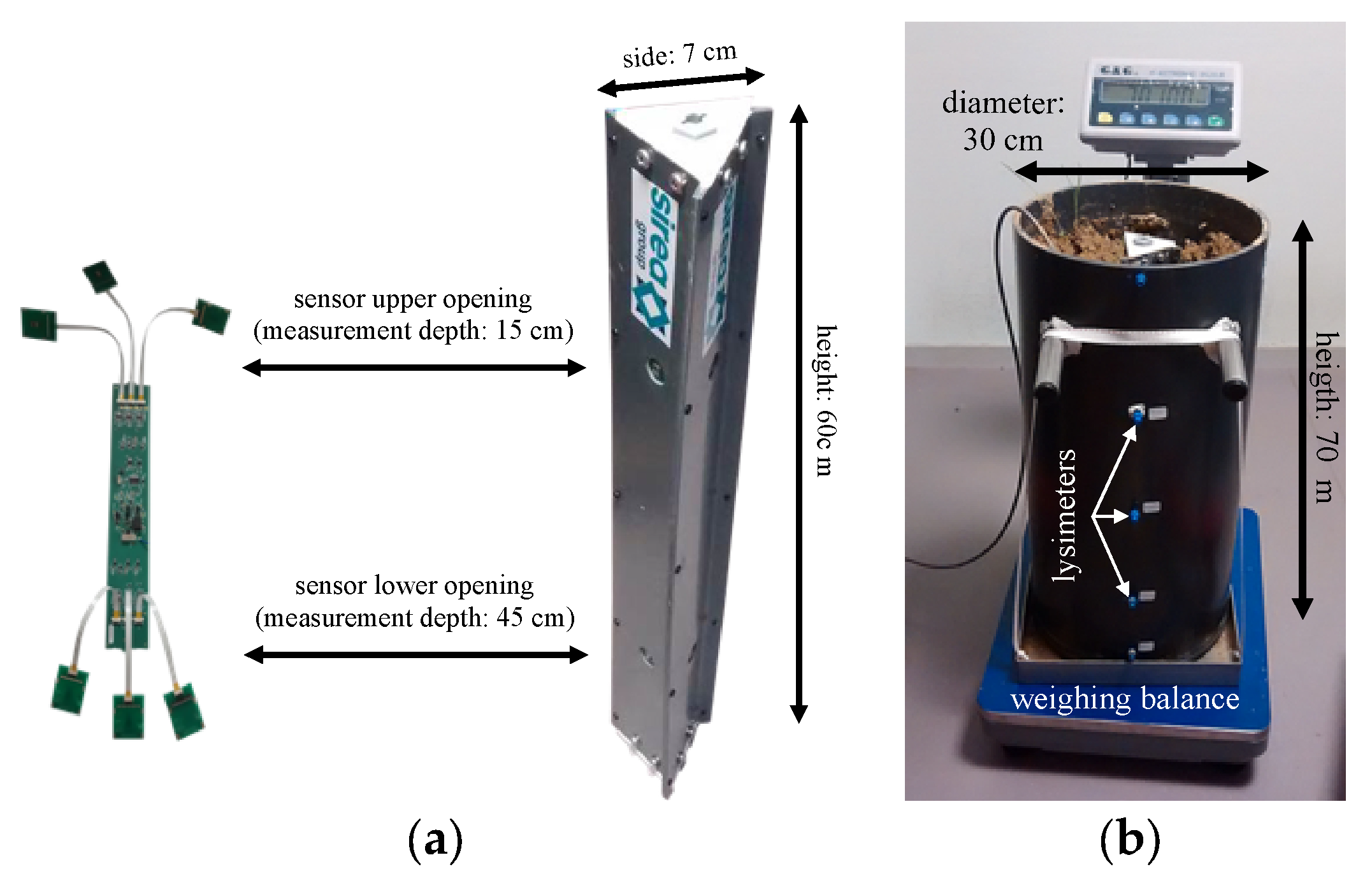
2.3. Realization of a Multi-ISFET Platform for Soil Analysis
2.4. Electrochemical Characterization of ChemFET-Based Sensors and Soil Measurements
- pH-ChemFET: sensitivity: 52 ± 2 mV/decade for pH ranging from 2 to 12,
- pNH4-ISFET: sensitivity: 56 ± 2 mV/decade in the [10−5–10−2 M] concentration range,
- pNO3-ISFET: sensitivity: 56 ± 2 mV/decade in the [10−5–10−2 M] concentration range.
- Day 0: start of the experiment;
- Day 0.82: addition of 2 liters of DI water to trigger the soil-sensor electrical contact;
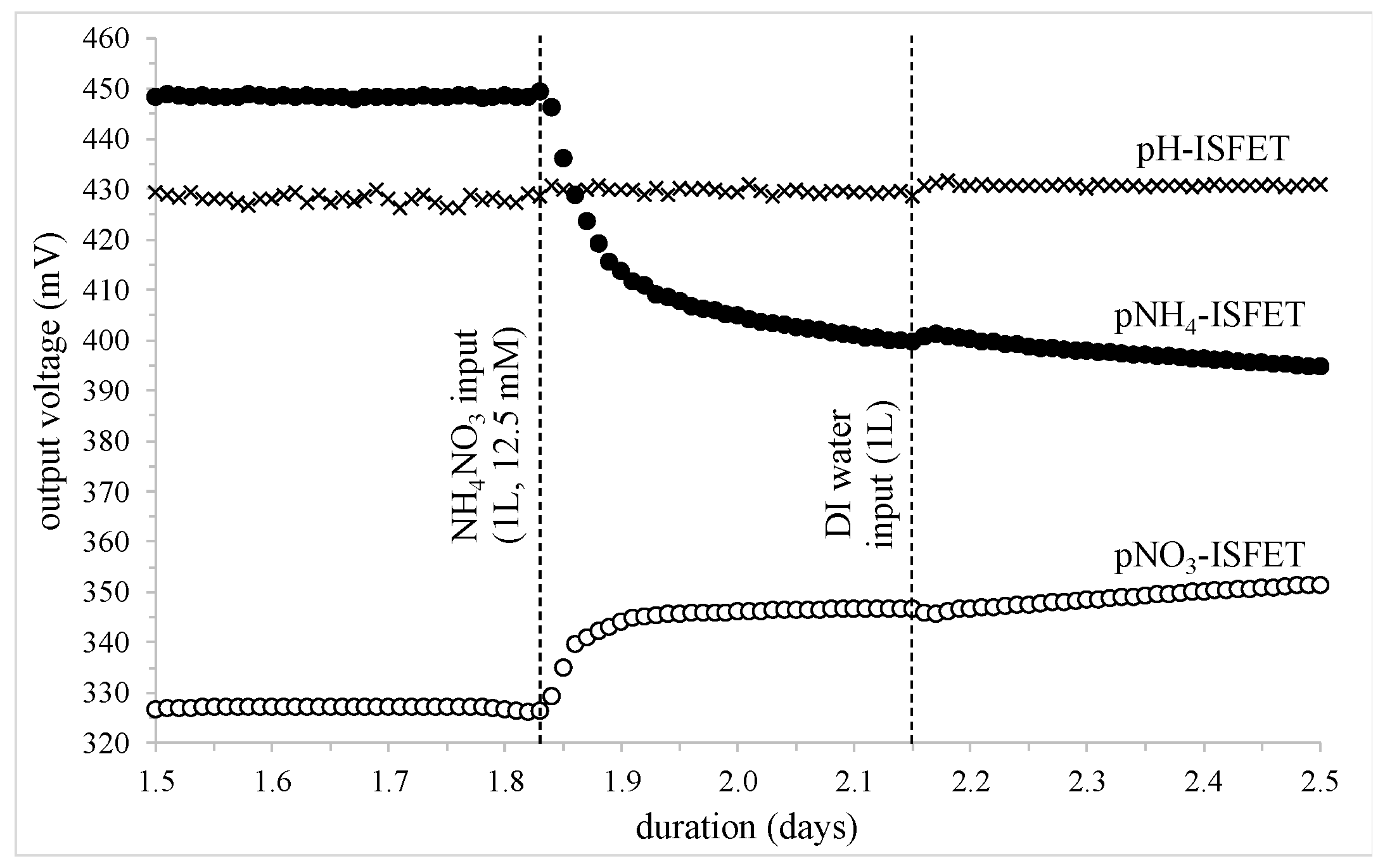
- Day 1.83: input of 1 liter of an NH4NO3 solution (1 g/L or 12.5 × 10−3 mole);
- Day 2.15: addition of 1 liter of DI water;
- Day 5 ± 0.04: addition of 3 liters of DI water to reach soil water saturation;
- Day 5.17: input of 1 liter of an NH4NO3 solution (1 g/L or 12.5 × 10−3 mole);
- Days 6.06 and 7.05: addition of 1 liter of DI water;
- Day 7.98: input of 1 liter of an NH4NO3 solution (1 g/L or 12.5 × 10−3 mole);
- Days 11.81 and 13.17: addition of 1 liter of DI water;
- Day 14.23: input of 1 liter of a tenfold-concentrated NH4NO3 solution (10 g/L or 125 × 10−3 mole) to check the final effectiveness of the ion-sensing procedure;
- Day 15: end of the experiment.
3. Results and Discussion
3.1. pH-ChemFET Characterization in Soil
- pH-ChemFET “−15 cm”: Vout = 432 ± 7 mV
- pH-ChemFET “−45 cm”: Vout = 411 ± 6 mV
3.2. Monitoring Nitrogen-Related Ionic Species in Soil
- pNH4-ISFET: voltage variation: −56 mV, [NH4+] multiplication ratio: ≈10
- pNO3-ISFET: voltage variation: +22 mV, [NO3−] multiplication ratio: ≈2.5
- pNH4-ISFET: voltage variation: +6 mV, [NH4+] multiplication ratio: ≈0.8
- pNO3-ISFET: voltage variation:−16 mV, [NO3−] multiplication ratio: ≈0.5

- pNH4-ISFET: voltage variation: −6 mV, [NH4+] multiplication ratio: ≈1.3
- pNO3-ISFET: voltage variation: +12 mV, [NO3−] multiplication ratio: ≈1.65
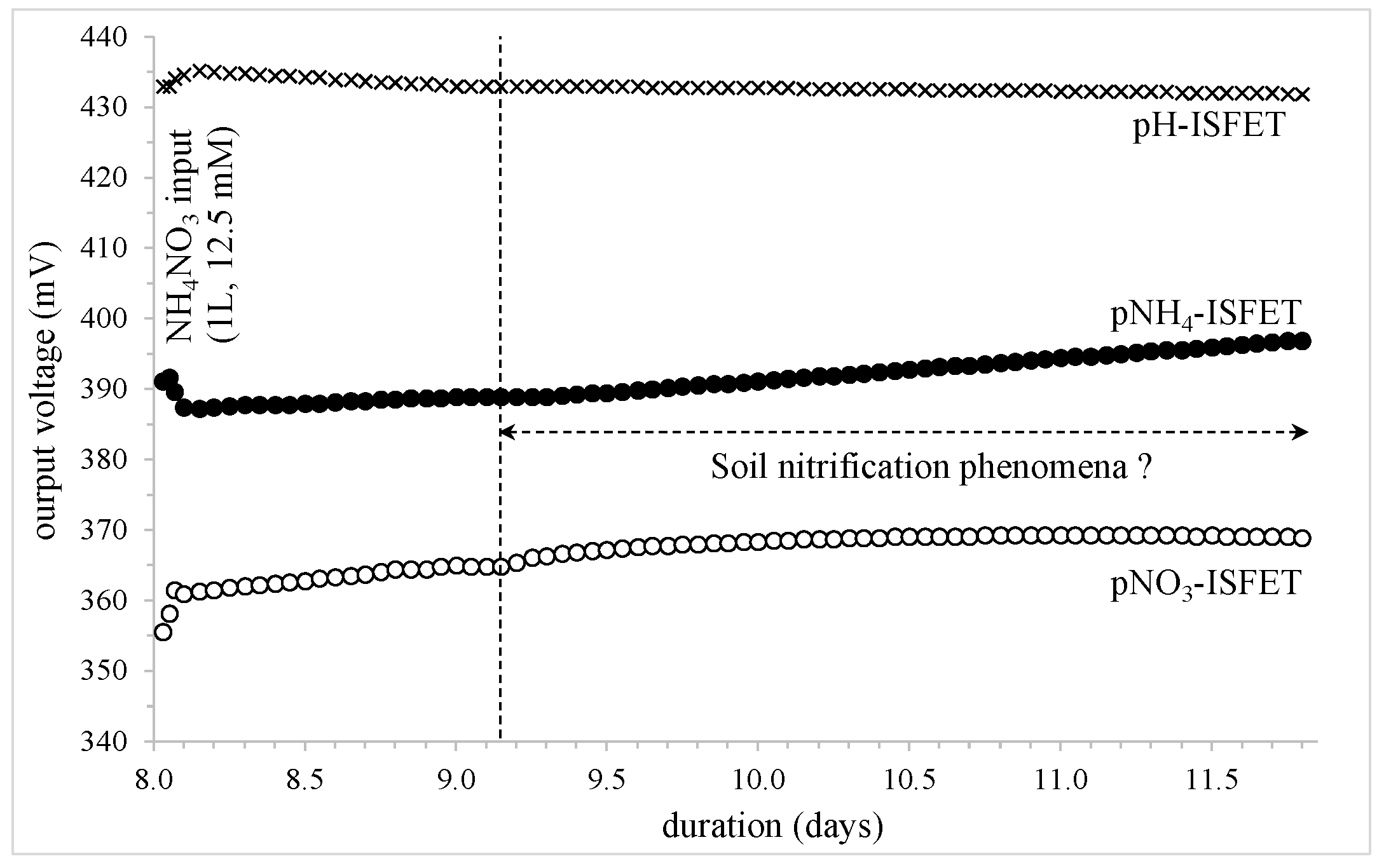
- pNH4-ISFET: voltage variation: +8 mV, [NH4+] multiplication ratio: ≈0.7
- pNO3-ISFET: voltage variation: +4 mV, [NO3−] multiplication ratio: ≈1.2
- pNO3-ISFET: voltage variation: +30 mV, [NO3−] multiplication ratio: ≈3.5
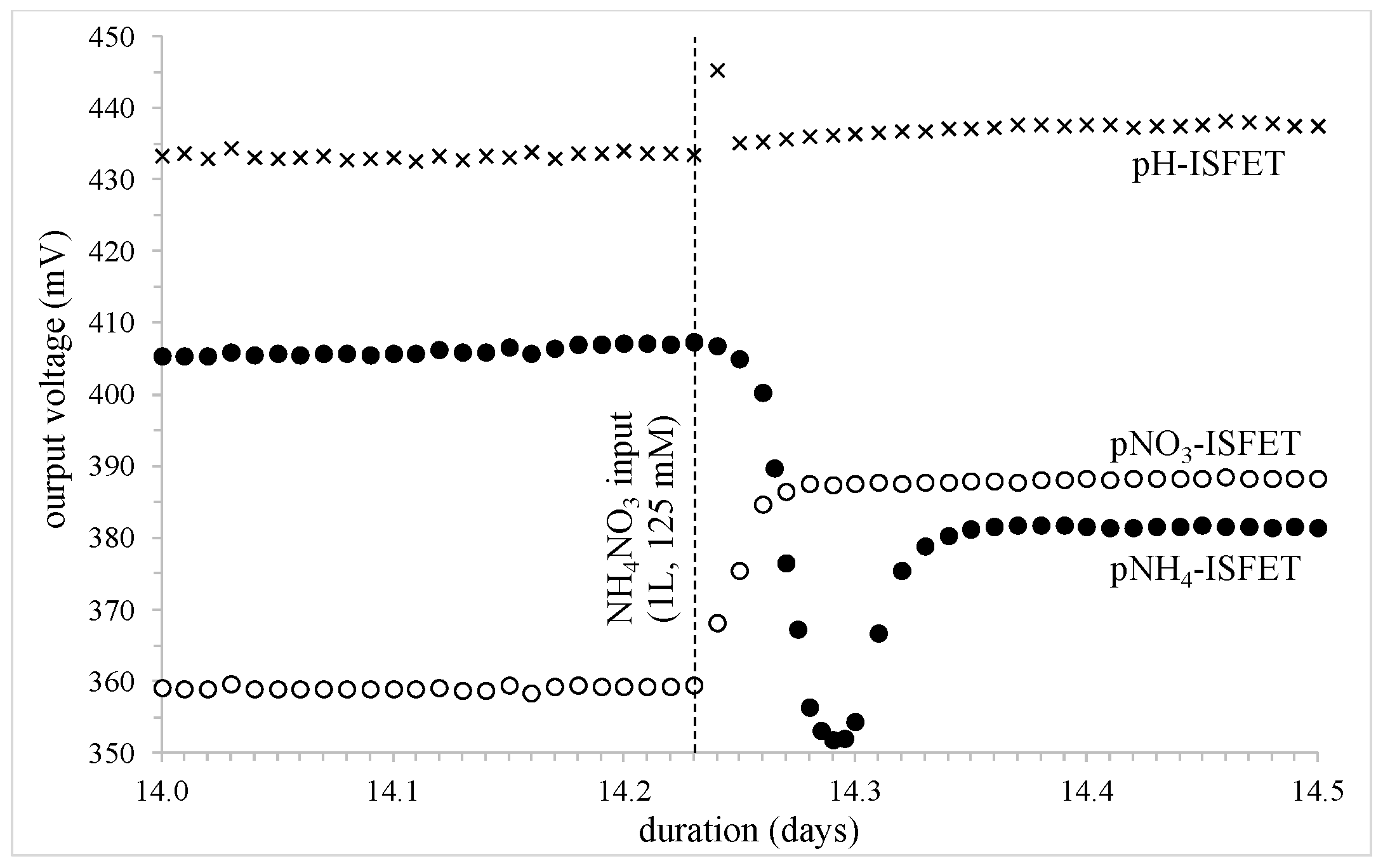
- pNH4-ISFET: voltage variation: −25 mV, [NO3−] multiplication ratio: ≈2.8
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawkesford, M. Chapter 6: Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 135–189. [Google Scholar]
- Andrews, M.; Raven, J.A.; Lea, P.J. Do plant needs nitrate? The mechanisms by which nitrogen forms affect plants. Ann. Appl. Biol. 2013, 163, 174–199. [Google Scholar] [CrossRef]
- Postgate, J.R. The nitrogen cycle—Biological nitrogen fixation: Fundamentals. Philos. Trans. R. Soc. B Biol. Sci. 1982, 296, 375–385. [Google Scholar]
- FAOSTAT, Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/ (accessed on 10 May 2017).
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Billen, G.; Beusen, A.; Bouwman, L.; Garnier, J. Anthropogenic nitrogen autography and heterotrophy of the world’s watersheds: Past, present and future trends. Glob. Biogeochem. Cycles 2010, 24 GB0A11. [Google Scholar]
- Billen, G.; Garnier, J.; Lassaletta, L. The nitrogen cascade from agricultural soils to the sea: Modelling nitrogen transfers at regional watershed and global scales. Philos. Trans. R. Soc. B Biol. Sci. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycle Agroecosyst. 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Report COM(2002)407, Environment, European Commission. Available online: http://ec.europa.eu/environment/water/water-nitrates/reports.html (accessed on 11 May 2017).
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- European Environment Agency. Exposure of Ecosystems to Acidification, Eutrophication and Ozone; European Environment Agency: Copenhagen, Denmark, 2017; Available online: https://www.eea.europa.eu/data-and-maps/indicators/exposure-of-ecosystems-to-acidification-3/assessment-2 (accessed on 21 August 2017).
- Kuang, B.; Mahmood, H.S.; Quraishi, M.Z.; Hoogmoed, W.B.; Mouazen, A.M.; van Henten, E.J. Sensing soil properties in the laboratory, in-situ and on-line: A review. Adv. Agron. 2012, 114, 155–223. [Google Scholar]
- Wesoly, M.; Przewodowski, W.; Ciosek-Skibinska, P. Electronic noses and electronic tongues for the agricultural purposes. Trends Anal. Chem. 2023, 164, 117082.1–22. [Google Scholar] [CrossRef]
- Conklin, A.R., Jr. Chapter 7: Soil and soil solution sampling, sample, transport and storage. In Introduction to Soil Chemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; pp. 135–158. [Google Scholar]
- Adsett, J.; Thottan, J.; Sibley, K.J. Development of an automated on-the-go soil nitrate monitoring system. Appl. Eng. Agric. 1999, 15, 351–356. [Google Scholar] [CrossRef]
- Birrell, S.J.; Hummel, J.W. Real-time multi ISFET/FIA soil analysis system with automatic sample extraction. Comput. Electron. Agric. 2001, 32, 45–67. [Google Scholar] [CrossRef]
- Adamchuk, V.I.; Lund, E.D.; Sethuramasamyraja, B.; Morgan, M.T.; Dobermann, A.; Marx, D.B. Direct measurement of soil chemical properties on-the-go using ion-selective electrodes. Comput. Electron. Agric. 2005, 48, 272–294. [Google Scholar] [CrossRef]
- Sibley, K.J.; Astatkie, T.; Brewster, G.; Struik, P.C.; Adsett, J.F.; Pruski, K. Field-scale validation of an automated soil nitrate extraction and measurement system. Precis. Agric. 2009, 10, 162–174. [Google Scholar] [CrossRef]
- Sinfield, J.V.; Fagerman, D.; Colic, O. Evaluation of sensing technologies for the on-the-go detection of macro-nutrients in cultivated soils. Comput. Electron. Agric. 2010, 70, 1–18. [Google Scholar] [CrossRef]
- Biyani, M.; Biyani, R.; Tsuchihashi, T.; Takamura, Y.; Ushijima, H.; Tamiya, E.; Biyani, M. DEP-on-go for simultaneous sensing of multiple heavy metals pollutants in environmental samples. Sensors 2016, 17, 45. [Google Scholar] [CrossRef]
- Yildirim, S. A variable extraction providing methods for on-the-go soil nitrate analysis systems. J. Agric. Sci. 2016, 22, 455–461. [Google Scholar]
- Artigas, J.; Jimenez, C.; Lemos, S.G.; Nogueira, A.R.A.; Torre-Neto, A.; Alonso, J. Development of a screen-printed thick-film nitrate sensor based on a graphite-epoxy composite for agricultural applications. Sens. Actuators B 2003, B88, 337–344. [Google Scholar] [CrossRef]
- Futagawa, M.; Iwasaki, T.; Murata, H.; Ishida, M.; Sawada, K. A miniature integrated multimodal sensor for measuring pH, EC and temperature for precision agriculture. Sensors 2012, 12, 8338–8354. [Google Scholar] [CrossRef]
- Tully, K.L.; Weil, R. Ion-selective electrode offers accurate, inexpensive method for analyzing soil solution nitrate in remote regions. Commun. Soil Sci. Plant Anal. 2014, 45, 1974–1980. [Google Scholar] [CrossRef]
- Caron, W.-O.; Lamhamedi, M.S.; Viens, J.; Messaddeq, Y. Practical application of electrochemical nitrate sensor under laboratory and forest nursery conditions. Sensors 2016, 16, 1190. [Google Scholar] [CrossRef]
- Jiang, H.; Ali, M.A.; Jiao, Y.; Yang, B.; Dong, L. In-situ, real-time monitoring of nutrient uptake on plant chip integrated with nutrient sensor. In Proceedings of the 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS 2017), Kaohsiung, Taiwan, 18–22 June 2017; pp. 289–292. [Google Scholar]
- Yeshno, E.; Arnon, S.; Dahan, O. Real-time monitoring of nitrate in soils as a key for optimization of agricultural productivity and prevention of groundwater pollution. Hydrol. Earth Syst. Sci. 2019, 23, 3997–4010. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Ali, M.A.; Dong, L.; Wang, X.; Archontoulis, S.V.; Schnable, J.; Castellano, M.J. Continuous in situ soil nitrate sensors: The importance of high-resolution measurements across time and a comparison with salt extraction-based methods. Soil Sci. Soc. Am. J. 2021, 85, 667–690. [Google Scholar] [CrossRef]
- Siemering, G.S.; Vanderleest, C.P.; Arriaga, F.J. Autonomous high-throughput in-situ soil nitrogen flux measurement system. Environ. Monit. Assess. 2022, 194, 680. [Google Scholar] [CrossRef]
- Joly, M.; Marlet, M.; Durieu, C.; Bene, C.; Launay, J. Temple-Boyer. Study of chemical field effect transistors for the detection of ammonium and nitrate ions in liquid and soil samples. Sens. Actuators B 2022, B351, 130949. [Google Scholar] [CrossRef]
- Archbold, G.; Parra, C.; Carillo, H.; Mouazen, A.M. Towards the implementation of ISFET sensors for in-situ and real-time chemical analyses in soils: A practical review. Comput. Electron. Agric. 2023, 209, 107802. [Google Scholar] [CrossRef]
- Campanella, L.; Colapicchioni, C.; Crescentini, G.; Sammartino, M.P.; Su, Y.; Tomassetti, M. Sensitive membrane ISFETs for nitrate analysis in waters. Sens. Actuators B 1995, B27, 329–335. [Google Scholar] [CrossRef]
- Wroblewski, W.; Chudy, M.; Dybko, A.; Brzozka, Z. NH4+-sensitive chemically modified field effect transistors based on siloxane membranes for flow-cell applications. Anal. Chim. Acta 1999, 401, 105–110. [Google Scholar] [CrossRef]
- Wroblewski, W.; Chudy, M.; Dybko, A. Nitrate-selective chemically modified field effect transistors for flow-cell applications. Anal. Chim. Acta 2000, 416, 97–104. [Google Scholar] [CrossRef]
- Chudy, M.; Wroblewski, W.; Dybko, A.; Brzozska, Z. Multi-ion analysis on versatile sensor head. Sens. Actuators B 2001, B78, 320–335. [Google Scholar] [CrossRef]
- Artigas, J.; Beltran, A.; Jimenez, C.; Baldi, A.; Mas, R.; Dominguez, C.; Alonso, J. Application of ion field-effect-transistor-based sensors to soil analysis. Comput. Electron. Agric. 2001, 31, 281–293. [Google Scholar] [CrossRef]
- Masadome, T.; Mishikawa, M.; Wakida, S. Fabrication and characterization of polymer-based microchip integrated with NH4+-ISFET using a small diameter wire as a template of channel. Anal. Lett. 2004, 37, 377–384. [Google Scholar] [CrossRef]
- Temple-Boyer, P.; Launay, J.; Humenyuk, I.; Conto, T.D.; Martinez, A.; Bériet, C.; Grisel, A. Study of front-side connected chemical field effect transistor for water analysis. Microelectron. Reliab. 2004, 44, 443–447. [Google Scholar] [CrossRef]
- Kim, H.J.; Hummel, J.W.; Birrell, S.J. Evaluation of nitrate and potassium ion-selective membranes for soil macronutrient sensing. Am. Soc. Agric. Biol. Eng. 2006, 49, 597–606. [Google Scholar]
- Humenuyk, I.; Torbiéro, B.; Souleille, S.A.; Colin, R.; Dollat, X.; Franc, B.; Martinez, A.; Temple-Boyer, P. Development of pNH4-ISFET microsensors for water analysis. Microelectron. J. 2006, 37, 475–479. [Google Scholar] [CrossRef][Green Version]
- Wakida, S.-I.; Okomura, T.; Shibutani, Y.; Liu, J. Highly sensitive nitrate-sensing materials for ion-selective field-effect transistors for single-drop rain analysis. Sens. Mater. 2007, 19, 235–247. [Google Scholar]
- Lehmann, U.; Grisel, A. Miniature multi-sensor probe for soil nutrient monitoring. Procedia Eng. 2014, 87, 1429–1432. [Google Scholar] [CrossRef]
- Cazalé, A.; Sant, W.; Launay, J.; Ginot, F.; Temple-Boyer, P. Study of field effect transistors for the sodium ion detection using fluoropolysiloxane-based sensitive layers. Sens. Actuators B 2013, B177, 515–521. [Google Scholar] [CrossRef]
- Sant, W.; Temple-Boyer, P.; Chanié, E.; Launay, J.; Martinez, A. On-line monitoring of urea using enzymatic field effect transistors. Sens. Actuators B 2011, B160, 59–64. [Google Scholar] [CrossRef][Green Version]
- Matthiesen, H. In-situ measurements of soil pH. J. Archaeol. Sci. 2004, 31, 1373–1381. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joly, M.; Marlet, M.; Barreau, D.; Jourdan, A.; Durieu, C.; Launay, J.; Temple-Boyer, P. Monitoring of Ammonium and Nitrate Ions in Soil Using Ion-Sensitive Potentiometric Microsensors. Sensors 2024, 24, 7143. https://doi.org/10.3390/s24227143
Joly M, Marlet M, Barreau D, Jourdan A, Durieu C, Launay J, Temple-Boyer P. Monitoring of Ammonium and Nitrate Ions in Soil Using Ion-Sensitive Potentiometric Microsensors. Sensors. 2024; 24(22):7143. https://doi.org/10.3390/s24227143
Chicago/Turabian StyleJoly, Matthieu, Maurane Marlet, David Barreau, Arnaud Jourdan, Céline Durieu, Jérôme Launay, and Pierre Temple-Boyer. 2024. "Monitoring of Ammonium and Nitrate Ions in Soil Using Ion-Sensitive Potentiometric Microsensors" Sensors 24, no. 22: 7143. https://doi.org/10.3390/s24227143
APA StyleJoly, M., Marlet, M., Barreau, D., Jourdan, A., Durieu, C., Launay, J., & Temple-Boyer, P. (2024). Monitoring of Ammonium and Nitrate Ions in Soil Using Ion-Sensitive Potentiometric Microsensors. Sensors, 24(22), 7143. https://doi.org/10.3390/s24227143



