Research on the Flow Characteristics and Reaction Mechanisms of Lateral Flow Immunoassay under Non-Uniform Flow
Abstract
:1. Introduction
2. Mathematical Modeling
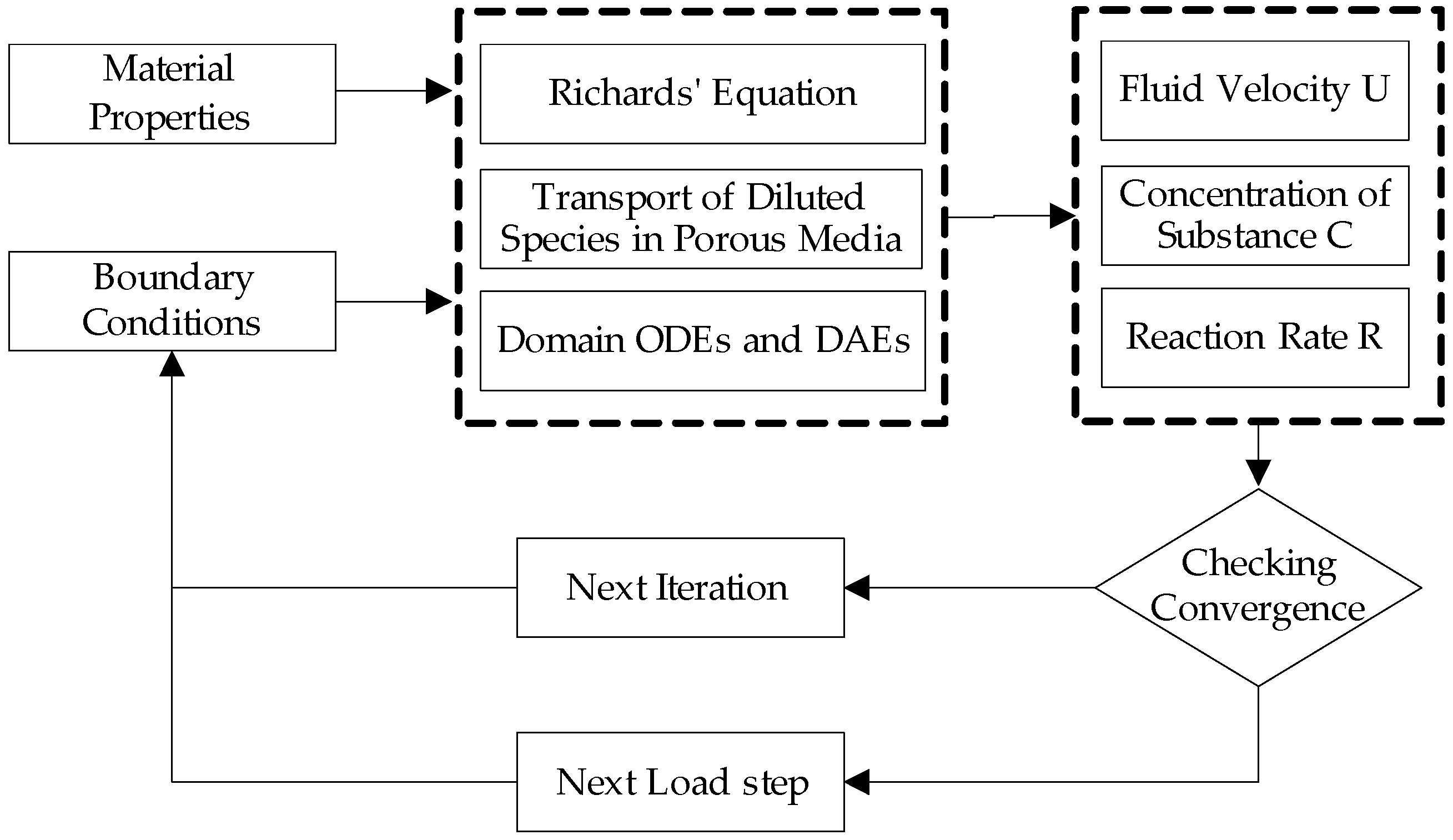
2.1. Fluid Dynamics Model
2.2. Transport of Dilute Species in Porous Media
2.3. Reaction Kinetics Model
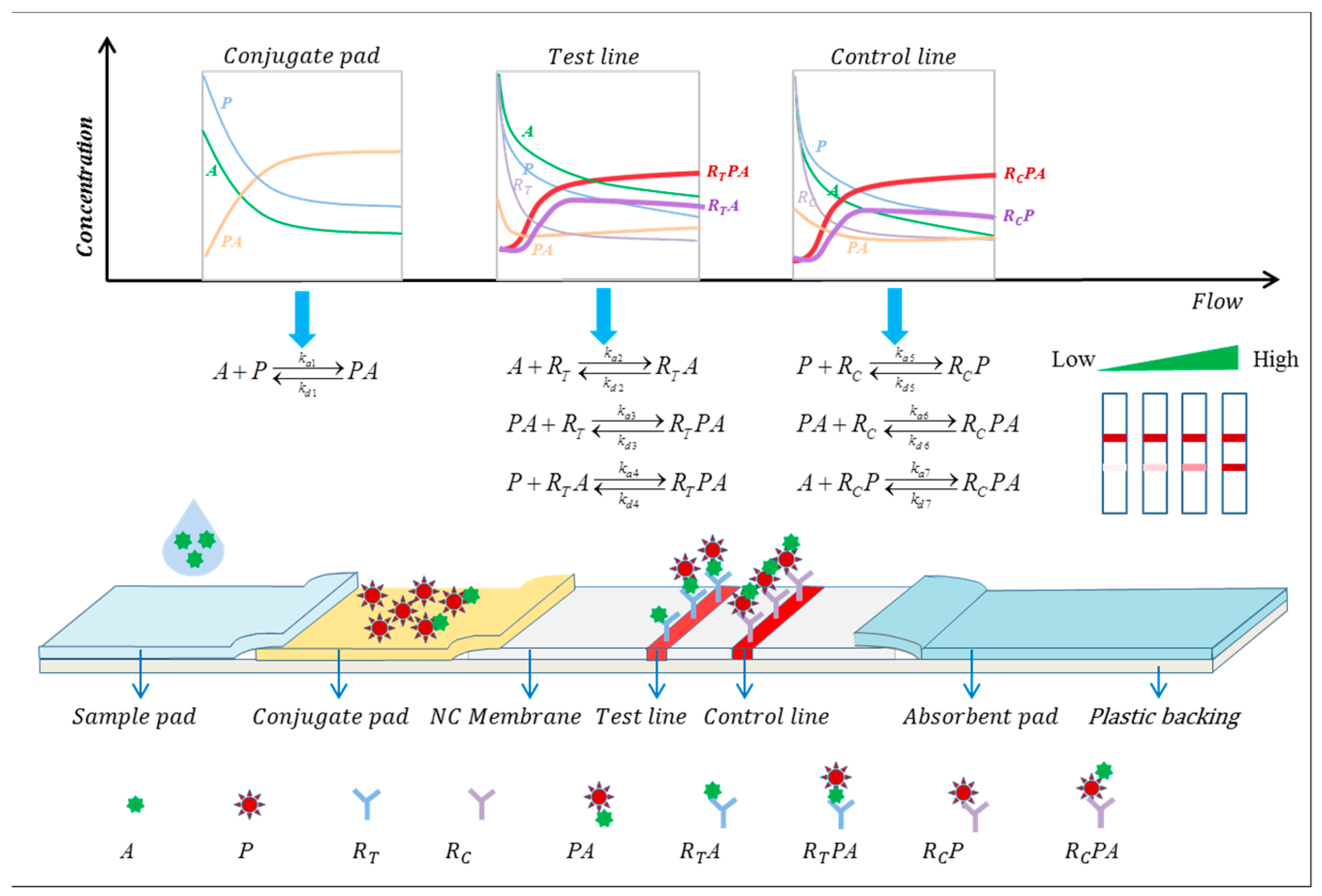
- Antigens consist of single molecules and exist in homogeneous form, as do antibodies.
- One report particle combines one target analyte.
- Binding is consistent, without positive or negative allosteric effects (binding of one site on the analytes does not affect the binding of another site to the antibody).
- The reaction is a first-order reversible interaction, with concentrations of reactants reaching a steady state over time.
- There is no non-specific binding, such as binding to the reaction vessel walls.
- The rate constants of the reaction are constant, meaning they do not change with variations in reagent and sample concentrations during the reaction process.
2.3.1. Sandwich LFIA Reaction Kinetics Model
2.3.2. Competitive LFIA Reaction Kinetics Model
3. Finite Element Simulation
3.1. Simulation Approach
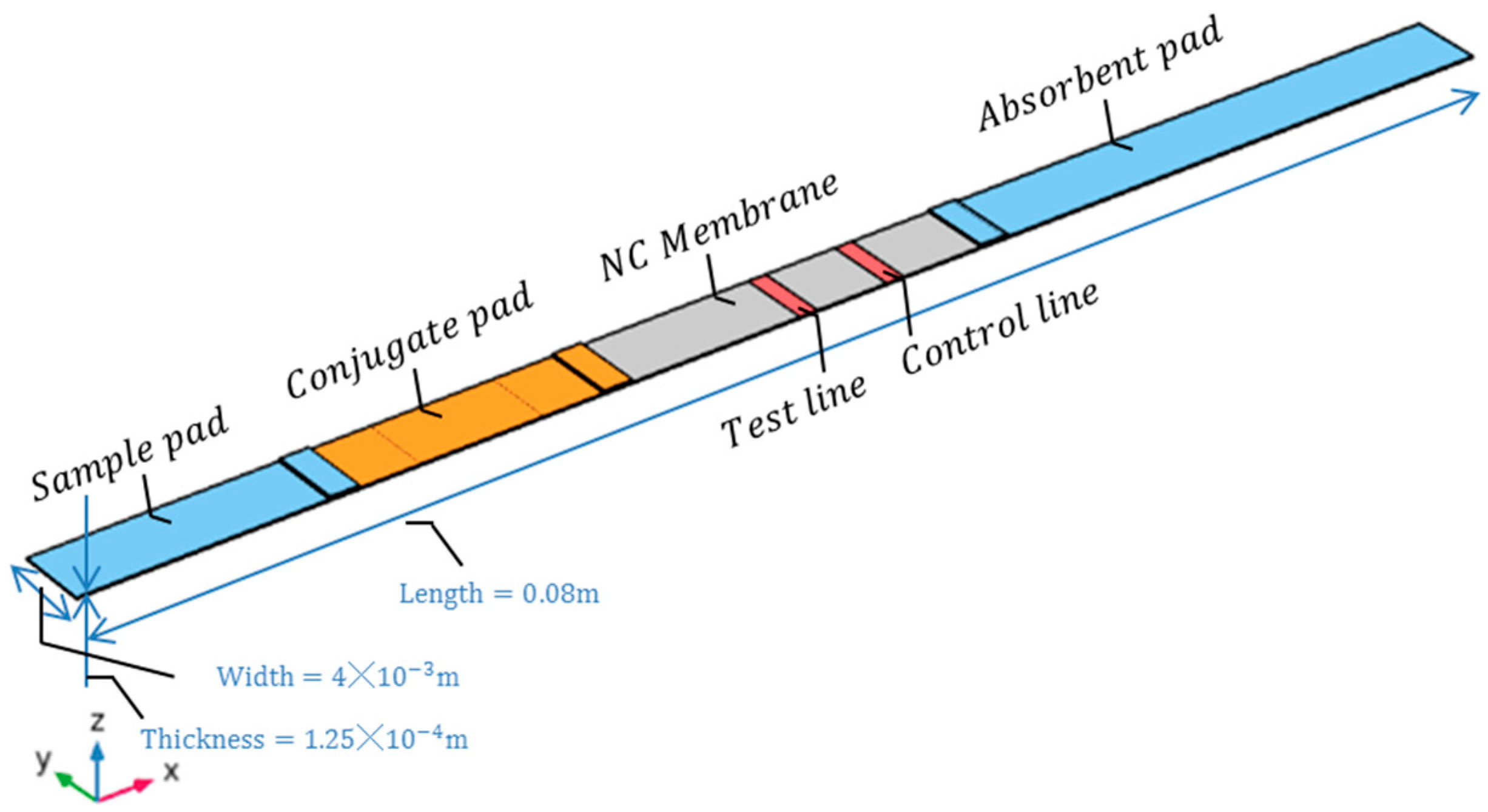
3.2. Model Parameter Settings, Initial Conditions, and Boundary Conditions
3.3. Parameter Definitions
4. Results and discussion
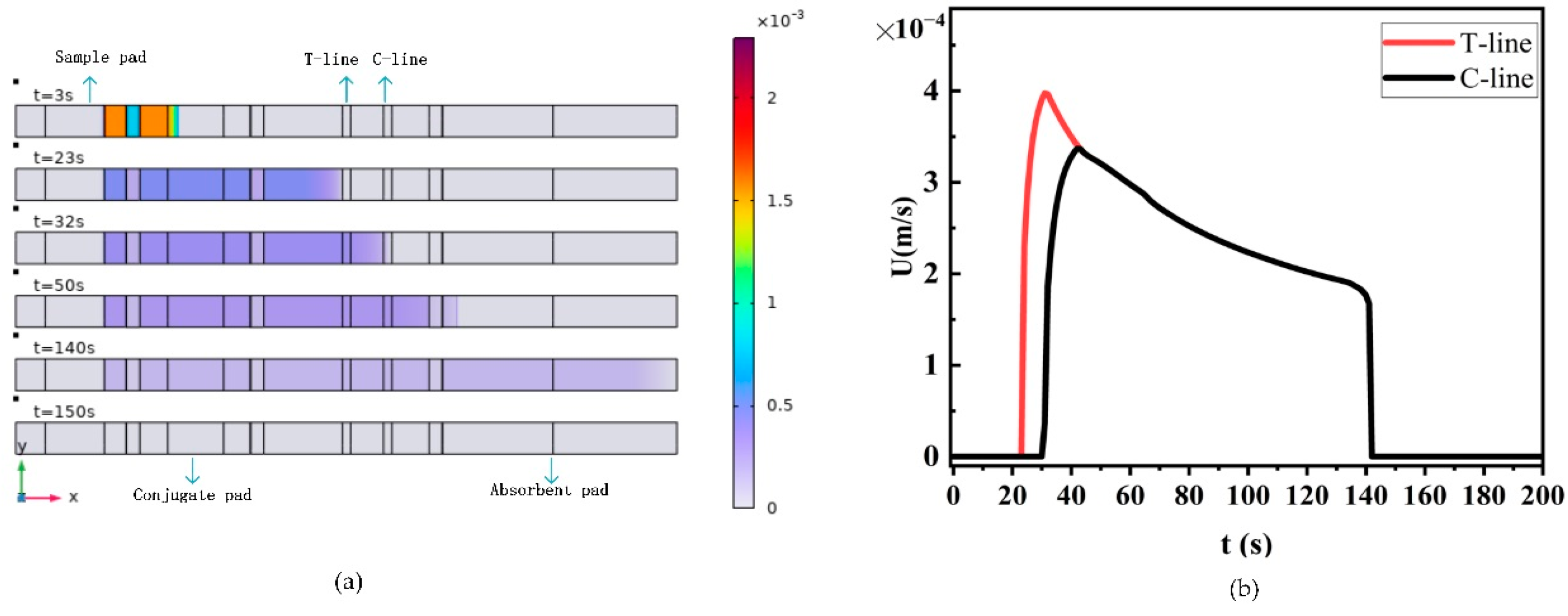
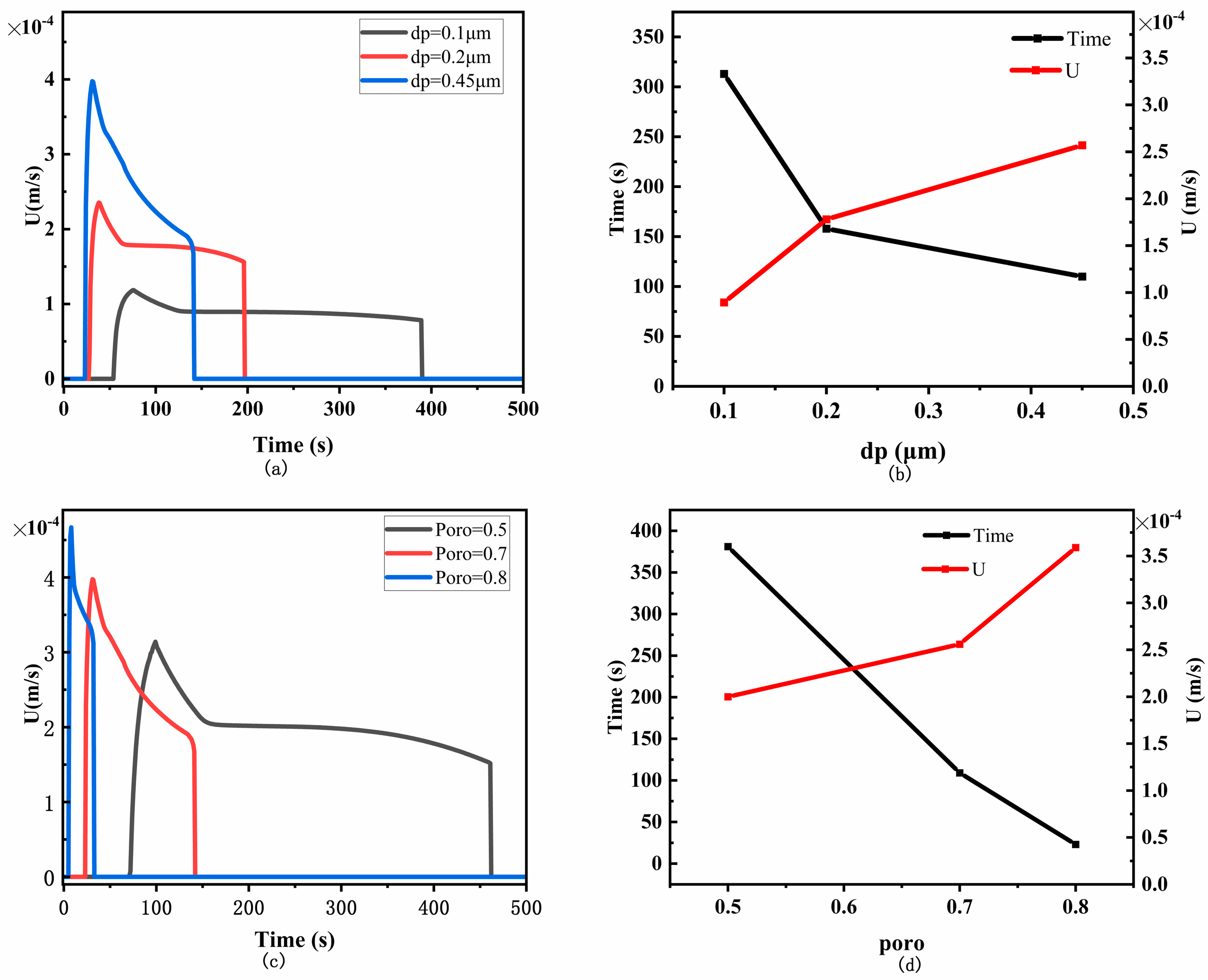
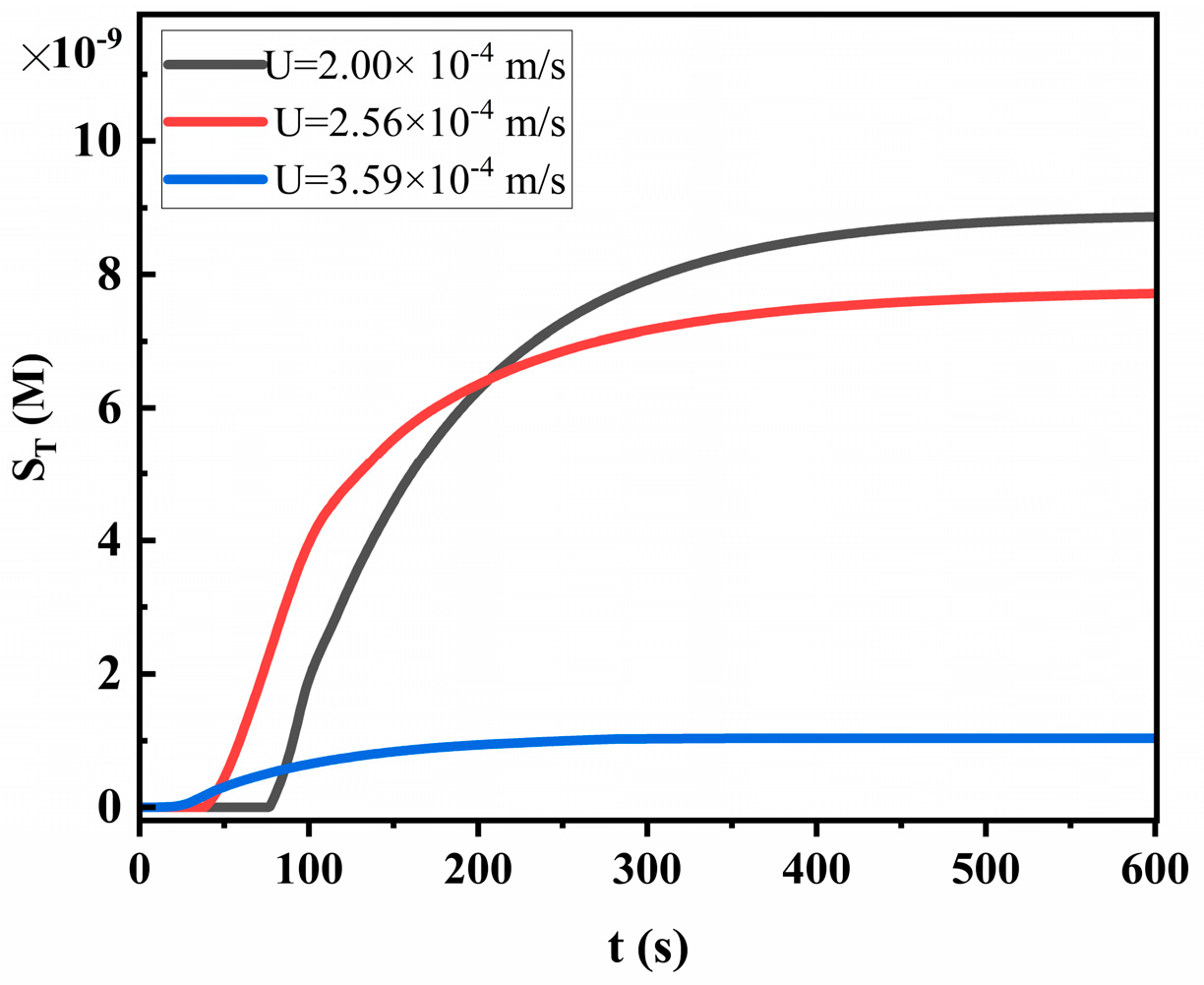
4.1. The Effect of Flow Velocity on LFIA Performance
4.2. Sandwich LFIA
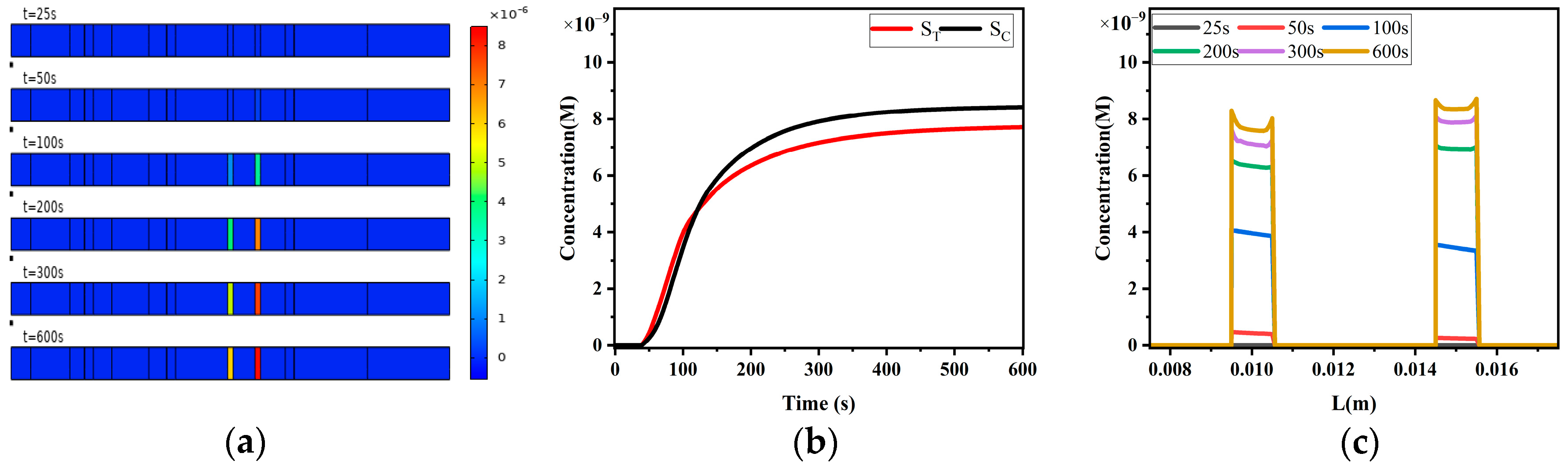
4.2.1. Sandwich LFIA Reaction Process
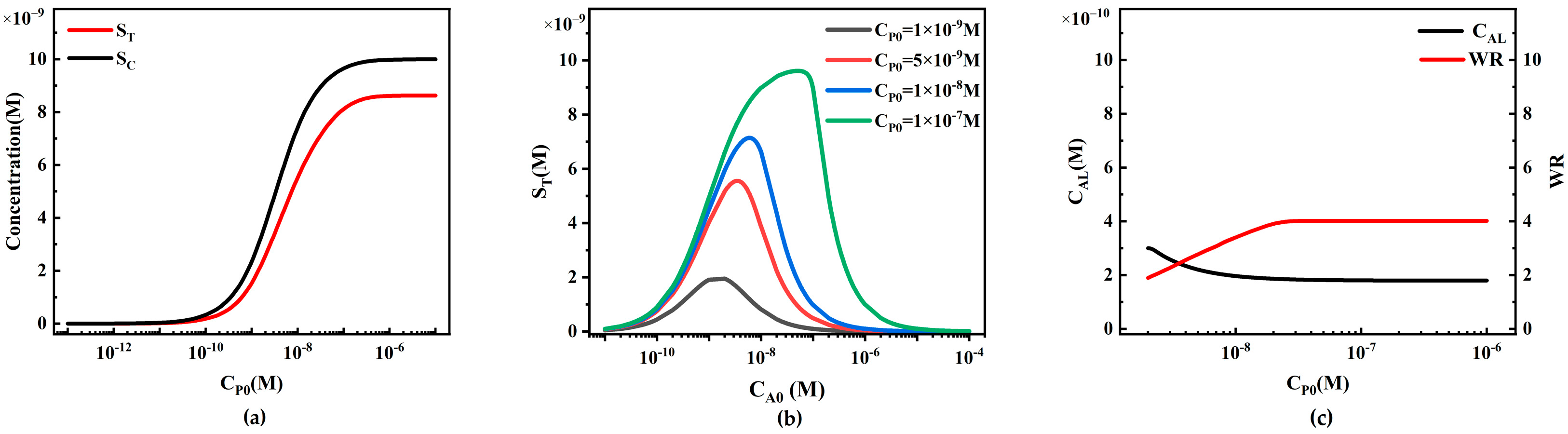
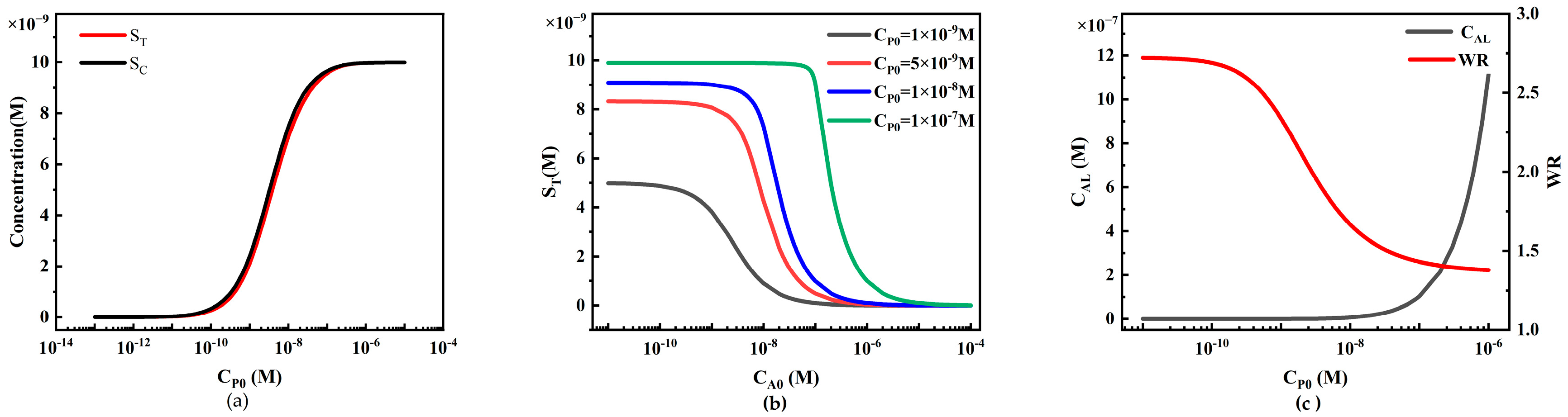
4.2.2. The Influence of Target Analyte Concentration on ST and SC in Sandwich LFIA
4.2.3. The Influence of Reporter Particle Concentration on Sandwich LFIA Performance
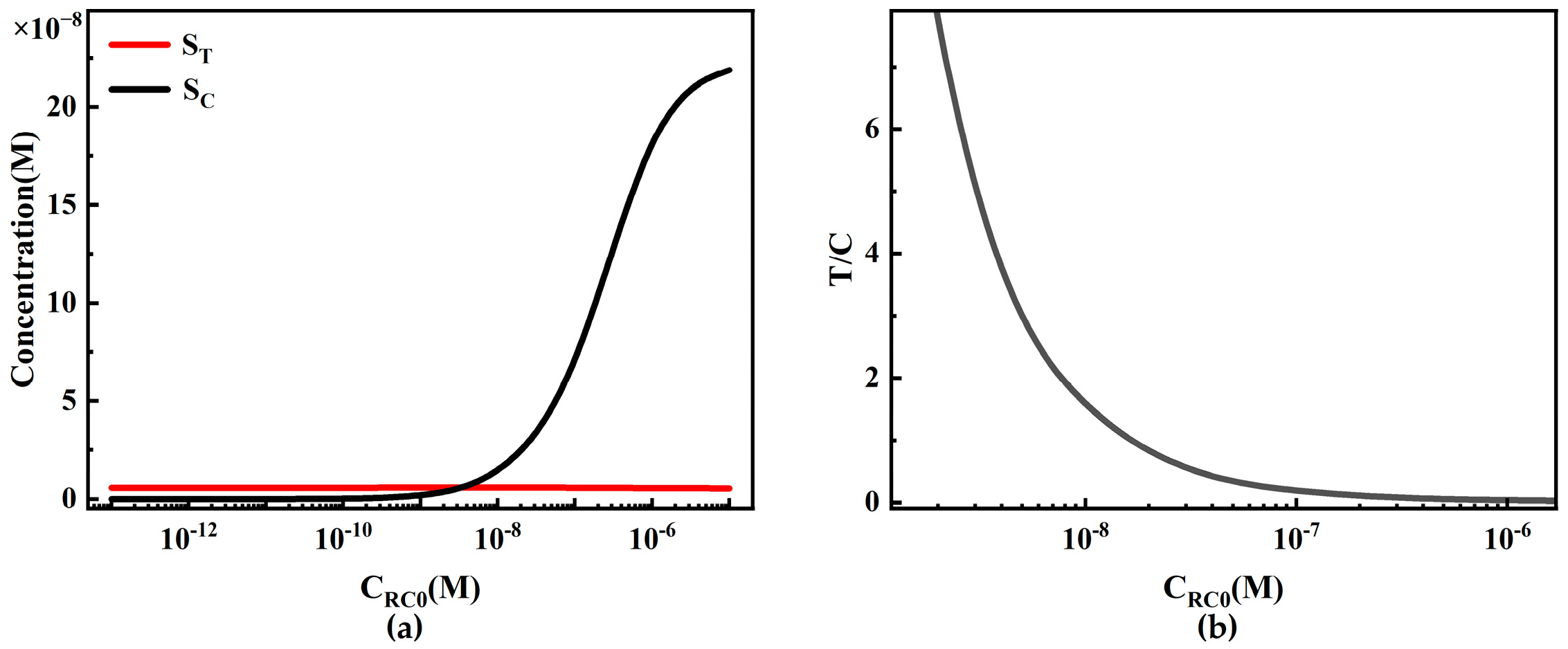
4.2.4. The Influence of Initial Capture Probe Concentration on Sandwich LFIA Performance
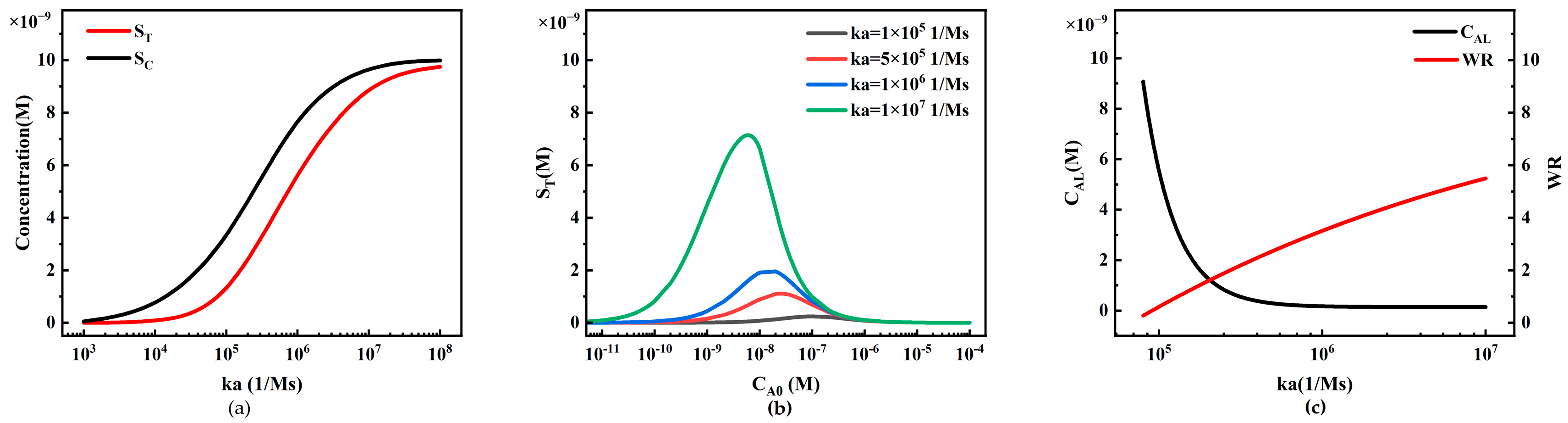
4.2.5. The Influence of Reaction Rate Constants on Sandwich LFIA Performance
4.3. Competitive LFIA
4.3.1. Competitive LFIA Reaction Process
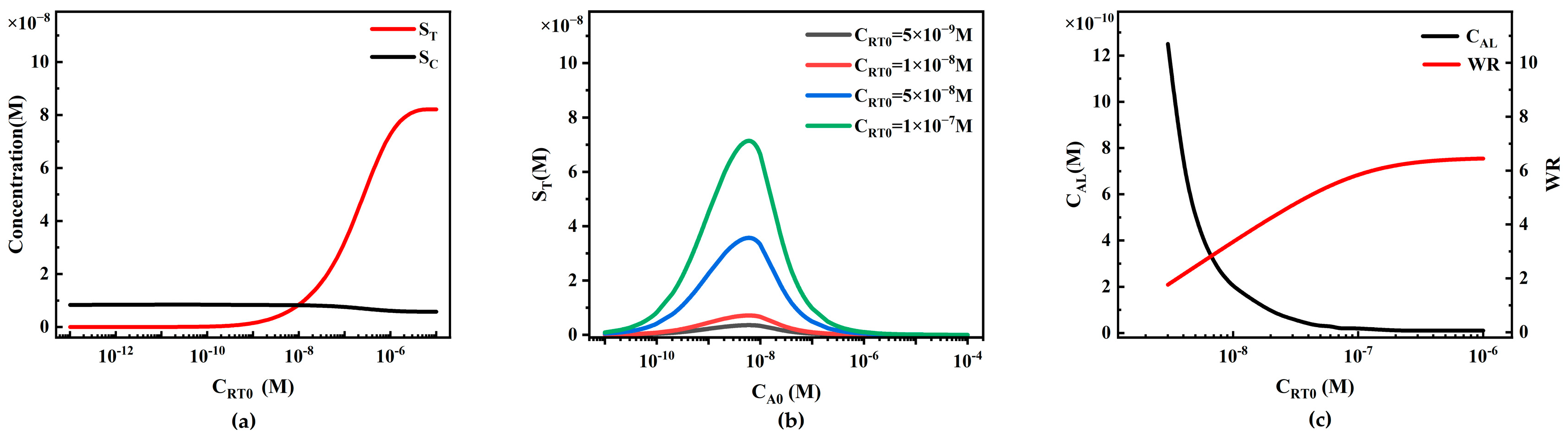
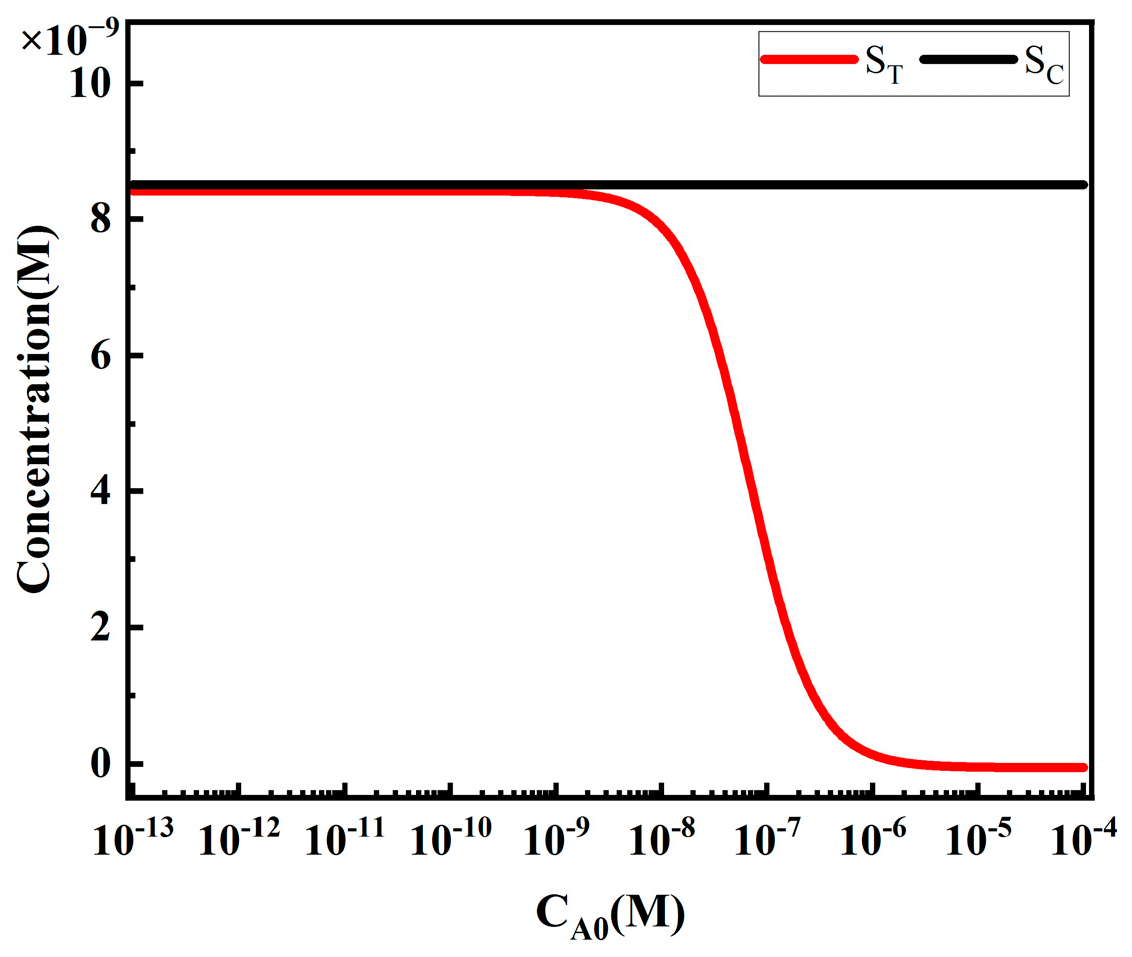
4.3.2. The Influence of Target Analyte Concentration on ST and SC in Competitive LFIA
4.3.3. The Influence of Reporter Particle Concentration on Competitive LFIA Performance
4.3.4. The Influence of Capture Probe Initial Concentration on Competitive LFIA Performance
4.3.5. The Influence of Reaction Constants on Competitive LFIA Performance
5. Conclusions
- (1)
- The sample flow velocity decreases exponentially with the distance from the sample front to the origin. Increasing the pore size and porosity of the membrane both increase the capillary flow velocity, thus reducing the sensitivity of LFIA.
- (2)
- In sandwich LFIA, appropriately increasing the report particle concentration CP0, increasing the initial concentration of T-line capture probe CRT0, increasing the binding rate ka, and decreasing the dissociation rate kd are all beneficial for reducing the detection limit and broadening the working range of LFIA. The initial concentration of C-line capture probe CRC0 has little effect on LFIA performance but lowers the T/C ratio.
- (3)
- For competitive LFIA, increasing the report particle concentration CP0, increasing the binding rate ka, and decreasing the dissociation rate kd may adversely affect the detection limit and working range of LFIA. Under the indicators of this paper, the effect of T-line CRT0 on LFIA performance is insensitive.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors 2021, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Song, J.R.; Sun, C.X.; Zuo, W.C.; Dai, J.J.; Ju, Y.M. Recent advances of lateral flow immunoassay for bacterial detection: Capture-antibody-independent strategies and high-sensitivity detection technologies. TrAC Trends Anal. Chem. 2023, 166, 16. [Google Scholar]
- Barshevskaya, L.V.; Sotnikov, D.V.; Zherdev, A.V.; Khassenov, B.B.; Baltin, K.K.; Eskendirova, S.Z.; Mukanov, K.K.; Mukantayev, K.K.; Dzantiev, B.B. Triple Immunochromatographic System for Simultaneous Serodiagnosis of Bovine Brucellosis, Tuberculosis, and Leukemia. Biosensors 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Dai, E.H.; Xiao, R.; Zhou, Z.H.; Zhang, M.L.; Bai, Z.K.; Shao, Y.; Qi, K.Z.; Tu, J.; Wang, C.W.; et al. Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of anti-SARS-CoV-2 IgM/IgG in clinical samples. Sens. Actuator B Chem. 2021, 329, 10. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Barshevskaya, L.V.; Bartosh, A.V.; Zherdev, A.V.; Dzantiev, B.B. Double Competitive Immunodetection of Small Analyte: Realization for Highly Sensitive Lateral Flow Immunoassay of Chloramphenicol. Biosensors 2022, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, W.B.; Wang, Z.D.; Wang, X.W.; Cui, L.; Huang, W.Y.; Zhu, Z.Y.; Liu, Z.J. Highly efficient detection of deoxynivalenol and zearalenone in the aqueous environment based on nanoenzyme-mediated lateral flow immunoassay combined with smartphone. J. Environ. Chem. Eng. 2023, 11, 9. [Google Scholar] [CrossRef]
- Willemsen, L.; Wichers, J.; Xu, M.; Van Hoof, R.; Van Dooremalen, C.; Van Amerongen, A.; Peters, J. Biosensing Chlorpyrifos in Environmental Water Samples by a Newly Developed Carbon Nanoparticle-Based Indirect Lateral Flow Assay. Biosensors 2022, 12, 13. [Google Scholar] [CrossRef]
- Hua, Q.C.; Liu, Z.W.; Wang, J.; Liang, Z.Q.; Zhou, Z.X.; Shen, X.; Lei, H.T.; Li, X.M. Magnetic immunochromatographic assay with smartphone-based readout device for the on-site detection of zearalenone in cereals. Food Control 2022, 134, 11. [Google Scholar] [CrossRef]
- Wild, D. (Ed.) The Immunoassay Handbook: Theory and Applications of Ligand Binding, ELISA and Related Techniques; People’s Medical Publishing House: Beijing, China, 2021; pp. 6–8. [Google Scholar]
- Datta, P. Immunoassay design. In Accurate Results in the Clinical Laboratory; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–73. [Google Scholar]
- Zhang, G. Rapid Detection Technology of Immunochromatographic Test Strips; Henan Science and Technology Press: Zhengzhou, China, 2015; pp. 8–11. [Google Scholar]
- Tong, L.; Li, D.Q.; Huang, M.; Huang, L.; Wang, J. Gold-Silver Alloy Nanoparticle-Incorporated Pitaya-Type Silica Nanohybrids for Sensitive Competitive Lateral Flow Immunoassay. Anal. Chem. 2023, 95, 17318–17327. [Google Scholar] [CrossRef]
- Jia, J.H.; Ao, L.J.; Luo, Y.X.; Liao, T.; Huang, L.; Zhuo, D.; Jiang, C.X.; Wang, J.; Hu, J. Quantum dots assembly enhanced and dual-antigen sandwich structured lateral flow immunoassay of SARS-CoV-2 antibody with simultaneously high sensitivity and specificity. Biosens. Bioelectron. 2022, 198, 8. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.; Jia, S.T.; Zhao, L.L.; Jia, T.T.; Xue, C.H. Electrospun nitrocellulose membrane for immunochromatographic test strip with high sensitivity. Microchim. Acta 2020, 187, 644. [Google Scholar] [CrossRef]
- Lin, D.; Li, B.; Fu, L.; Qi, J.; Xia, C.; Zhang, Y.; Chen, L. A novel polymer-based nitrocellulose platform for implementing a multiplexed microfluidic paper-based enzyme-linked immunosorbent assay. Microsyst. Nanoeng. 2022, 8, 53. [Google Scholar] [CrossRef]
- Christopoulou, N.M.; Kalogianni, D.P.; Christopoulos, T.K. Macromolecular crowding agents enhance the sensitivity of lateral flow immunoassays. Biosens. Bioelectron. 2022, 218, 8. [Google Scholar] [CrossRef]
- Liu, X.J.; Chen, Y.Q.; Bu, T.; Deng, Z.I.; Zhao, L.; Tian, Y.L.; Jia, C.H.; Li, Y.C.; Wang, R.; Wang, J.L.; et al. Nanosheet antibody mimics based label-free and dual-readout lateral flow immunoassay for Salmonella enteritidis rapid detection. Biosens. Bioelectron. 2023, 229, 7. [Google Scholar] [CrossRef]
- Zadehkafi, A.; Siavashi, M.; Asiaei, S.; Bidgoli, M.R. Simple geometrical modifications for substantial color intensity and detection limit enhancements in lateral-flow immunochromatographic assays. J. Chromatogr. B 2019, 1110, 1–8. [Google Scholar] [CrossRef]
- Díaz-González, M.; de la Escosura-Muñiz, A. Strip modification and alternative architectures for signal amplification in nanoparticle-based lateral flow assays. Anal. Bioanal. Chem. 2021, 413, 4111–4117. [Google Scholar] [CrossRef]
- Schaumburg, F.; Kler, P.A.; Berli, C.L.A. Numerical prototyping of lateral flow biosensors. Sens. Actuator B Chem. 2018, 259, 1099–1107. [Google Scholar] [CrossRef]
- Jeon, M.J.; Kim, S.-K.; Hwang, S.-H.; Lee, J.U.; Sim, S.J. Lateral flow immunoassay based on surface-enhanced Raman scattering using pH-induced phage-templated hierarchical plasmonic assembly for point-of-care diagnosis of infectious disease. Biosens. Bioelectron. 2024, 250, 116061. [Google Scholar] [CrossRef] [PubMed]
- Kunpatee, K.; Khantasup, K.; Komolpis, K.; Yakoh, A.; Nuanualsuwan, S.; Sain, M.M.; Chaiyo, S. Ratiometric electrochemical lateral flow immunoassay for the detection of Streptococcus suis serotype 2. Biosens. Bioelectron. 2023, 242, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hu, H.; Deng, S.L.; Xiao, X.Y.; Xiong, Y.H.; Peng, J.; Lai, W.H. An integrated colorimetric and photothermal lateral flow immunoassay based on bimetallic Ag-Au urchin-like hollow structures for the sensitive detection of E. coli O157:H7. Biosens. Bioelectron. 2023, 225, 10. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.; Buragohain, P.; Borse, V. Strategies for sensitivity enhancement of point-of-care devices. Biosens. Bioelectron. 2022, 10, 100098. [Google Scholar] [CrossRef]
- Gasperino, D.; Baughman, T.; Hsieh, H.V.; Bell, D.; Weigl, B.H. Improving Lateral Flow Assay Performance Using Computational Modeling. In Annual Review of Analytical Chemistry; Bohn, P.W., Pemberton, J.E., Eds.; Annual Reviews: Palo Alto, CA, USA, 2018; Volume 11, pp. 219–244. [Google Scholar]
- Qian, S.; Bau, H.H. A mathematical model of lateral flow bioreactions applied to sandwich assays. Anal. Biochem. 2003, 322, 89–98. [Google Scholar] [CrossRef]
- Qian, S.; Bau, H.H. Analysis of lateral flow biodetectors: Competitive format. Anal. Biochem. 2004, 326, 211–224. [Google Scholar] [CrossRef]
- Berli, C.L.A.; Kler, P.A. A quantitative model for lateral flow assays. Microfluid. Nanofluid. 2016, 20, 9. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Mathematical Model of Serodiagnostic Immunochromatographic Assay. Anal. Chem. 2017, 89, 4419–4427. [Google Scholar] [CrossRef] [PubMed]
- Sotnikov, D.V.; Byzova, N.A.; Zvereva, E.A.; Bartosh, A.V.; Zherdev, A.V.; Dzantiev, B.B. Mathematical modeling of immunochromatographic test systems in a competitive format: Analytical and numerical approaches. Biochem. Eng. J. 2020, 164, 9. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Li, A.; Feng, S.S.; Qu, Z.G.; Xu, F. The effect of report particle properties on lateral flow assays: A mathematical model. Sens. Actuator B Chem. 2017, 248, 699–707. [Google Scholar] [CrossRef]
- Liu, Z.; Qu, Z.G.; Tang, R.H.; He, X.C.; Yang, H.; Bai, D.; Xu, F. An improved detection limit and working range of lateral flow assays based on a mathematical model. Analyst 2018, 143, 2775–2783. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.C.; Li, A.; Qu, Z.G.; Xu, F. A two-dimensional mathematical model for analyzing the effects of capture probe properties on the performance of lateral flow assays. Analyst 2019, 144, 5394–5403. [Google Scholar] [CrossRef]
- Asadi, H.; Pourjafar-Chelikdani, M.; Khabazi, N.P.; Sadeghy, K. Quasi-steady imbibition of physiological liquids in paper-based microfluidic kits: Effect of shear-thinning. Phys. Fluids 2022, 34, 15. [Google Scholar] [CrossRef]
- Simulating Rapid Detection in COMSOL Multiphysics®. Available online: http://cn.comsol.com/blogs/modeling-a-rapid-detection-test-in-comsol-multiphysics/ (accessed on 16 August 2023).
- Tay, D.M.; Kim, S.; Hao, Y.; Yee, E.H.; Jia, H.; Vleck, S.M.; Sikes, H.D. Accelerating the optimization of vertical flow assay performance guided by a rational systematic model-based approach. Biosens. Bioelectron. 2023, 222, 114977. [Google Scholar] [CrossRef] [PubMed]
- Mendez, S.; Fenton, E.M.; Gallegos, G.R.; Petsev, D.N.; Sibbett, S.S.; Stone, H.A. Imbibition in porous membranes of complex shape: Quasi-stationary flow in thin rectangular segments. Langmuir 2010, 26, 1380–1385. [Google Scholar] [CrossRef] [PubMed]







| Parameters | Values | Descriptions |
|---|---|---|
| M_l | 2 [cm] | NC membrane length |
| M_th | 125 [m] | NC membrane thickness |
| L_th | 1 [mm] | Line thickness |
| Poro | 0.7 | Porosity of NC membrane |
| d_p | 0.45 [μm] | Pore diameter of NC membrane |
| 1.715 × 10−14 m2 | Membrane permeability of NC membrane | |
| Poro2 | 0.8 | Porosity of other porous materials |
| d_p2 | 1 [μm] | Pore diameter of other porous materials |
| 2 | 2.8444 × 10−13 m2 | Membrane permeability of other porous materials |
| Hw | 2 [mm] | Water head |
| p0 | −4.06 × 10−6 N/m2 | Richards equation initial phase pressure |
| C0 | 10−8 [M] | Concentration of substances |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, Y.; Niu, Q.; Wang, L.; Xing, C.; Wang, Q.; Bao, H. Research on the Flow Characteristics and Reaction Mechanisms of Lateral Flow Immunoassay under Non-Uniform Flow. Sensors 2024, 24, 1989. https://doi.org/10.3390/s24061989
Zhao X, Zhang Y, Niu Q, Wang L, Xing C, Wang Q, Bao H. Research on the Flow Characteristics and Reaction Mechanisms of Lateral Flow Immunoassay under Non-Uniform Flow. Sensors. 2024; 24(6):1989. https://doi.org/10.3390/s24061989
Chicago/Turabian StyleZhao, Xuyan, Yuan Zhang, Qunfeng Niu, Li Wang, Chenglong Xing, Qiao Wang, and Hui Bao. 2024. "Research on the Flow Characteristics and Reaction Mechanisms of Lateral Flow Immunoassay under Non-Uniform Flow" Sensors 24, no. 6: 1989. https://doi.org/10.3390/s24061989
APA StyleZhao, X., Zhang, Y., Niu, Q., Wang, L., Xing, C., Wang, Q., & Bao, H. (2024). Research on the Flow Characteristics and Reaction Mechanisms of Lateral Flow Immunoassay under Non-Uniform Flow. Sensors, 24(6), 1989. https://doi.org/10.3390/s24061989







