Hydrogen-Bond Acidic Materials in Acoustic Wave Sensors for Nerve Chemical Warfare Agents’ Detection
Abstract
1. Introduction
1.1. Sensors Equipped with Absorptive Layers
1.2. Linear Solvation Energy Relationship (LSER) Model
1.3. Mechanism of Sorption of Organophosphorus Compounds
2. Review of Hydrogen-Bond Acidic (HBA) Polymers
2.1. Linear Organic Polymers
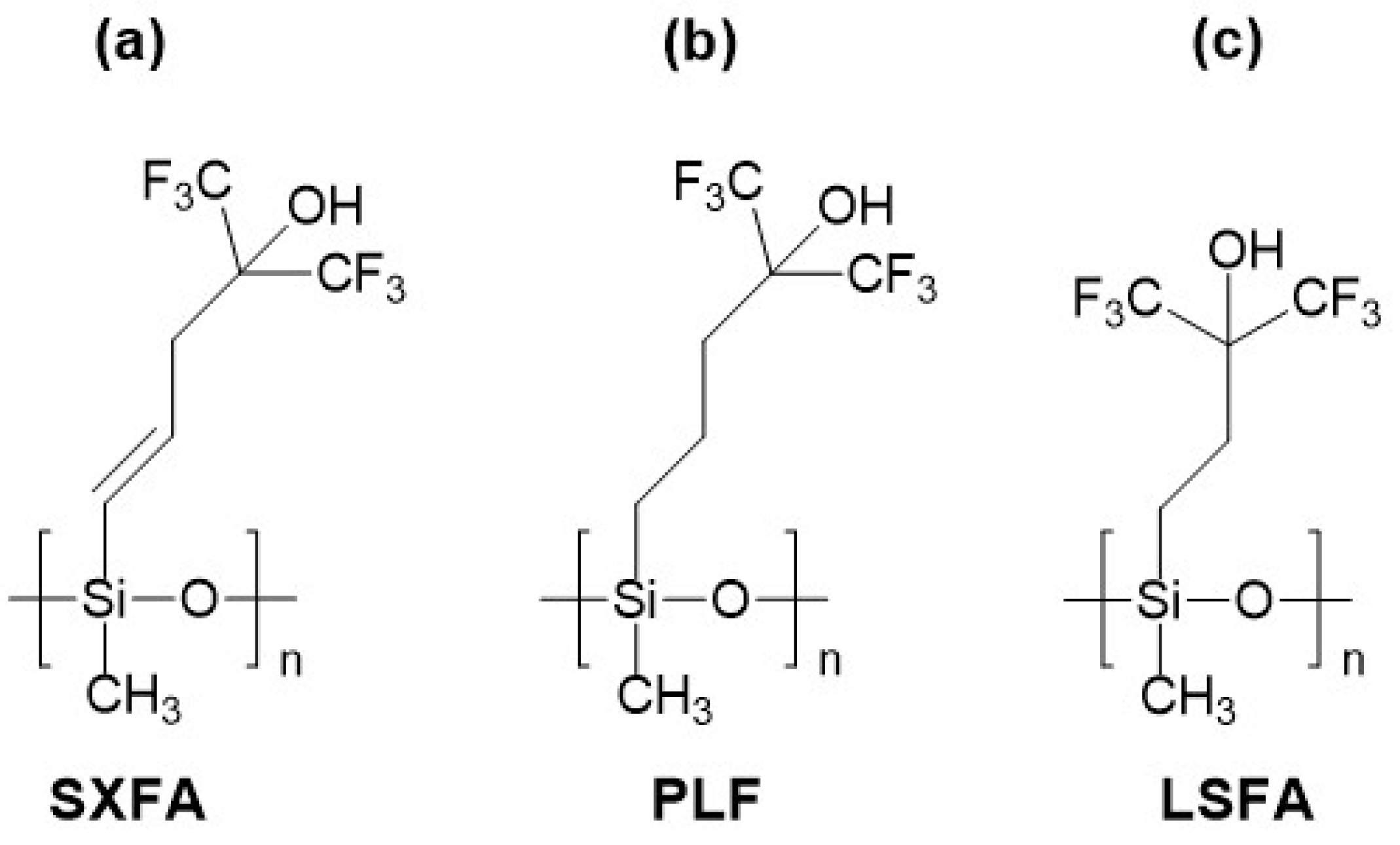
2.2. Linear Silicon-Containing Polymers with Aliphatic Fluoroalcohol Substituents
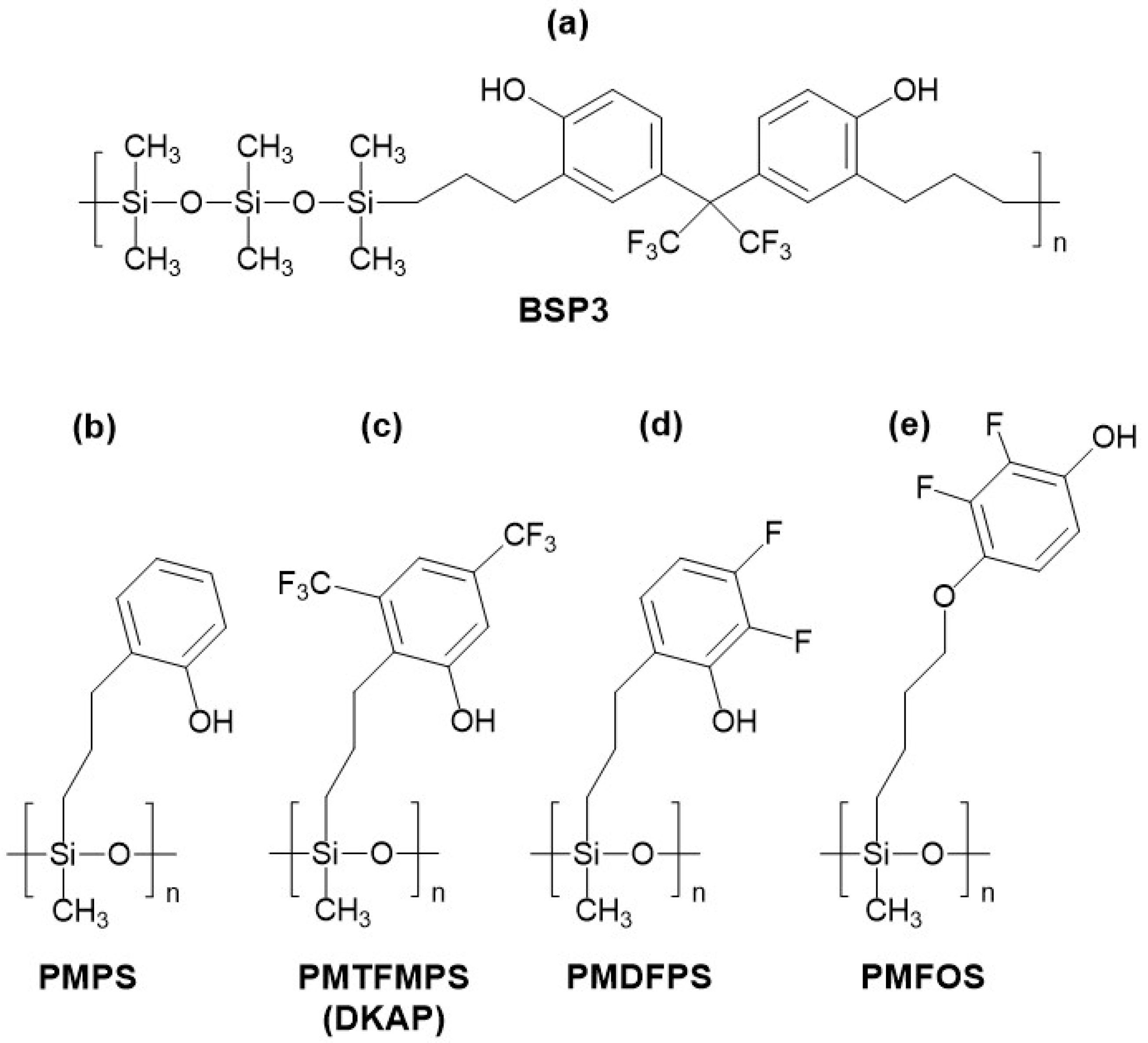
2.3. Linear Silicon-Containing Polymers with Phenol and Fluorophenol Substituents
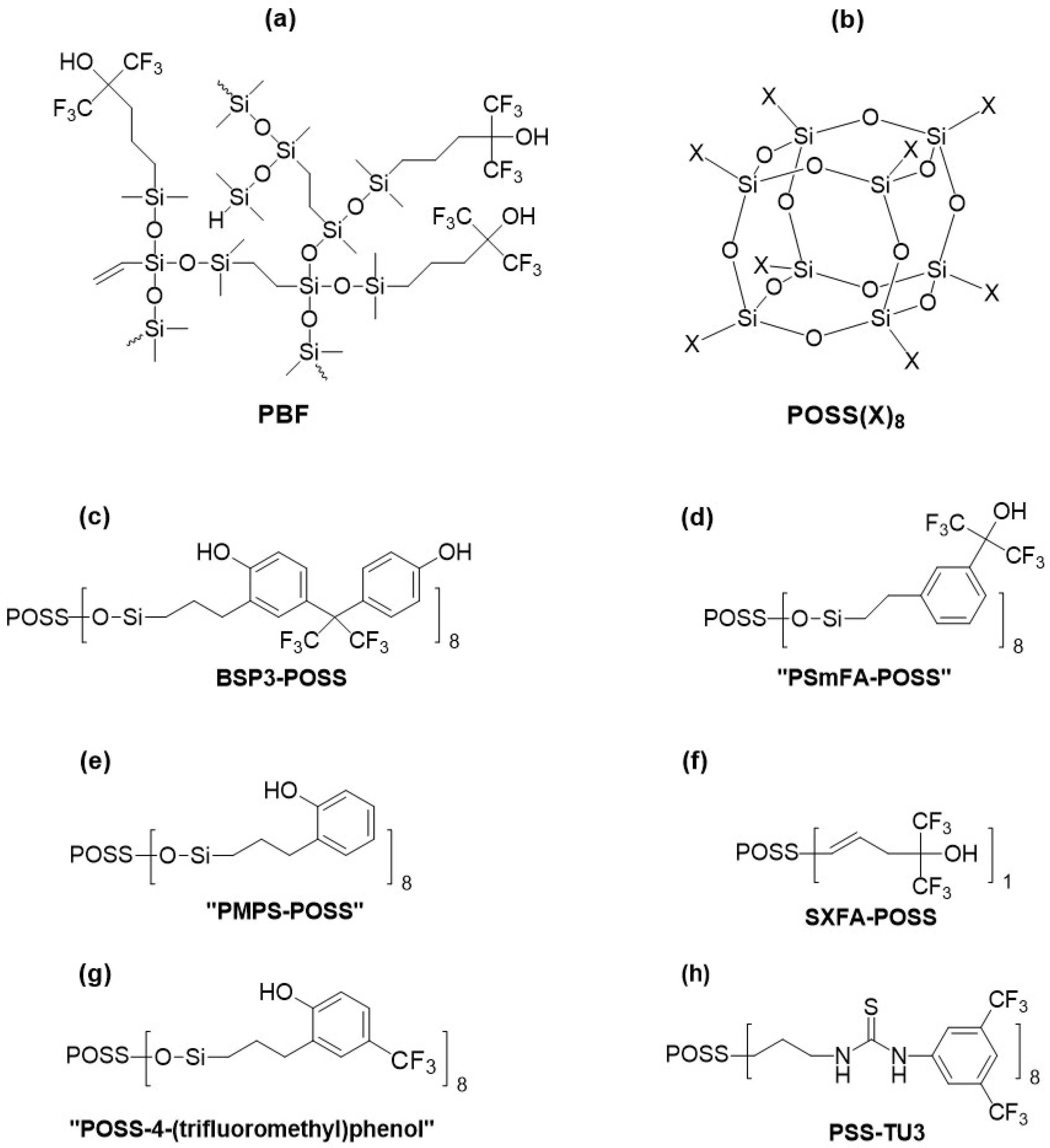
2.4. Silicon-Based Materials with Hyper-Branching and Spatial Architecture
2.5. A Summary of the Review of HBA Polymers
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giannoukos, S.; Brkić, B.; Taylor, S.; Marshall, A.; Verbeck, G.F. Chemical Sniffing Instrumentation for Security Applications. Chem. Rev. 2016, 116, 8146–8172. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Qualley, A.; Hughes, G.T.; Rubenstein, M.H.; Malloy, T.A.; Piatkowski, T. Improving Quantification of Tabun, Sarin, Soman, Cyclosarin, and Sulfur Mustard by Focusing Agents: A Field Portable Gas Chromatography-Mass Spectrometry Study. J. Chromatogr. A 2021, 1636, 461784. [Google Scholar] [CrossRef] [PubMed]
- Puton, J.; Namieśnik, J. Ion Mobility Spectrometry: Current Status and Application for Chemical Warfare Agents Detection. TrAC Trends Anal. Chem. 2016, 85, 10–20. [Google Scholar] [CrossRef]
- Yang, L.; Han, Q.; Cao, S.; Yang, J.; Yang, J.; Ding, M. Portable Solid Phase Micro-Extraction Coupled with Ion Mobility Spectrometry System for On-Site Analysis of Chemical Warfare Agents and Simulants in Water Samples. Sensors 2014, 14, 20963–20974. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Kanamori-Kataoka, M.; Tsuge, K.; Ohsawa, I.; Matsushita, K.; Sekiguchi, H.; Itoi, T.; Iura, K.; Sano, Y.; Yamashiro, S. Sensing Technology for Chemical-Warfare Agents and Its Evaluation Using Authentic Agents. Sens. Actuators B Chem. 2005, 108, 193–197. [Google Scholar] [CrossRef]
- Jin, Y.; Ka, D.; Jang, S.; Heo, D.; Seo, J.A.; Jung, H.; Jeong, K.; Lee, S. Fabrication of Graphene Based Durable Intelligent Personal Protective Clothing for Conventional and Non-Conventional Chemical Threats. Nanomaterials 2021, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Martín, A.; Nakagawa, T.; Barfidokht, A.; Lu, X.; Sempionatto, J.R.; Lyu, K.M.; Karajic, A.; Musameh, M.M.; Kyratzis, I.L.; et al. Detection of Vapor-Phase Organophosphate Threats Using Wearable Conformable Integrated Epidermal and Textile Wireless Biosensor Systems. Biosens. Bioelectron. 2018, 101, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Xie, G.; Chen, C.; Liu, Y.; Tai, H.; Jiang, Y.; Su, Y. Hierarchical piezoelectric composite film for self-powered moisture detection and wearable biomonitoring. Appl. Phys. Lett. 2024, 124, 053701. [Google Scholar] [CrossRef]
- Rosser, K.; Pavey, K.; FitzGerald, N.; Fatiaki, A.; Neumann, D.; Carr, D.; Hanlon, B.; Chahl, J. Autonomous Chemical Vapour Detection by Micro UAV. Remote Sens. 2015, 7, 16865–16882. [Google Scholar] [CrossRef]
- Dawoud, A.; Mia, R.; Motchaalangaram, J.A.; Miao, W.; Wallace, K. Toward Remote Detection of Chemical Warfare Simulants Using a Miniature Potentiostat. Micro 2024, 4, 49–60. [Google Scholar] [CrossRef]
- Pan, Y.; Mu, N.; Liu, B.; Cao, B.; Wang, W.; Yang, L. A Novel Surface Acoustic Wave Sensor Array Based on Wireless Communication Network. Sensors 2018, 18, 2977. [Google Scholar] [CrossRef] [PubMed]
- King, W.H. Piezoelectric Sorption Detector. Anal. Chem. 1964, 36, 1735–1739. [Google Scholar] [CrossRef]
- Pei, Z.; Ma, X.; Ding, P.; Zhang, W.; Luo, Z.; Li, G. Study of a QCM Dimethyl Methylphosphonate Sensor Based on a ZnO-Modified Nanowire-Structured Manganese Dioxide Film. Sensors 2010, 10, 8275–8290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, J.; Yang, M.; Gao, S.; Zuo, G.; Yan, C.; Cheng, Z. Single Crystal WO3 Nanoflakes as Quartz Crystal Microbalance Sensing Layer for Ultrafast Detection of Trace Sarin Simulant. Anal. Chim. Acta 2009, 654, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, H.; Wang, X.; He, J.; Yu, Y.; He, H. Flower-like Tungsten Oxide Particles: Synthesis, Characterization and Dimethyl Methylphosphonate Sensing Properties. Anal. Chim. Acta 2010, 675, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Alev, O.; Özdemir, O.; Goldenberg, E.; Çolakerol Arslan, L.; Büyükköse, S.; Öztürk, Z.Z. WS2 thin film based quartz crystal microbalance gas sensor for dimethyl methylphosphonate detection at room temperature. Thin Solid Film. 2022, 745, 139097. [Google Scholar] [CrossRef]
- Shaik, M.; Rao, V.K.; Ramana, G.V.; Halder, M.; Gutch, P.K.; Pandey, P.; Jain, R. P-Hexafluoroisopropanol Phenyl Functionalized Graphene for QCM Based Detection of Dimethyl Methylphosphonate, a Simulant of the Nerve Agent Sarin. RSC Adv. 2018, 8, 8240–8245. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Jha, P.; Chouksey, A.; Rawat, J.S.B.S.; Tandon, R.P.; Chaudhury, P.K. 4-(Hexafluoro-2-Hydroxy Isopropyl)Aniline Functionalized Highly Sensitive Flexible SWCNT Sensor for Detection of Nerve Agent Simulant Dimethyl Methylphosphonate. Mater. Chem. Phys. 2016, 181, 487–494. [Google Scholar] [CrossRef]
- Wu, Q.; Li, X.; Wang, X.; Yuan, Y.; Bu, X.; Wu, H.; Li, X.; Han, C.; Wang, X.; Liu, W. High-Performance p-Hexafluoroisopropanol Phenyl Functionalized Multi-Walled Carbon Nanotube Film on Surface Acoustic Wave Device for Organophosphorus Vapor Detection. Nanotechnology 2022, 33, 375501. [Google Scholar] [CrossRef]
- Vergara, A.V.; Pernites, R.B.; Pascua, S.; Binag, C.A.; Advincula, R.C. QCM Sensing of a Chemical Nerve Agent Analog via Electropolymerized Molecularly Imprinted Polythiophene Films. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 675–685. [Google Scholar] [CrossRef]
- Diauudin, F.N.; Rashid, J.I.A.; Knight, V.F.; Wan Yunus, W.M.Z.; Ong, K.K.; Kasim, N.A.M.; Abdul Halim, N.; Noor, S.A.M. A Review of Current Advances in the Detection of Organophosphorus Chemical Warfare Agents Based Biosensor Approaches. Sens. Bio-Sens. Res. 2019, 26, 100305. [Google Scholar] [CrossRef]
- Tasaltin, C.; Gurol, I.; Harbeck, M.; Musluoglu, E.; Ahsen, V.; Ozturk, Z.Z. Synthesis and DMMP Sensing Properties of Fluoroalkyloxy and Fluoroaryloxy Substituted Phthalocyanines in Acoustic Sensors. Sens. Actuators B Chem. 2010, 150, 781–787. [Google Scholar] [CrossRef]
- Wang, P.-H.; Yu, J.-H.; Li, Z.-J.; Ding, Z.-J.; Guo, L.; Du, B. Synthesis and Evaluation of a New Phthalocyanine as Sensor Material for Sarin Detection. Sens. Actuators B Chem. 2013, 188, 1306–1311. [Google Scholar] [CrossRef]
- Şen, Z.; Tarakci, D.K.; Gürol, I.; Ahsen, V.; Harbeck, M. Governing the Sorption and Sensing Properties of Titanium Phthalocyanines by Means of Axial Ligands. Sens. Actuators B Chem. 2016, 229, 581–586. [Google Scholar] [CrossRef]
- Hejczyk, T.; Wrotniak, J.; Magnuski, M.; Jakubik, W. Experimental and Numerical Acoustoelectric Investigation of the New SAW Structure with (RR)-P3HT Polymer in DMMP Detection. Arch. Acoust. 2023, 46, 313–322. [Google Scholar] [CrossRef]
- Jakubik, W.; Wrotniak, J.; Kaźmierczak-Bałata, A.; Stolarczyk, A.; Powroźnik, P. Light-Activated SAW Sensor Structures with Photoconductive Polymer Films for DMMP Detection. Sens. Actuators B Chem. 2023, 384, 133597. [Google Scholar] [CrossRef]
- Henry, W. Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures. Philos. Trans. R. Soc. 1803, 93, 29–43. [Google Scholar]
- Lin, R.-C.; Chen, Y.-C.; Chang, W.-T.; Cheng, C.-C.; Kao, K.-S. Highly Sensitive Mass Sensor Using Film Bulk Acoustic Resonator. Sens. Actuators A Phys. 2008, 147, 425–429. [Google Scholar] [CrossRef]
- Ballantine, D., Jr.; White, R.; Martin, S.; Ricco, A.; Frye, G.; Zellers, E.; Wohltjen, H. Acoustic Wave Sensors: Theory, Design, and Physico-Chemical Applications in Applications of Modern Acoustics; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Vitha, M.; Carr, P.W. The Chemical Interpretation and Practice of Linear Solvation Energy Relationships in Chromatography. J. Chromatogr. A 2006, 1126, 143–194. [Google Scholar] [CrossRef] [PubMed]
- Grate, J.W.; McGill, R.A.; Abraham, M.H. Chemically Selective Polymer Coatings for Acoustic Vapor Sensors and Arrays. In Proceedings of the IEEE 1992 Ultrasonics Symposium Proceedings, Tucson, AZ, USA, 20–23 October 1992; IEEE: Tucson, AZ, USA, 1992; pp. 275–279. [Google Scholar]
- Abraham, M.H.; Ibrahim, A.; Zissimos, A.M. Determination of Sets of Solute Descriptors from Chromatographic Measurements. J. Chromatogr. A 2004, 1037, 29–47. [Google Scholar] [CrossRef]
- Grate, J.W. Hydrogen-Bond Acidic Polymers for Chemical Vapor Sensing. Chem. Rev. 2008, 108, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, N.; Endo, S.; Brown, T.N.; Watanabe, N.; Bronner, G.; Abraham, M.H.; Goss, K.-U. UFZ-LSER Database v 3.2.1 [Internet]; Helmholtz Centre for Environmental Research-UFZ: Leipzig, Germany, 2017; Available online: https://www.ufz.de/index.php?en=31698&contentonly=1&m=0&lserd_data[mvc]=Public/start#searchresult (accessed on 4 March 2024).
- Abraham, M.H.; Acree, W.E., Jr. Descriptors for the Prediction of Partition Coefficients and Solubilities of Organophosphorus Compounds. Sep. Sci. Technol. 2013, 48, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.D.; Poole, C.F.; Abraham, M.H. Synthesis and Gas Chromatographic Evaluation of a High-Temperature Hydrogen-Bond Acid Stationary Phase. J. Chromatogr. A 1998, 805, 217–235. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA; London, UK, 2014. [Google Scholar]
- Matatagui, D.; Martí, J.; Fernández, M.J.; Fontecha, J.L.; Gutiérrez, J.; Gràcia, I.; Cané, C.; Horrillo, M.C. Chemical Warfare Agents Simulants Detection with an Optimized SAW Sensor Array. Sens. Actuators B Chem. 2011, 154, 199–205. [Google Scholar] [CrossRef]
- Grate, J.W.; Wise, B.M.; Abraham, M.H. Method for Unknown Vapor Characterization and Classification Using a Multivariate Sorption Detector. Initial Derivation and Modeling Based on Polymer-Coated Acoustic Wave Sensor Arrays and Linear Solvation Energy Relationships. Anal. Chem. 1999, 71, 4544–4553. [Google Scholar] [CrossRef]
- Matatagui, D.; Martí, J.; Fernández, M.J.; Fontecha, J.L.; Santos, J.P.; Gràcia, I.; Cané, C.; Horrillo, M.C. Love-Wave Sensor Array to Detect, Discriminate and Classify Chemical Warfare Agent Simulants. Sens. Actuators B Chem. 2012, 175, 173–178. [Google Scholar] [CrossRef]
- Grabka, M.; Jasek, K.; Choma, J. Application of Poly(bis-cyanopropylsiloxane) in Surface Acoustic Wave Sensors to Organophosphorus Chemical Warfare Agent Detection. Environ. Pollut. Control 2018, 40, 9–16. [Google Scholar]
- Zellers, E.T.; Han, M. Effects of Temperature and Humidity on the Performance of Polymer-Coated Surface Acoustic Wave Vapor Sensor Arrays. Anal. Chem. 1996, 68, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.W. 1984 Scientific Conference on Chemical Defense Research; Aberdeen Proving Ground: Harford County, MD, USA, 1984. [Google Scholar]
- Ting, S.P.; Pearce, E.M.; Kwei, T.K. Compatibility Studies of Poly(styrene-co-Vinylphenyl Hexafluorodimethyl Carbinol) with Bisphenol A Polycarbonate, Poly(butyl Methacrylate) and Poly(2,6-dimethyl-1,4-Phenylene Oxide). J. Polym. Sci. Part C Polym. Lett. 1980, 18, 201. [Google Scholar] [CrossRef]
- Barlow, J.W.; Cassidy, P.E.; Lloyd, D.R.; You, C.-J.; Chang, Y.; Wong, P.C.; Noriyan, J. Polymer Sorbents for Phosphorus Esters: II. Hydrogen Bond Driven Sorption in Fluoro-Carbinol Substituted Polystyrene. Polym. Eng. Sci. 1987, 27, 703–715. [Google Scholar] [CrossRef]
- Chang, Y.; Noriyan, J.; Lloyd, D.R.; Barlow, J.W. Polymer Sorbents for Phosphorus Esters: I. Selection of Polymers by Analog Calorimetry. Polym. Eng. Sci. 1987, 27, 693–702. [Google Scholar] [CrossRef]
- Snow, A.W.; Sprague, L.G.; Soulen, R.L.; Grate, J.W.; Wohltjen, H. Synthesis and Evaluation of Hexafluorodimethylcarbinol Functionalized Polymers as Microsensor Coatings. J. Appl. Polym. Sci. 1991, 43, 1659–1671. [Google Scholar] [CrossRef]
- Ballantine, D.S.; Rose, S.L.; Grate, J.W.; Wohltjen, H. Correlation of Surface Acoustic Wave Device Coating Responses with Solubility Properties and Chemical Structure Using Pattern Recognition. Anal. Chem. 1986, 58, 3058–3066. [Google Scholar] [CrossRef]
- Field, D.E. Fluorinated Polyepoxy and Polyurethane Coatings. J. Coat. Technol. 1976, 48, 43. [Google Scholar]
- Rebière, D.; Déjous, C.; Pistré, J.; Planade, R.; Lipskier, J.-F.; Robin, P. Surface Acoustic Wave Detection of Organophosphorus Compounds with Fluoropolyol Coatings. Sens. Actuators B Chem. 1997, 43, 34–39. [Google Scholar] [CrossRef]
- Rebière, D.; Déjous, C.; Pistré, J.; Lipskier, J.-F.; Planade, R. Synthesis and Evaluation of Fluoropolyol Isomers as Saw Microsensor Coatings: Role of Humidity and Temperature. Sens. Actuators B Chem. 1998, 49, 139–145. [Google Scholar] [CrossRef]
- Gupta, D.C.; Gutch, P.K.; Lal, G.; Vyas, K.D.; Jaiswal, D.K. Fluorinated Epoxy Resins-Based Sorbent Coating Materials for Quartz Piezoelectric Crystal Detector. Def. Sci. J. 2004, 54, 229–234. [Google Scholar] [CrossRef][Green Version]
- Wohltjen, H.; Snow, A.W.; Barger, W.R.; Ballantine, D.S. Trace Chemical Vapor Detection Using SAW Delay Line Oscillators. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1987, 34, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Grate, J.W.; Snow, A.; Ballantine, D.S.; Wohltjen, H.; Abraham, M.H.; McGill, R.A.; Sasson, P. Determination of Partition Coefficients from Surface Acoustic Wave Vapor Sensor Responses and Correlation with Gas-Liquid Chromatographic Partition Coefficients. Anal. Chem. 1988, 60, 869–875. [Google Scholar] [CrossRef]
- Grate, J.W.; Rose-Pehrsson, S.L.; Venezky, D.L.; Klusty, M.; Wohltjen, H. Smart Sensor System for Trace Organophosphorus and Organosulfur Vapor Detection Employing a Temperature-Controlled Array of Surface Acoustic Wave Sensors, Automated Sample Preconcentration, and Pattern Recognition Available. Anal. Chem. 1993, 65, 1868–1881. [Google Scholar] [CrossRef]
- Agent GB (Sarin) Results—AEGL Program. Available online: https://www.epa.gov/aegl/agent-gb-sarin-results-aegl-program (accessed on 25 February 2024).
- Zhang, C.; Qu, L.; Wang, Y.; Xu, T.; Zhang, C. Thermal Insulation and Stability of Polysiloxane Foams Containing Hydroxyl-Terminated Polydimethylsiloxanes. RSC Adv. 2018, 8, 9901–9909. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Andonian-Haftvan, J.; Du, C.M.; Diart, V.; Whiting, G.S.; Grate, J.W.; McGill, R.A. Hydrogen Bonding. Part 29. Characterization of 14 Sorbent Coatings for Chemical Microsensors Using a New Solvation Equation. J. Chem. Soc. Perkin Trans. 1995, 2, 369–378. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; Li, S.; Liu, M.; Pan, Y. Advances in SXFA-Coated SAW Chemical Sensors for Organophosphorous Compound Detection. Sensors 2011, 11, 1526–1541. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, G.; Guo, T.; Liu, X.; Zhang, C.; Yang, J.; Cao, B.; Zhang, C.; Wang, W. Environmental Characteristics of Surface Acoustic Wave Devices for Sensing Organophosphorus Vapor. Sens. Actuators B Chem. 2020, 315, 127986. [Google Scholar] [CrossRef]
- McGill, R.A.; Chrisey, D.B.; Mlsna, T.E.; Stepnowski, J.L.; Chung, R.; Cotal, H. Performance optimization of surface acoustic wave chemical sensors. In Proceedings of the International Frequency Control Symposium, Orlando, FL, USA, 30 May 1997; pp. 140–156. [Google Scholar] [CrossRef]
- McGill, R.A.; Chung, R.; Chrisey, D.B.; Dorsey, P.C.; Matthews, P.; Pique, A.; Mlsna, T.E.; Stepnowski, J.L. Performance Optimization of Surface Acoustic Wave Chemical Sensors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1998, 45, 1370–1380. [Google Scholar] [CrossRef]
- Piqué, A.; Auyeung, R.C.Y.; Stepnowski, J.L.; Weir, D.W.; Arnold, C.B.; McGill, R.A.; Chrisey, D.B. Laser Processing of Polymer Thin Films for Chemical Sensor Applications. Surf. Coat. Technol. 2003, 163–164, 293–299. [Google Scholar] [CrossRef]
- Singh, H.; Raj, V.B.; Kumar, J.; Durani, F.; Mishra, M.; Nimal, A.T.; Sharma, M.U. SAW Mono Sensor for Identification of Harmful Vapors Using PCA and ANN. Process Saf. Environ. Prot. 2016, 102, 577–588. [Google Scholar] [CrossRef]
- Yan, C.; Qin, M.; Guo, T.; Zhang, L.; Yang, J.; Pan, Y. Synthesis and Application of Polymer SXFA in the Detection of Organophosphine Agents with a SAW Sensor. Polymers 2024, 16, 784. [Google Scholar] [CrossRef] [PubMed]
- Demathieu, C.; Chehimi, M.M.; Lipskier, J.-F. Inverse Gas Chromatographic Characterization of Functionalized Polysiloxanes. Relevance to Sensors Technology. Sens. Actuators B Chem. 2000, 62, 1–7. [Google Scholar] [CrossRef]
- Zimmermann, C.; Rebière, D.; Déjous, C.; Pistré, J.; Chastaing, E.; Planade, R. A Love-Wave Gas Sensor Coated with Functionalized Polysiloxane for Sensing Organophosphorus Compounds. Sens. Actuators B Chem. 2001, 76, 86–94. [Google Scholar] [CrossRef]
- Mazein, P.; Zimmermann, C.; Rebière, D.; Déjous, C.; Pistré, J.; Planade, R. Dynamic Analysis of Love Waves Sensors Responses: Application to Organophosphorus Compounds in Dry and Wet Air. Sens. Actuators B Chem. 2003, 95, 51–57. [Google Scholar] [CrossRef]
- Zimmermann, C.; Mazein, P.; Rebiere, D.; Dejous, C.; Pistre, J.; Planade, R. Detection of GB and DMMP Vapors by Love Wave Acoustic Sensors Using Strong Acidic Fluoride Polymers. IEEE Sens. J. 2004, 4, 479–488. [Google Scholar] [CrossRef]
- Zimmermann, C.; Mazein, P.; Rebière, D.; Déjous, C.; Pistré, J.; Planade, R. Effect of relative humidity on the performance of organo-phosphorus Love-wave sensors using functionalized polysiloxane with fluoride group. In Proceedings of the Eurosensors XVI Proceedings, Prague, Czech Republic, 15–18 September 2002; pp. 1123–1126. [Google Scholar]
- Wang, Y.; Du, X.; Li, Y.; Long, Y.; Qiu, D.; Tai, H.; Tang, X.; Jiang, Y. A Simple Route to Functionalize Siloxane Polymers for DMMP Sensing. J. Appl. Polym. Sci. 2013, 130, 4516–4520. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Y.; Du, X.; Cheng, L.; Wu, P.; Jiang, Y. The Different Sensitive Behaviors of a Hydrogen-Bond Acidic Polymer-Coated SAW Sensor for Chemical Warfare Agents and Their Simulants. Sensors 2015, 15, 18302–18314. [Google Scholar] [CrossRef] [PubMed]
- Grabka, M.; Jasek, K.; Pasternak, M. Application of Polymethyl [4-(2,3-Difluoro-4-Hydroxyphenoxy)Butyl] Siloxane in Surface Acoustic Wave Gas Sensors for Dimethyl Methylphosphonate Detection. Sens. Actuators B Chem. 2021, 329, 129216. [Google Scholar] [CrossRef]
- Abraham, M.H. Scales of Solute Hydrogen-Bonding: Their Construction and Application to Physicochemical and Biochemical Processes. Chem. Soc. Rev. 1993, 22, 73–83. [Google Scholar] [CrossRef]
- Grate, J.W.; Abraham, M.H. Solubility Interactions and the Design of Chemically Selective Sorbent Coatings for Chemical Sensors and Arrays. Sens. Actuators B Chem. 1991, 3, 85–111. [Google Scholar] [CrossRef]
- Grate, J.W.; Kaganove, S.N.; Patrash, S.J.; Craig, R.; Bliss, M. Hybrid Organic/Inorganic Copolymers with Strongly Hydrogen-Bond Acidic Properties for Acoustic Wave and Optical Sensors. Chem. Mater. 1997, 9, 1201–1207. [Google Scholar] [CrossRef]
- Abraham, M.H.; Hamerton, I.; Rose, J.B.; Grate, J.W. Hydrogen Bonding. Part 18. Gas–Liquid Chromatographic Measurements for the Design and Selection of Some Hydrogen Bond Acidic Phases Suitable for Use as Coatings on Piezoelectric Sorption Detectors. J. Chem. Soc. Perkin Trans. 1991, 2, 1417–1423. [Google Scholar] [CrossRef]
- Grate, J.W.; Patrash, S.J.; Kaganove, S.N.; Wise, B.M. Hydrogen Bond Acidic Polymers for Surface Acoustic Wave Vapor Sensors and Arrays. Anal. Chem. 1999, 71, 1033–1040. [Google Scholar] [CrossRef]
- Wang, W.; Hu, H.; Chen, G.; Xie, X.; He, S. Optimization of a BSP3-Coated Surface Acoustic Wave Chemical Sensor. IEEE Sens. J. 2015, 15, 6730–6737. [Google Scholar] [CrossRef]
- Du, X.; Ying, Z.; Jiang, Y.; Liu, Z.; Yang, T.; Xie, G. Synthesis and Evaluation of a New Polysiloxane as SAW Sensor Coatings for DMMP Detection. Sens. Actuators B Chem. 2008, 134, 409–413. [Google Scholar] [CrossRef]
- He, W.; Liu, Z.; Du, X.; Jiang, Y.; Xiao, D. Analytical Application of Poly{methyl[3-(2-Hydroxy-3,4-Difluoro)Phenyl]Propyl Siloxane} as a QCM Coating for DMMP Detection. Talanta 2008, 76, 698–702. [Google Scholar] [CrossRef]
- Du, X.; Wang, Z.; Huang, J.; Tao, S.; Tang, X.; Jiang, Y. A New Polysiloxane Coating on QCM Sensor for DMMP Vapor Detection. J. Mater. Sci. 2009, 44, 5872–5876. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C.; Keeley, D.L.; Dvornic, P.R.; Keinath, S.E.; McCrea, K.R. Hydrogen-Bond Acidic Polyhedral Oligosilsesquioxane Filled Polymer Coatings for Surface Acoustic Wave Sensors. J. Appl. Polym. Sci. 2007, 104, 3171–3182. [Google Scholar] [CrossRef]
- Lewis, P.R.; Manginell, P.; Adkins, D.R.; Kottenstette, R.J.; Wheeler, D.R.; Sokolowski, S.S.; Trudell, D.E.; Byrnes, J.E.; Okandan, M.; Bauer, J.M.; et al. Recent Advancements in the Gas-Phase MicroChemLab. IEEE Sens. J. 2006, 6, 784–795. [Google Scholar] [CrossRef]
- Wang, Y.; Du, X.; Long, Y.; Tang, X.; Tai, H.; Jiang, Y. The Response Comparison of a Hydrogen-Bond Acidic Polymer to Sarin, Soman and Dimethyl Methyl Phosphonate Based on a Surface Acoustic Wave Sensor. Anal. Methods 2014, 6, 1951–1955. [Google Scholar] [CrossRef]
- Holmes, M.A.; Mackay, M.E.; Giunta, R.K. Nanoparticles for Dewetting Suppression of Thin Polymer Films Used in Chemical Sensors. J. Nanopart. Res. 2007, 9, 753–763. [Google Scholar] [CrossRef]
- Grabka, M.; Jasek, K.; Witkiewicz, Z. Inverse Gas Chromatographic Evaluation of Polysiloxanes Containing Phenolic and Fluorophenolic Functional Groups for Use in Gas Sensors. Talanta 2021, 234, 122711. [Google Scholar] [CrossRef] [PubMed]
- Grabka, M.; Kula, P.; Szala, M.; Jasek, K.; Czerwiński, M. Fluorophenol-Containing Hydrogen-Bond Acidic Polysiloxane for Gas Sensing-Synthesis and Characterization. Polymers 2022, 14, 1147. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C.; Hu, J.; Kaganove, S.N.; Keinath, S.E.; Keeley, D.L.; Dvornic, P.R. Hydrogen-Bond Acidic Hyperbranched Polymers for Surface Acoustic Wave (SAW) Sensors. Chem. Mater. 2004, 16, 5357–5364. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C.; Keeley, D.L.; Voit, B.; Eichhorn, K.-J.; Mikhaylova, Y. Hyperbranched Polyesters with Internal and Exo-Presented Hydrogen-Bond Acidic Sensor Groups for Surface Acoustic Wave Sensors. J. Appl. Polym. Sci. 2008, 107, 1401–1406. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Kim, J.; Seo, B.-I.; Kim, J.-H. SAW Chemical Array Device Coated with Polymeric Sensing Materials for the Detection of Nerve Agents. Sensors 2020, 20, 7028. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.-G.; Jang, H.-C.; Choi, H.-S.; Lee, Y.-J.; Kim, J.-H. High Performance and Reusable SAW Sensor Coated with Thiourea-Decorated POSS with Different Functional Groups for DMMP Detection. Coatings 2023, 13, 348. [Google Scholar] [CrossRef]
- Sferopoulos, R. A Review of Chemical Warfare Agent (CWAs) Detector Technologies and Commercial-Off-The-Shelf Items; Australian Government, Department of Defence: Victoria, BC, Australia, 2009.
- CBRN-Chemical Warfare Agent (CWA) Detectors. Available online: https://enmet.com/product-category/cbrn/ (accessed on 25 February 2024).
- Chen, D.; Wang, J.; Li, D.; Liu, Y.; Song, H.; Liu, Q. A Poly(Vinylidene Fluoride)-Coated ZnO Film Bulk Acoustic Resonator for Nerve Gas Detection. J. Micromech. Microeng. 2011, 21, 085017. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Dickert, F.L. An Overview of High Frequency Acoustic Sensors—QCMs, SAWs and FBARs—Chemical and Biochemical Applications. Sensors 2019, 19, 4395. [Google Scholar] [CrossRef]


| Substance Codename/CAS | LSER Descriptors | Source | ||||
|---|---|---|---|---|---|---|
| E | S | A | B | L | ||
| GA/77-81-6 | 0.34 | 1.14 | 0.05 | 1.29 | 4.78 | [34] |
| GB/107-44-8 | 0 | 0.74 | 0 | 0.81 | 3.10 | |
| GD/96-64-0 | 0 | 0.74 | 0 | 0.81 | 4.35 | |
| VX/50782-69-9 | 0.50 | 1.19 | 0 | 1.63 | 7.53 | |
| DFP/55-91-4 | −0.05 | 0.42 | 0 | 0.86 | 3.83 | |
| DMMP/756-79-6 | 0.21 | 1.62 | 0 | 1.01 | 3.90 | [34] |
| Group of Materials | Name (Acronym) | Type of SAW Device/Center Frequency [MHz] | Layer Thickness [nm]/[kHz] | Temp. [°C]/Humid. [%] | DMMP Conc. [mg/m3] | Response [kHz]/ LOD [mg/m3] | Source |
|---|---|---|---|---|---|---|---|
| Aliphatic fluoroalcohol substituents, linear backbone | FPOL | Delay line/158 | N/A */250 | 30/0 ** | 1.1 | 1.3/1 | [55] |
| SXFA | Delay line/150 | N/A/N/A | 13/47 | 0.8 | 1.6/0.12 | [60] | |
| SXFA | Resonator/417 | 15/500 | N/A/N/A | 0.1 | 2.2/N/A | [63] | |
| SXFA | Delay line/300 | 38/N/A | 14/23 | 0.6 | 1.6/0.004 | [59] | |
| PLF | Resonator/434 | 21/765 | 15/0 | 1 | 3.2/N/A | [71] | |
| PLF | Resonator/195 | N/A/100 | 30/0 | 1.5 | 0.62/0.07 | [73] | |
| LSFA | Resonator/434 | 20.5/740 | 15/0 | 1 | 10/N/A | [71] | |
| Phenol and fluorophenol substituents, linear backbone | BSP-3 | Resonator/300 | 76/170 | 20/35 | 1.5 | 4/0.004 | [79] |
| PMPS | Resonator/434 | N/A/600 | 25/70 | 316 | 16/N/A | [80] | |
| DKAP | Resonator/434 | N/A/1200 | 20/N/A | 1 | 11/N/A | [85] | |
| PMFOS | Resonator/195 | N/A/100 | 30/0 | 1.5 | 0.5/0.07 | [73] | |
| Hyper-branched or spatial architecture | HB-PCSOX-BSP3 | N/A/500 | N/A/500 | 28/N/A | 0.09 | 3.6/N/A | [89] |
| HB-PCSOX-PMPS | N/A/500 | N/A/500 | 28/N/A | 0.09 | 2.7/N/A | [89] | |
| PSS-TU3 | Delay line/250 | N/A/N/A | N/A/N/A | 1.0 | 14.7/N/A | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabka, M.; Jasek, K.; Witkiewicz, Z. Hydrogen-Bond Acidic Materials in Acoustic Wave Sensors for Nerve Chemical Warfare Agents’ Detection. Sensors 2024, 24, 2477. https://doi.org/10.3390/s24082477
Grabka M, Jasek K, Witkiewicz Z. Hydrogen-Bond Acidic Materials in Acoustic Wave Sensors for Nerve Chemical Warfare Agents’ Detection. Sensors. 2024; 24(8):2477. https://doi.org/10.3390/s24082477
Chicago/Turabian StyleGrabka, Michał, Krzysztof Jasek, and Zygfryd Witkiewicz. 2024. "Hydrogen-Bond Acidic Materials in Acoustic Wave Sensors for Nerve Chemical Warfare Agents’ Detection" Sensors 24, no. 8: 2477. https://doi.org/10.3390/s24082477
APA StyleGrabka, M., Jasek, K., & Witkiewicz, Z. (2024). Hydrogen-Bond Acidic Materials in Acoustic Wave Sensors for Nerve Chemical Warfare Agents’ Detection. Sensors, 24(8), 2477. https://doi.org/10.3390/s24082477







