Abstract
Aerosols, as well as suspended particulate matter, impact atmospheric pollution, the climate, and human health, directly or indirectly. Particle size, chemical composition, and other aerosol characteristics are determinant factors for atmospheric pollution dynamics and more. In the last decade, low-cost devices have been widely used in instrumentation to measure aerosols. However, they present some issues, such as the problem of discriminating whether the aerosol is composed of liquid particles or solid. This issue could lead to errors in the estimation of mass concentration in monitoring environments where there is fog. In this study, we investigate the use of an optical particle counter (OPC) coupled to a quartz crystal microbalance with an integrated microheater (H-QCM) to enhance measurement performances. The H-QCM was used not only to measure the collected mass on its surface but also, by using the integrated microheater, it was able to heat the collected mass by performing heating cycles. In particular, we tested the developed system with aerosolized saline solutions of sodium chloride (NaCl), with three decreasing concentrations of salt and three electronic cigarette solutions (e-liquid), with different concentrations of propylene glycol and glycerin mixtures. The results showed that the OPC coherently counted the salt dilution effects, and the H-QCM output confirmed the presence of liquid and solid particles in the aerosols. In the case of e-liquid aerosols, the OPC counted the particles, and the HQCM output highlighted that in the aerosol, there were no solid particles but a liquid phase only. These findings contribute to the refinement of aerosol measurement methodologies by low-cost sensors, fostering a more comprehensive understanding.
1. Introduction
An aerosol is a colloidal suspension of fine solid or liquid particles in a gas and has been investigated in several research fields. In medicine, it is mainly studied as a method for drug delivery [1]. In material and chemical science, it is studied as a method to produce or functionalize materials [2]. Aerosols, as well as particulate matter or suspended particulate matter (PMx), impact atmospheric pollution, the climate, and human health, either directly or indirectly. For these reasons, the number of publications on atmospheric aerosols has dramatically increased in the last decade [3]. Aerosols play an important role in environmental pollution, and the measurements of the particles’ sizes or their chemical composition are crucial for understanding atmospheric pollution dynamics [4,5,6]. Knowledge of aerosols is continually deepening thanks to scientific research, but concepts such as deliquescence transition, efflorescence, coagulation, nucleation, or hygroscopic growth of salt are not fully understood when the size is submicrometric [7].
In atmospheric pollution monitoring (or PM), aerosols are among the most important pollutants, and several devices are available to perform automatic measurements, such as scanning mobility particle sizer (SMPS), quartz crystal microbalance cascade, beta gauge, and other vibrating devices [8,9,10]. While these devices provide accurate and reproducible responses, they often occupy significant volumes, and their management costs are not sustainable for use in large-scale mapping applications. In fact, most of these mentioned measurement systems require a sampling system upstream of the instrument to treat the air sample (e.g., dryers, thermal conditioning sampler, filters, etc.), which cannot be easily miniaturized and have a non-negligible impact on costs and energy consumption [11,12]. For these reasons, the research community is addressing the study and development of low-cost sensors or sensor systems [13]. These devices, with their small size, low cost, and low power consumption, are suitable for application scenarios where a large number of sampling points are needed [14,15,16]. Optical particle counters (OPCs) and quartz crystal microbalances (QCMs) are low-cost devices widely employed to measure and study aerosols. OPCs count particles with an equivalent diameter in certain ranges using the laser scattering method [17]. QCMs measure the thickness or mass of collected substance on its electrode surface by ultrasonic vibration of the piezoelectric crystal [18,19,20]. These devices are widely used in biosensing, medical, space, and pollutant monitoring applications [21,22,23,24].
As described by Görner et al., a well-calibrated OPC is suitable for aerosol mass concentration monitoring in the workplace [25]. Additionally, in a paper by Hand, Jenny L., and Sonia M. Kreidenweis, OPC data were used to implement a new data analysis method to retrieve the refractive index and effective density from aerosol size distribution data [26].
However, measurements carried out only by OPC can be influenced by the different density or composition of the aerosol, particularly when data about mass concentration are desirable. In fact, to calculate the mass concentration of aerosols, the OPC uses a fixed particle mass density value that can vary depending on the aerosol composition [27,28]. QCM and OPC are powerful tools that can provide valuable information about the properties and behaviour of particles in a wide range of applications. Using them together can provide even more comprehensive insights. As described in the research paper by Kyeong-Rak Lee et al. [29], an OPC and QCM can be used together in certain applications to provide complementary information about the properties and behaviour of particles.
In this work, we propose combining a low-cost OPC with a modified QCM and integrating a microheater and a microresistance temperature detector on its surface (H-QCM) as reported in our previous paper [30]. The developed sensor system produces both particle counting and the possibility of obtaining information regarding the presence of liquid or solid phases in the analyzed aerosol sample. In particular, we tested the developed system with aerosolized saline solutions of sodium chloride (NaCl), with three decreasing concentrations of salt and three electronic cigarette solutions (e-liquid), with different concentrations of propylene glycol and glycerin mixtures. The results highlighted the possibility of using the QCM combined with OPC to determine the presence or absence of a solid phase in the tested aerosol.
This improvement could be applied in real cases where the measurement of aerosols (or PM) may not be entirely reliable using only low-cost OPCs, without any kind of sample processing system, due to the aerosol composition or interference agents such as fog or oil vapours.
2. Methods and Materials
2.1. Solid and Liquid Aerosols
An aerosol is a colloidal suspension of fine solid or liquid particles in a gas [6,31]. An example of a solid aerosol is aerosol salt. Specifically, NaCl aerosol refers to a type of aerosol containing sodium chloride particles generated via various methods such as salt sprays, nebulizers, or humidifiers. These particles vary in size, ranging from fractions of a sub-micrometre to a few millimetres, and they may be uniformly or unevenly distributed throughout the gas.
The salty particles of NaCl easily absorb moisture from the air, making them susceptible to deliquescence and efflorescence. These phenomena occur when the salty particles, now in a wet form, either dissolve into a liquid solution (deliquescence) or leave behind salt crystals on a solid surface as the water evaporates (efflorescence). The incidence of these dynamics depends on factors like humidity and temperature [32].
Instead, an example of a liquid aerosol is the aerosolization of a mixture of propylene glycol (PG) and vegetable glycerin (VG), commonly used as e-liquid for electronic cigarettes. This mixture (when vaporized) produces an aerosol that users inhale. Properties of this aerosol depend on the ratio of PG to VG in the e-liquid. PG produces small liquid particles and less dense aerosol than VG, while VG produces bigger particles and a denser aerosol [33,34].
The OPC is able to detect particles using light scattering and faces a fundamental limitation in differentiating the physical state of the particles. While it provides valuable information about particle size in aerosols, it cannot distinguish between solid and liquid particles solely based on light scattering [25,29].
To address this limitation, especially for the study of liquid and saline aerosols, a combination of the OPC and the H-QCM [30,35] could be very interesting.
The salt particles may initially be found in an aerosol phase, and as soon as they come into contact with the QCM surface, they may undergo phase transition and form a solid deposit.
According to Sauerbrey’s Equation (1), the deposition of salt particles on the QCM surface can lead to an increase in the mass of the QCM, which can be measured as a change in the resonant frequency of the crystal as follows:
Δf refers to the frequency shift due to the changing mass Δm; Cf represents a constant, where f0 is the fundamental resonating frequency; vq is the velocity of propagation of the transverse wave in the plane of the quartz; ρq is the density of the quartz; and A is the effective area of the electrode [36].
The deposition of aerosol salt on an H-QCM can be influenced by various factors, including the concentration and size distribution of the salt particles in the aerosol, the relative humidity of the air, and the temperature of the H-QCM surface [35]. In fact, considering these parameters, phenomena like efflorescence and deliquescence could potentially occur.
Propylene glycol (PG) and vegetable glycerin (VG) can be mixed to produce a liquid aerosol that is detectable on a quartz crystal microbalance (QCM). In this case, detecting liquid particulate matter introduces additional considerations. Liquids may exhibit behaviours such as wetting or adherence, affecting how they distribute and adhere to the QCM surface. This can result in a more nuanced change in mass compared to solid particles. Additionally, it is important to note that under certain conditions, the liquid aerosol could evaporate from the QCM surface.
While the core principles of mass detection can be applied to both solid and liquid particulate matter on a QCM [37], their specific dynamics and interactions can differ. Understanding these subtleties is crucial for interpreting QCM data accurately in studies involving both solid and liquid aerosols to obtain analytical results.
2.2. Reagents and Samples Preparation
The chemicals used to prepare the samples, which included two different sets of solutions, were vegetable glycerol (VG, CAS No. 56-81-5), propylene glycol (PG, CAS No. 57-55-6), and sodium chloride (NaCl, CAS No. 7647-14-5) purchased from Merck (Darmstadt, Germany). A stock solution of NaCl (0.15 M, approximately 0.90%) at a physiological concentration (NaClphy) was prepared by weighing 2.2104 g of NaCl in 250 mL of distilled water (H2Odist) as the solvent. Two additional solutions were prepared by diluting the stock solution by half (NaCl 1:2, 0.075 M) and tenfold (NaCl 1:10, 0.015 M), respectively. After aerosolization, this set of solutions (NaClphy, NaCl1:2, NaCl1:10) simulated saline aerosols, where fine particles of NaCl were suspended in the air at different concentrations.
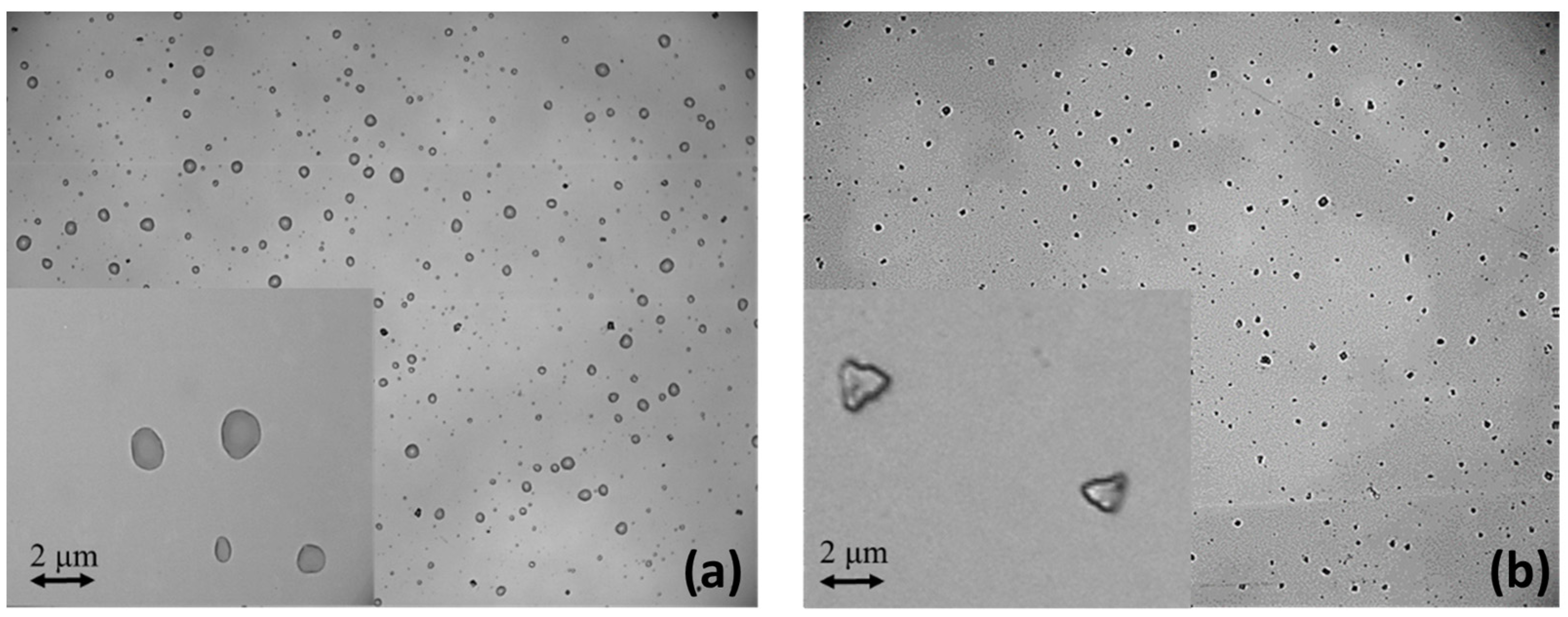
In addition, another set of three solutions was prepared using different ratios of propylene glycol (PG) and glycerol (VG): liq80:20, liq50:50, and liq20:80 of PG and VG, respectively. This time, liquid aerosols were generated, where fine particles of PG:VG were suspended in the air. To summarize, six samples were prepared, three of which are related to solid aerosol (NaClphy, NaCl1:2, and NaCl1:10) and three of which are related to liquid aerosol (liq80:20, liq50:50, and liq20:80). In order to observe the differences between liquid droplets and solid particulate in the samples, a solution of NaCl and one of e-liquid were nebulized over an optically polished quartz slice. Details of the nebulization system will be described in the following paragraph. Using an optical microscope (Leica DM2700 M, By Leica, Milan, Italy) with a 100×/0.85 magnification objective and a 22 mm field of view, liquid droplets of PG:VG were observed (Figure 1a), while solid particles of NaCl were observed (Figure 1b) after aerosolization on a quartz crystal slice. In particular, the photo on the right was captured after a heating treatment of the nebulized sample quartz. In fact, without heating, even the sample with NaCl appeared in the form of liquid droplets.
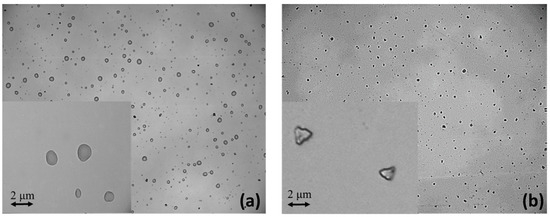
Figure 1.
Microscope images of liquid droplets of PG:VG (a) and solid particles of NaCl deposited over quartz crystal slice after aerosolization (b).
2.3. Measurement Setup
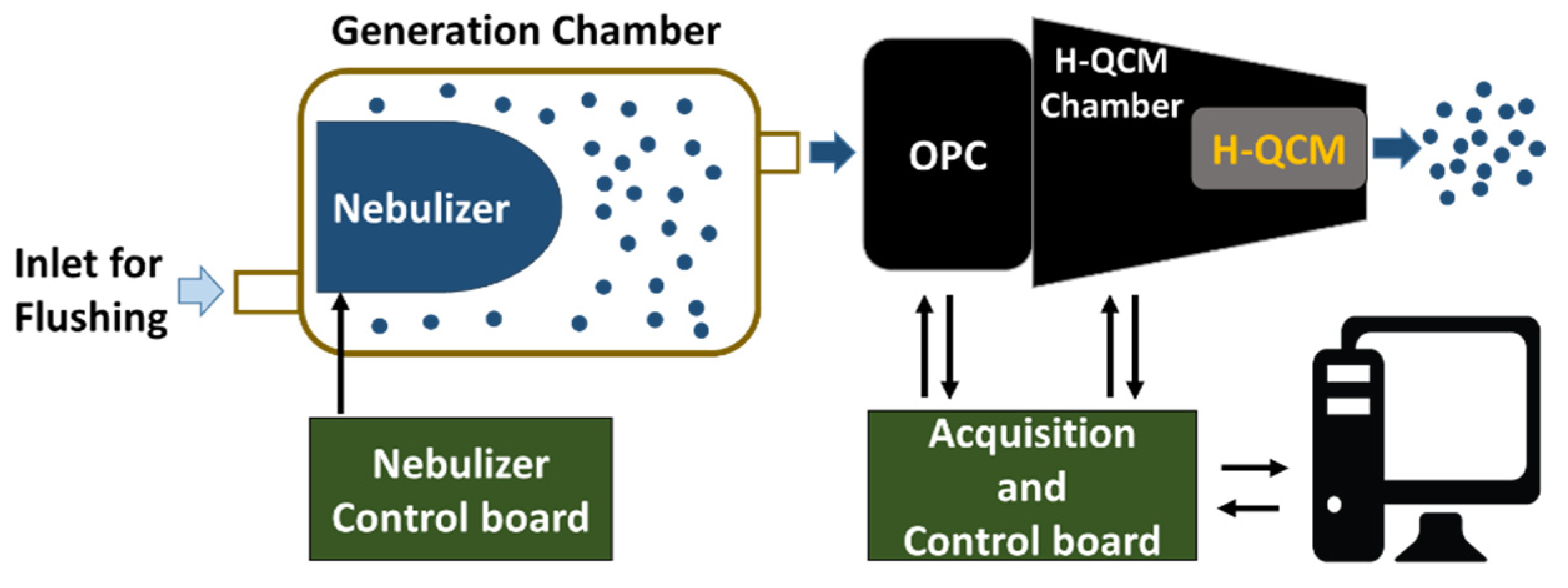
For these experiments, an OPC (OPC-N2 by Alphasense Ltd., Great Notley, Braintree Essex CM77 7AA, Rayne, UK) and an H-QCM were used together to provide complementary information about the properties of aerosols (e.g., liquid or solid phases). Figure 2 reports the scheme of the measurement setup used to perform all tests presented (Figure S1 in the Supplementary Materials shows a photograph of the setup).
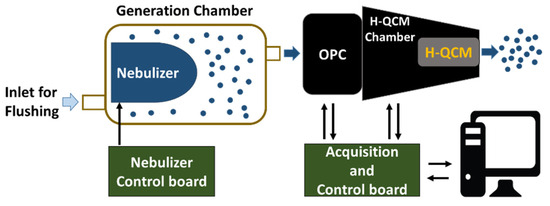
Figure 2.
Measurement setup schematic block. The nebulizer was based on ultrasonic vibrating mesh, and the OPC outlet was connected to H-QCM by a suitable adapter (H-QCM chamber).
Samples were aerosolized using a piezo vibrating mesh nebulizer (1100 holes, 6 um of diameter, and 110 kHz of vibrating frequency) [38] placed inside a chamber (generation chamber) and controlled by a nebulizer control board. The OPC withdrew an aerosol sample (for a duration of 1 min) from the generation chamber using its integrated fan. Following this, the sampled aerosol reached the H-QCM connected to the output of the OPC by a suitable mechanical adapter (H-QCM chamber). The OPC data and the resonant frequency of H-QCM were acquired by the acquisition and control board. Finally, a PC unit was used to store all the data. The frequency shift ΔF = f(t) − f0 was used to signify output data of H-QCM where f0 was the resonant frequency obtained without any mass deposition. Figure S2 in the Supplementary Materials shows a detailed view of the developed sensor system.
As previously described, the H-QCM, based on AT-cut quartz crystal, was used to measure the mass of the aerosol sampled by OPC. At the same time, the integrated heater of the H-QCM heated the collected mass on the surface of the sensor in order to discern the aerosol characteristics. The microheater was a double omega-shaped thin film (one on the top and one on the bottom of the crystal) that was connected to a temperature controller that measured the temperature and regulated the power supplied. The temperature of the heater was calculated by the controller using a calibration curve concerning the heater electrical resistance and temperature (measured by several micro-thermocouples during the calibration activities) [39,40]. During the heating, the crystal frequency changed following the behaviour of AT-cut crystal, and then, after the heating, the frequency returned to the previous value [41]. The graph reported in Figure S3 of the Supplementary Materials illustrates the frequency shift observed at various temperatures provided by the integrated heater without deposited mass. The steps of temperature were set by the acquisition and control board to perform the calibration. A maximum value of = 2909 Hz was reached for = 180 °C when 1.1 W of power was provided to the heater in the presence of an air flux produced by the OPC fan (dynamic behaviour of the frequency during the heating step is reported in the Supplementary Materials in Figure S4).
3. Results and Discussions
The following results were obtained during the test following the nebulization of saline aerosol (solid aerosol), and PG/VG aerosol (liquid aerosol) will be presented. In particular, in Section 3.1 and Section 3.3, we reported the data obtained from the OPC, and in Section 3.2 and Section 3.4, the results concerning the H-QCM.
3.1. Measurements of the Saline Aerosol with OPC
When an aerosol containing salt particles is introduced to an OPC, the salt particles may be detected and counted as individual particles. The detection of salt particles depends on several factors including the size and concentration of the particles and the refractive index. Salt particles typically have a high refractive index compared to other aerosol particles, which can make them more easily detectable by an OPC [42,43].
The Count Mean Diameter (CMD) was derived from measurements taken with the OPC for the three solutions; it can be obtained by calculating a weighted average based on the number of particles in each size range. Then, CMD provides an estimation of the average size of saline aerosol particles, allowing us to gain a better understanding of the particle size distribution [44].
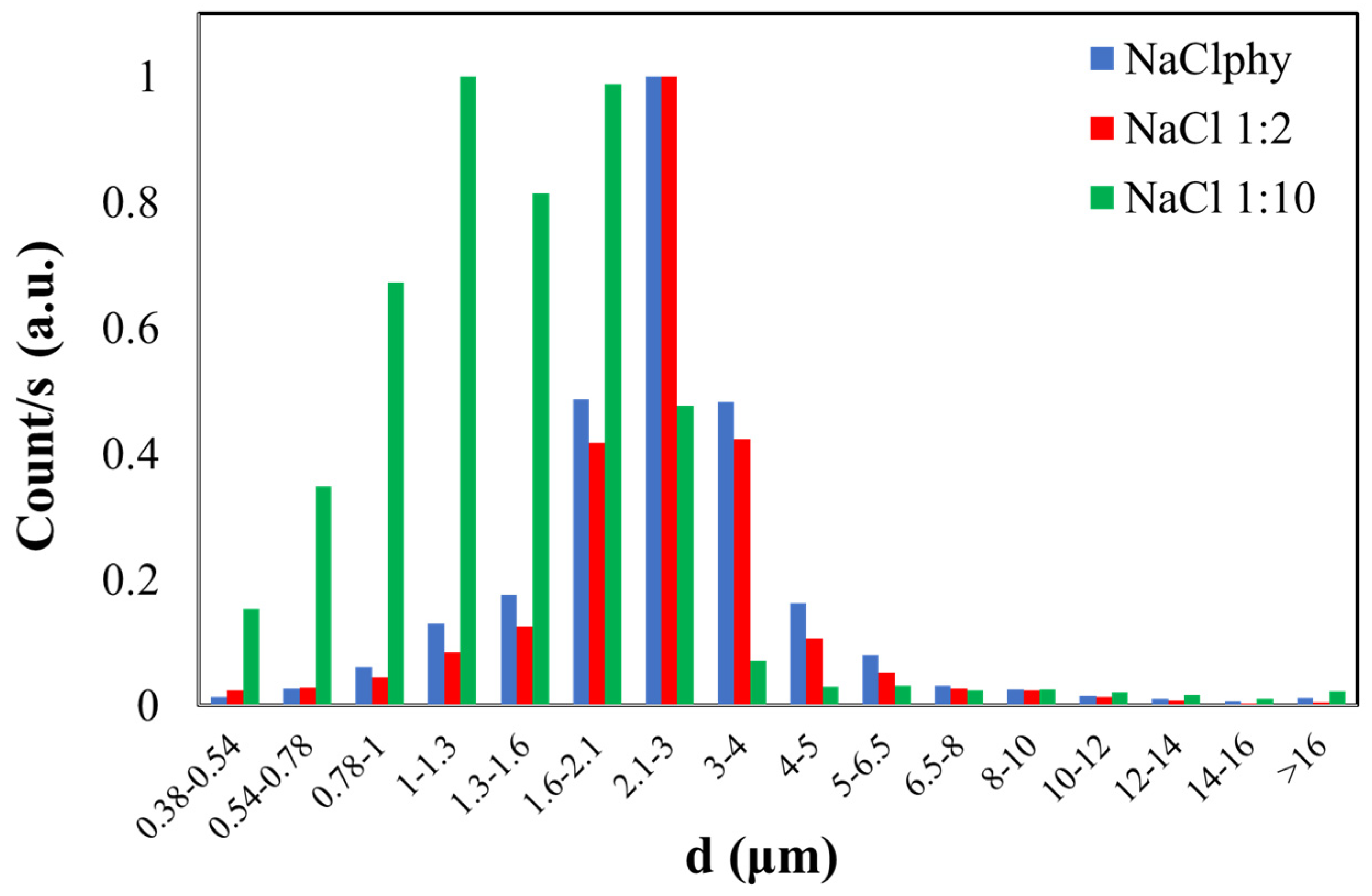
The counts related to the three NaCl solutions were plotted (Figure 3). Specifically, on the y-axis, normalized counts are plotted, and on the x-axis, the bins corresponding to different diameters are represented (Figure S5 of the Supplementary Materials reports an example of the counts as a function of the aerodynamic diameter (d) of NaCl 1:10 and distilled water, H2Odist).
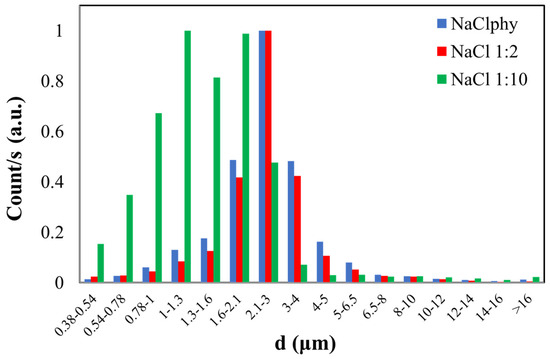
Figure 3.
Normalized graph of counts as a function of the aerodynamic diameter (d) of NaCl particles with different concentrations (NaClphy, NaCl1:2, and NaCl1:10).
The blue histogram represents the size distribution of particles in the NaCl physiological solution where the calculated CMD is 2.90 ± 0.45 µm for this sample. The red histogram refers to the dimensional distribution of the NaCl1:2 solution, which has half the concentration compared to the physiological one. In this case, a CMD of 2.83 ± 0.44 µm was obtained. The graph of the latest solution diluted with a 1:10 ratio to the physiological one (NaCl1:10) is represented by the green histogram. The calculated CMD is 1.75 ± 0.39 µm, and this value slightly deviates from the first two, considering experimental error as well. This result may be related to the dilution of this latest solution compared to the initial one.
Both NaClphy and NaCl1:2 exhibit a CMD value that is comparable within experimental error, indicating a comparable size distribution. The particles in the more concentrated solution may initially have larger sizes due to the higher solute concentration. Consequently, during nebulization, the OPC may detect both larger and smaller particles, resulting in a broader size distribution. Conversely, in the case of the diluted solution, the particles may be initially smaller. As the droplets fragment during nebulization, predominantly smaller particles may be generated. The OPC is likely to primarily detect smaller particles, leading to a size distribution that is more concentrated around smaller diameters.
3.2. Measurements of the Saline Aerosol with H-QCM
The H-QCM offers a complementary approach, measuring frequency variations during the collection of aerosols to discriminate between solid and liquid phases. The collection of saline aerosol particles on its electrode induces a frequency shift correlated to the added mass, as shown in Equation (1). The H-QCM provides a real-time and sensitive method for monitoring the dynamics of saline aerosol particles. Furthermore, the heating process facilitated by the integrated heater enables the evaporation of the solvent (H2Odist) from the prepared solutions. This allows for the correlation of the frequency shift exclusively to the solid mass deposited on the quartz. This approach allows for the discrimination between the presence of solid and liquid particulates on the surface.
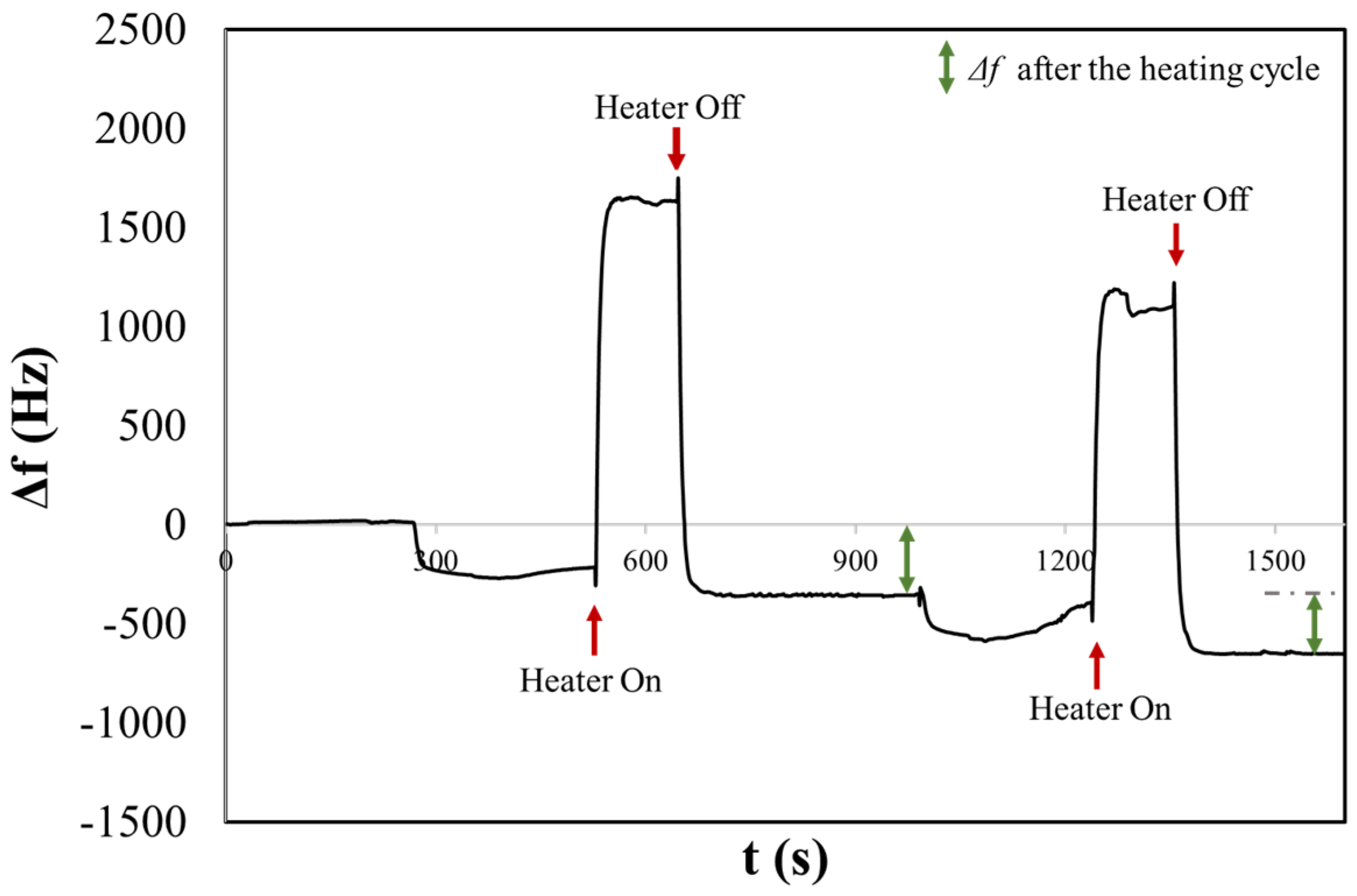
Figure 4 shows the frequency shift chronogram obtained for the NaClphy sample. Before turning on the heater, the observed Δf relates to an aerosol deposition, a mix of solid and liquid phases. In this state, the signal does not settle, unlike after heating. This could be due to the dynamic processes on the H-QCM surface, where both solid NaCl and liquid H2O are present. In fact, when salt arrives in the form of an aerosol, it will not be in its solid crystalline state but will be surrounded by a certain concentration of water [45]. This condition can result in surface dynamics that delay the stabilization of the signal. For instance, phenomena such as deliquescence and aggregation, where salt particles may cluster together or adsorb water on the surface, can occur. This can result in the formation of more complex structures or the creation of a thicker and more homogeneous layer. Furthermore, variations in surface tension may still occur, affecting the water’s ability to wet the surface or influencing other behaviours related to surface properties [46].
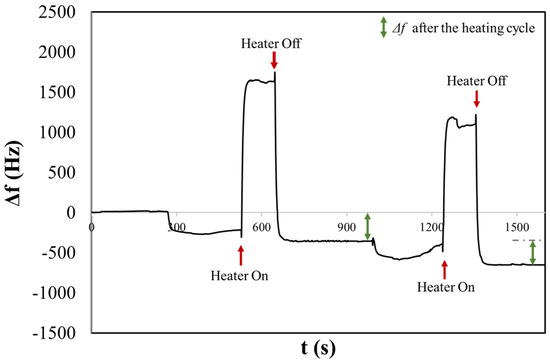
Figure 4.
The chronogram of NaCl physiological aerosol shows two consecutive depositions before and after heating on a H-QCM.
After three minutes, the surface heater was turned on, resulting in a rapid positive variation in Δf and was turned off after two minutes (rapid decreasing and stabilizing at a frequency of Δf = 354 Hz). The reached temperature resulted in the evaporation of water, and NaCl was present as a solid crystalline form. During this process, the compound may undergo changes in its crystal structure. The phenomenon of efflorescence is often associated with the evaporation of water containing dissolved salts, which leads to the crystallization of salts on the surface of the material. The frequency shift remains stable after the heating cycle. In fact, when heated, the bound water is released in the form of vapour, leaving behind anhydrous sodium chloride, which is devoid of water. A second measurement was performed consecutively, and once again, it was possible to observe the first and second rapid variation in the frequency following the activation and deactivation of the heater, stabilizing at a frequency of Δf = 301 Hz.
It is possible to note that before heating cycles in both measurements, the frequency shift is smaller compared to that observed after heating. This could be correlated with the sizes of the salt crystals that initially contain hydration water. The dimensions of some crystals might be larger than 2 microns and therefore may not be detected by the 10 MHz QCM, as discussed in our previous article [47]. Conversely, after heating, a larger frequency shift suggests the evaporation of H2O from the salt particle, resulting in smaller dimensions that can be detected by the QCM. This is consistent with the results obtained from the OPC, where the CMD for NaClphy was measured to be 2.90 ± 0.45 µm.
Figures S6 and S7 of the Supplementary Materials present examples of chronograms of the NaCl1:2 and NaCl1:10 saline solutions. The behaviour of the NaCl1:2 solution is entirely analogous to that of the physiological solution, including the dimensional effect, consistent with the values obtained from the calculation of the CMD (2.90 µm and 2.83 µm, respectively). For NaCl1:10 aerosols, the majority of the crystals are presumably below 2 µm, as detected by measurements with the OPC, resulting in a CMD of 1.75 µm. For this reason, the decrease in Δf is related to the evaporation of water and, consequently, to the loss of mass from the quartz crystal surface (Equation (1)). The integration of the OPC and H-QCM enhances our understanding of the particulate matter an aerosol is composed of. The OPC, which relies on light scattering, may encounter challenges when dealing with salt particles, as discussed in the preceding paragraphs regarding humidity and aggregation phenomena. Meanwhile, the H-QCM, with its heated surface, provides real-time sensitivity and the ability to discriminate between solid and liquid phases. The analysis of NaCl solutions emphasizes OPC’s capability to detect diverse particle sizes, complemented by the H-QCM’s observations of frequency changes during nebulization and particle dynamics.
3.3. Measurements of the Liquid Aerosol with OPC
When a liquid aerosol passes through the OPC, the droplets interact with the laser beam, operating similarly to when dealing with saline aerosols.
This implies that measurements obtained using an OPC do not discriminate between aerosol phases. To obtain data that closely represents real conditions, a correction for the density of the different particulate matter detected is necessary. Indeed, one of the main limitations of the OPC is that particles of different substances may have different densities. This means that even if two particles have the same optical diameter, they could have different volumes or aerodynamic masses due to their density [48]. Also, in this case, the integration of an OPC with a H-QCM could enhance the understanding of the aerosol’s characteristics.
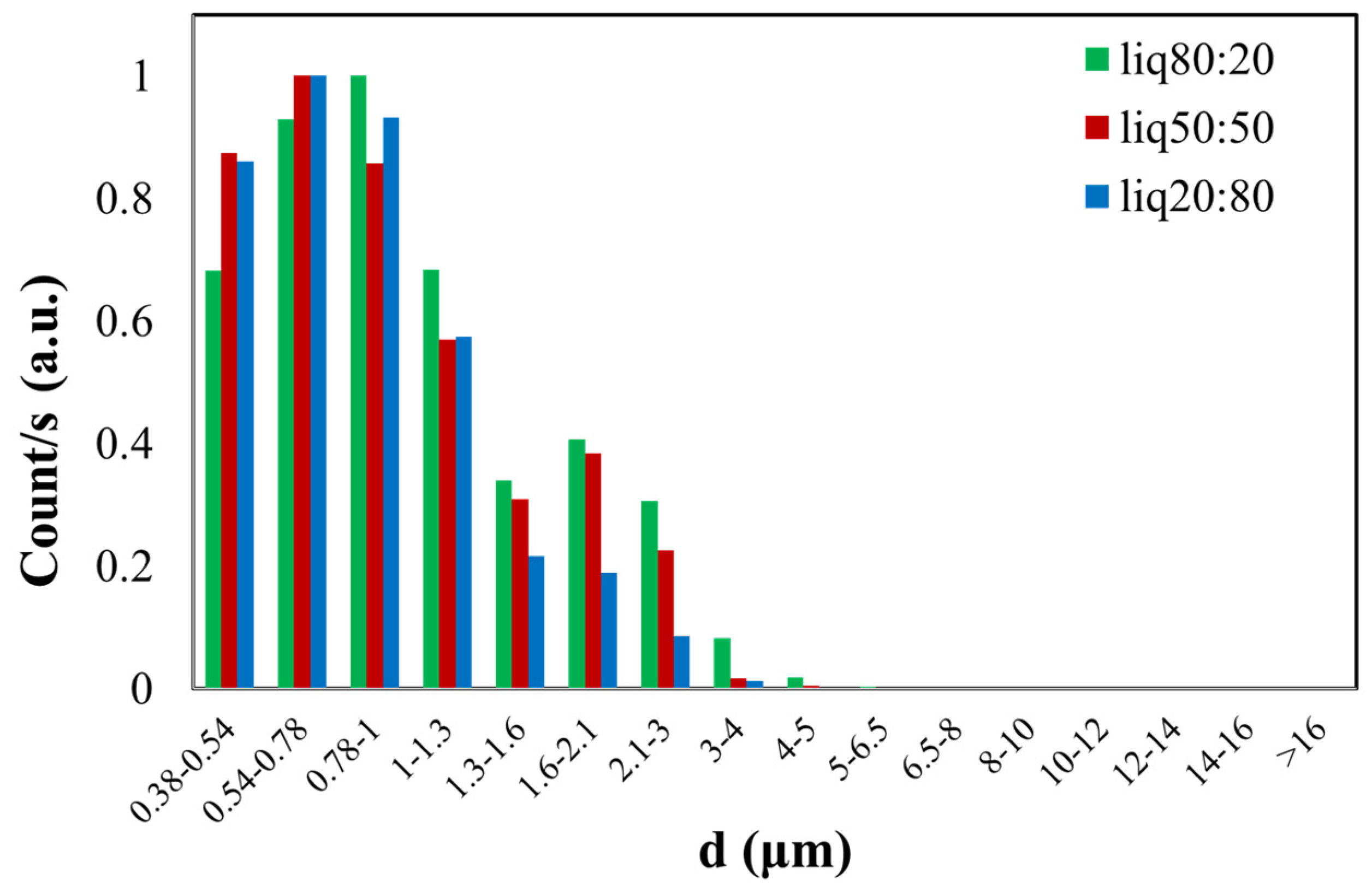
In Figure 5, the green histogram shows the size distribution of the PG/VG liquid aerosol in an 80:20 ratio, with a found CMD of 0.90 ± 0.17 µm. The red histogram represents the dimensional distribution of the 50:50 PG/VG, resulting in a CMD of 1.01 ± 0.15 µm. In the last case, the blue histogram illustrates the normalized count distribution of the PG/VG 20:80, yielding a CMD of 1.13 ± 0.15 µm. The dimensional distribution is nearly similar for all three samples with different ratios of PG/VG, considering the obtained CMD value and the calculated errors.
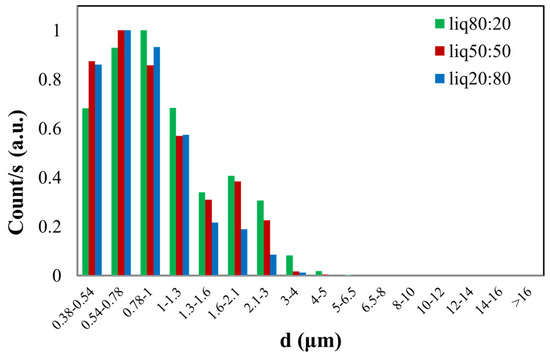
Figure 5.
Normalized graph of counts as a function of the aerodynamic diameter (d) of e-liquid droplets with different PG/VG ratios (liq80:20, liq50:50, liq20:80).
3.4. Measurements of the Liquid Aerosol with QCM
The liquid aerosol induces a frequency shift of H-QCM related to the deposition of mass, although the liquid aerosol is expected to undergo natural evaporation (at room temperature) from the crystal surface over a specific time frame. However, by utilizing the integrated heater, it becomes possible to accelerate the evaporation of liquid with high evaporation temperature. This allows us to discern whether the phase of an aerosol detected by the H-QCM is solid or liquid, a distinction that is not achievable with a standalone OPC (Section 3.3).
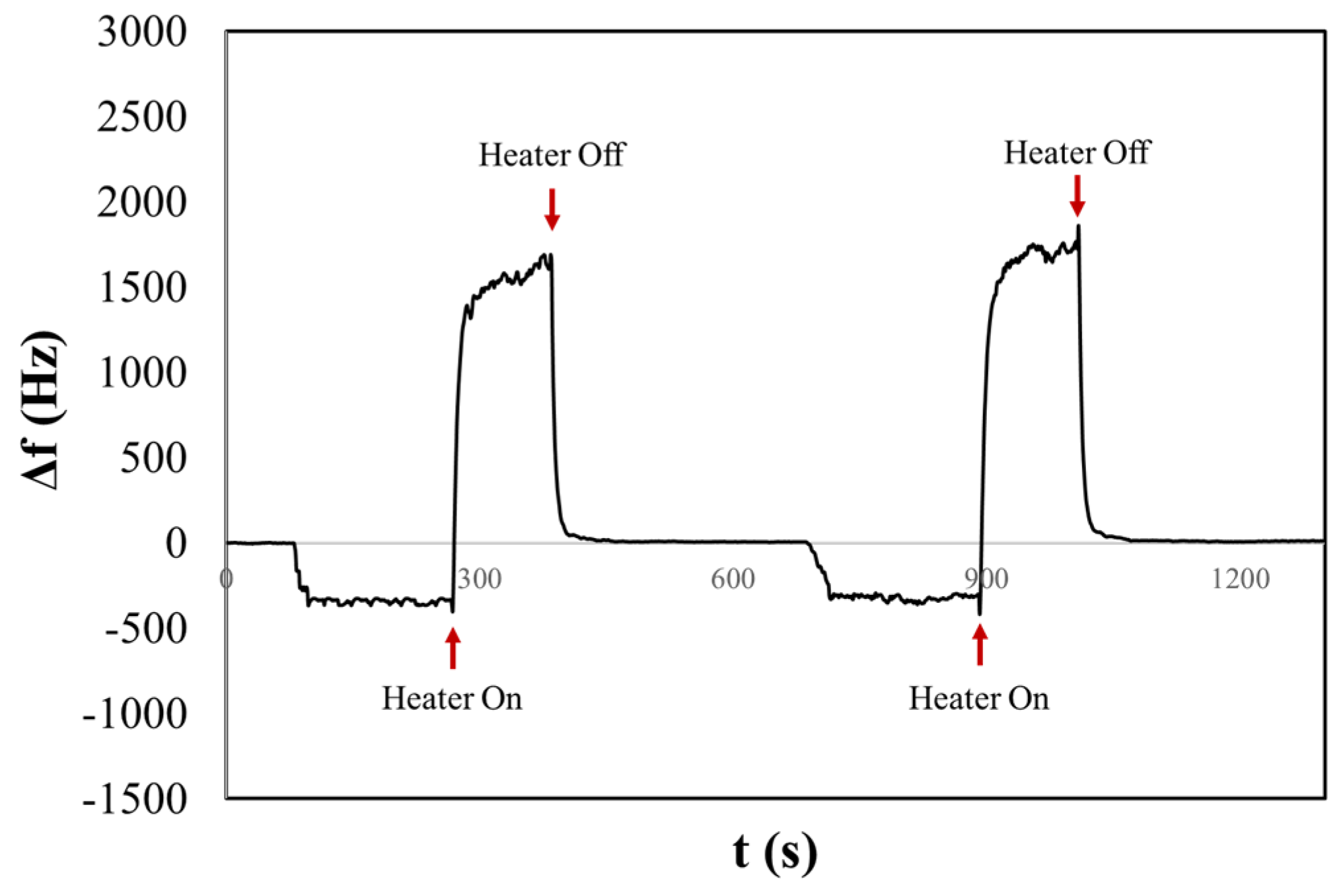
In Figure 6, we present the resulting chronogram of two distinct depositions before and after heating. The aerosol generated within the nebulization chamber was aspirated for one minute by the pump of the OPC. Subsequently, the aerosol flow was directed onto the surface of the QCM. After three minutes of stabilizing the frequency shift, the heater was activated, reaching a temperature of about 170 °C in two minutes. This was followed by a waiting period of five minutes to ensure signal stabilization. The experiment reveals that before activating the heater, a frequency shift occurs that might be mistakenly associated with the presence of solid particulate (Δf ≠ 0). However, during the heating process, the liquid aerosol droplets evaporate from the surface, restoring the initial resonance frequency. We obtained the same behaviour for the liq80:20 and liq20:80 samples. Figures S8 and S9 in Supplementary Materials show examples of chronograms obtained for the other two PG/VG liquid solutions. Table 1 summarizes the results of experiments of saline and liquid aerosols. In particular, the table reports average values and deviations obtained by several repetitions of measurements.
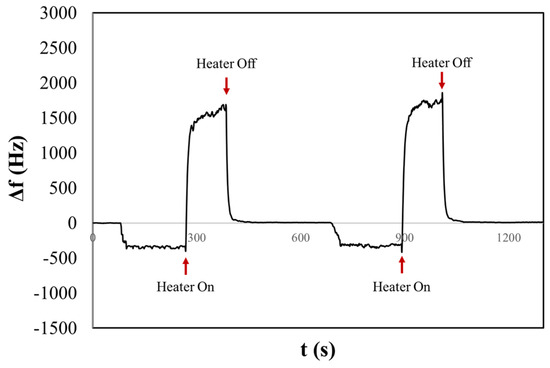
Figure 6.
The chronogram of PG/VG 50:50 shows two consecutive depositions before and after heating on a H-QCM.

Table 1.
Summary of the average frequency shifts for saline and liquid aerosols.
Regarding the saline aerosol, it is evident that before heating, the average value of frequency shift () does not follow the trend of salt concentration in the solutions. In fact, the should be higher for the more concentrated saline solution and lower for the one diluted by a factor of ten. Additionally, the average frequency value shows a significant standard deviation, reflecting the possibility of different surface dynamics processes at the interface (e.g., the crystal electrode surface and sample). These dynamics could contribute to lower reproducibility of the deposition when both solid and liquid phases are present.
After heating cycles, the values not only align with the saline concentration trend in different solutions (NaCl phy > NaCl 1:2 > NaCl 1:10) but also exhibit a lower standard deviation compared to the previous measurements. This indicates improved reproducibility of measurements after heating cycles.
In the case of liquid aerosols, the observed values might be mistakenly attributed to the measurements of solid particles. However, after heating, restoration of the initial frequency indicates the absence of residual mass on the surface of the H-QCM, associated with the evaporation of the liquid. This information obtained by using H-QCM could be used as important feedback for OPC measurements. In simpler terms, if, after heating cycles, the frequency shift persists, the OPC data are correct and are not affected by the presence of a liquid phase of aerosol. In future work, we will analyze the performances of the H-QCM (e.g., sensitivity, the limit of detection, reproducibility, etc.) to assess the possibility of correcting the OPC output data when expressed in terms of mass concentration (µg/m3).
The proposed OPC+H-QCM device could be employed in the field of PM measurement, particularly in environments with marine aerosol or fog, which limits the use of simple OPCs without the use of an air sample treatment system before performing the count. Moreover, such systems require minimal space and energy for their operation.
In the chemical processing industry, exhaust fumes from process reactor stacks simultaneously contain aerosols with different phases. The OPC+H-QCM device could be useful for analyzing these fumes, enabling a thermogravimetric analysis (TGA) alongside total particulate matter dimensional counting.
Although this study presents preliminary results, the proposed tool could be useful for analyzing the fumes produced by e-cigarettes. It could help in identifying solid phases inhaled by users and correlating the quantity and sizes of the particles to some potential pathologies.
4. Conclusions
This study investigated the responses of an OPC coupled with an H-QCM to saline and liquid aerosols. Regarding the saline aerosol, the OPC demonstrated that the CMD values for NaClphy and NaCl 1:2 were comparable within experimental error, suggesting similar size distributions, while NaCl 1:10 exhibited a slightly different trend, potentially attributed to dilution effects. The H-QCM, focusing on the saline aerosol, elucidated the dynamics during and after heating. In particular before heating, the frequency shifts suggested the coexistence of solid and liquid phases, influenced by surface phenomena such as deliquescence and aggregation. After heating, the separation of solid and liquid phases became evident, resulting in improved reproducibility in frequency shifts and allowing for a clearer interpretation of the deposition process. In the case of liquid aerosols (PG/VG mixtures), the OPC provided consistent CMD values across different ratios, emphasizing the need for density corrections when OPC is used alone. Conversely, the H-QCM demonstrated its ability to discriminate between solid and liquid phases. Overall, this study highlights the complementary strengths of OPC and H-QCM in aerosol analysis. While the OPC is utilized for size distribution characterization, the H-QCM provides real-time insights into phase discrimination aerosols. These findings contribute to the refinement of aerosol measurement using a low-cost sensor, fostering a more comprehensive understanding.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/s24082500/s1. Figure S1. Measurement setup with the nebulization chamber and the coupled OPC+H-QCM system. Figure S2. Photograph of the developed sensor system (a). The H-QCM was fixed by a support in the H-QCM measurement chamber (b). Figure S3. H-QCM frequency shifts versus temperature (12 increasing steps) without deposited mass. The frequency and temperature errors were 1 Hz and 0.1 °C, respectively. Figure S4. The H-QCM exhibited frequency variations as temperature increased, beginning from 22 °C. At the maximum temperature reached, approximately 181 °C, the frequency shift was about 3000 Hz. Figure S5. Counts as a function of the aerodynamic diameter (d) of NaCl 1:10 and distilled water (H2Odist). Figure S6. Chronogram of frequency shift due to two consecutive NaCl1:2 aerosol measurements. In particular, the graph highlighted the behaviour before and after heating cycles. Figure S7. Chronogram of frequency shift due to two consecutive NaCl1:10 aerosol measurements. In particular, the graph highlighted the behaviour before and after heating cycles. Figure S8. Chronogram of frequency shift due to two consecutive liq80:20 aerosol measurements. In particular, the graph highlighted the behaviour before and after heating cycles. Figure S9. Chronogram of frequency shift due to two consecutive liq20:80 aerosol measurements. In particular, the graph highlighted the behavior before and after heating cycles.
Author Contributions
Conceptualization, E.Z. and M.A.M.; Investigation, E.Z. and M.A.M.; Data curation, A.C. and P.P.; Resources, E.Z.; Supervision, E.Z. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data that support the findings of this study are available after the reasonable request to the corresponding author.
Acknowledgments
The authors thank Alessandro Capocecera for his technical contribution to the development of both the software interface and Arduino scripts.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dolovich, M.B.; Dhand, R. Aerosol drug delivery: Developments in device design and clinical use. Lancet 2011, 377, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Gurav, A.; Kodas, T.; Pluym, T.; Xiong, Y. Aerosol processing of materials. Aerosol Sci. Technol. 1993, 19, 411–452. [Google Scholar] [CrossRef]
- Pöschl, U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew. Chem. Int. Ed. Engl. 2005, 44, 7520–7540. [Google Scholar] [CrossRef] [PubMed]
- Myhre, G.; Myhre, C.E.L.; Samset, B.H.; Storelvmo, T. Aerosols and their Relation to Global Climate and Climate Sensitivity. Nat. Educ. Knowl. 2013, 4, 7. [Google Scholar]
- Spurny, K.R. Methods of Aerosol Measurement before the 1960s. Aerosol Sci. Technol. 1998, 29, 329–349. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Tare, V.; Chinnam, N.; Srivastava, A.K.; Dey, S.; Agarwal, A.; Lal, S. Measurements of atmospheric parameters during Indian Space Research Organization Geosphere Biosphere Programme Land Campaign II at a typical location in the Ganga basin: 1. Physical and optical properties. J. Geophys. Res. 2006, 111, D23209. [Google Scholar] [CrossRef]
- Cheng, Y.; Su, H.; Koop, T.; Mikhailov, E.; Pöschl, U. Size dependence of phase transitions in aerosol nanoparticles. Nat. Commun. 2015, 6, 5923. [Google Scholar] [CrossRef] [PubMed]
- McMurry, P.H. A review of atmospheric aerosol measurements. Atmos. Environ. 2000, 34, 1959–1999. [Google Scholar] [CrossRef]
- Amaral, S.S.; de Carvalho Costa, J.A., Jr.; Pinheiro, M.A.M.C. An overview of particulate matter measurement instruments. Atmosphere 2015, 6, 1327–1345. [Google Scholar] [CrossRef]
- Kangasluoma, J.; Cai, R.; Jiang, J.; Deng, C.; Stolzenburg, D.; Ahonen, L.R.; Lehtipalo, K. Overview of measurements and current instrumentation for 1–10 nm aerosol particle number size distributions. J. Aerosol Sci. 2020, 148, 105584. [Google Scholar] [CrossRef]
- Vincent, J.H. Aerosol Sampling: Science, Standards, Instrumentation and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Wiedensohler, A.; Birmili, W.; Putaud, J.P.; Ogren, J. Recommendations for aerosol sampling. In Aerosol Science: Technology and Applications; Wiley: Hoboken, NJ, USA, 2013; pp. 45–59. [Google Scholar] [CrossRef]
- Sousan, S.; Koehler, K.; Thomas, G.; Park, J.H.; Hillman, M.; Halterman, A.; Peters, T.M. Inter-comparison of low-cost sensors for measuring the mass concentration of occupational aerosols. Aerosol Sci. Technol. 2016, 50, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.R.; Malings, C.; Pandis, S.N.; Presto, A.A.; McNeill, V.F.; Westervelt, D.M.; Subramanian, R. From low-cost sensors to high-quality data: A summary of challenges and best practices for effectively calibrating low-cost particulate matter mass sensors. J. Aerosol Sci. 2021, 158, 105833. [Google Scholar] [CrossRef]
- Alfano, B.; Barretta, L.; Del Giudice, A.; De Vito, S.; Di Francia, G.; Esposito, E.; Polichetti, T. A review of low-cost particulate matter sensors from the developers’ perspectives. Sensors 2020, 20, 6819. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Zhang, H.; Hammer, M.; Zhan, Y.; Kenney, D.; Martin, R.V.; Biswas, P. Integrating fixed monitoring systems with low-cost sensors to create high-resolution air quality maps for the Northern China Plain Region. ACS Earth Space Chem. 2021, 5, 3022–3035. [Google Scholar] [CrossRef]
- Liu Benjamin, Y.H.; Berglund, R.N.; Agarwal, J.K. Experimental studies of optical particle counters. Atmos. Environ. 1967 1974, 8, 717–732. [Google Scholar]
- Steinem, C.; Janshoff, A. Piezoelectric Sensors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 5. [Google Scholar]
- Crilley, L.R.; Shaw, M.; Pound, R.; Kramer, L.J.; Price, R.; Young, S.; Lewis, A.C.; Pope, F.D. Evaluation of a low-cost optical particle counter (Alphasense OPC-N2) for ambient air monitoring, Atmos. Meas. Tech. 2018, 11, 709–720. [Google Scholar] [CrossRef]
- Chen, M.; Romay, F.J.; Li, L.; Naqwi, A.; Marple, V.A. A novel quartz crystal cascade impactor for real-time aerosol mass distribution measurement. Aerosol Sci. Technol. 2016, 50, 971–983. [Google Scholar] [CrossRef]
- Vashist, S.K.; Vashist, P. Recent Advances in Quartz Crystal Microbalance-Based Sensors. J. Sens. 2011, 2011, 571405. [Google Scholar] [CrossRef]
- Afzal, A.; Mujahid, A.; Schirhagl, R.; Bajwa, S.Z.; Latif, U.; Feroz, S. Gravimetric Viral Diagnostics: QCM Based Biosensors for Early Detection of Viruses. Chemosensors 2017, 5, 7. [Google Scholar] [CrossRef]
- Scaccabarozzi, D.; Saggin, B.; Tarabini, M.; Palomba, E.; Longobardo, A.; Zampetti, E. Thermo-mechanical design and testing of a microbalance for space applications. Adv. Space Res. 2014, 54, 2386–2397. [Google Scholar] [CrossRef]
- Zampetti, E.; Papa, P.; Bearzotti, A.; Macagnano, A. Pocket Mercury-Vapour Detection System Employing a Preconcentrator Based on Au-TiO2 Nanomaterials. Sensors 2021, 21, 8255. [Google Scholar] [CrossRef] [PubMed]
- Görner, P.; Simon, X.; Bémer, D.; Lidén, G. Workplace aerosol mass concentration measurement using optical particle counters. J. Environ. Monit. 2012, 14, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Hand, J.L.; Sonia, M.K. A new method for retrieving particle refractive index and effective density from aerosol size distribution data. Aerosol Sci. Technol. 2002, 36, 1012–1026. [Google Scholar] [CrossRef]
- McMeeking, G.R.; Kreidenweis, S.M.; Carrico, C.M.; Lee, T.; Collett, J.L., Jr.; Malm, W.C. Observations of smoke-influenced aerosol during the Yosemite Aerosol Characterization Study: Size distributions and chemical composition. J. Geophys. Res. Atmos. 2005, 110. [Google Scholar] [CrossRef]
- Crilley, L.R.; Singh, A.; Kramer, L.J.; Shaw, M.D.; Alam, M.S.; Apte, J.S.; Pope, F.D. Effect of aerosol composition on the performance of low-cost optical particle counter correction factors. Atmos. Meas. Tech. 2020, 13, 1181–1193. [Google Scholar] [CrossRef]
- Lee, K.-R.; Kim, Y.-J. Portable multilateral measurement system employing Optical Particle Counter and one-stage Quartz Crystal Microbalance to measure PM10. Sens. Actuators A Phys. 2022, 333, 113272. [Google Scholar] [CrossRef]
- Zampetti, E.; Macagnano, A.; Papa, P.; Bearzotti, A.; Petracchini, F.; Paciucci, L.; Pirrone, N. Exploitation of an integrated microheater on QCM sensor in particulate matter measurements. Sens. Actuators A Phys. 2017, 264, 205–211. [Google Scholar] [CrossRef]
- Moosmüller, H.; Chakrabarty, R.K.; Arnott, W.P. Aerosol light absorption and its measurement: A review. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 844–878. [Google Scholar] [CrossRef]
- Li, X.; Gupta, D.; Eom, H.J.; Kim, H.; Ro, C.U. Deliquescence and efflorescence behavior of individual NaCl and KCl mixture aerosol particles. Atmos. Environ. 2014, 82, 36–43. [Google Scholar] [CrossRef]
- Li, L.; Lee, E.S.; Nguyen, C.; Zhu, Y. Effects of propylene glycol, vegetable glycerin, and nicotine on emissions and dynamics of electronic cigarette aerosols. Aerosol Sci. Technol. 2020, 54, 1270–1281. [Google Scholar] [CrossRef]
- Jordt, S.E.; Jabba, S.; Ghoreshi, K.; Smith, G.J.; Morris, J.B. Propylene Glycol and Glycerin in E-Cigarettes Elicit Respiratory Irritation Responses and Modulate Human Sensory Irritant Receptor Function. Am. J. Respir. Crit. Care Med. 2019, 199, A4169. [Google Scholar]
- Jang, I.R.; Jung, S.I.; Lee, G.; Park, I.; Kim, S.B.; Kim, H.J. Quartz crystal microbalance with thermally-controlled surface adhesion for an efficient fine dust collection and sensing. J. Hazard. Mater. 2022, 424, 127560. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wigung Diinner Schichten und zur Mikrowigung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Gordon, J.G. The oscillation frequency of a quartz resonator in contact with liquid. Anal. Chim. Acta 1985, 175, 99–105. [Google Scholar] [CrossRef]
- Moon, S.-H.; Chang, K.H.; Park, H.M.; Park, B.J.; Yoo, S.K.; Nam, K.C. Effects of Driving Frequency and Voltage on the Performances of Vibrating Mesh Nebulizers. Appl. Sci. 2021, 11, 1296. [Google Scholar] [CrossRef]
- Scaccabarozzi, D.; Saggin, B.; Magni, M.; Corti, M.G.; Zampetti, E.; Palomba, E.; Longobardo, A.; Dirri, F. Calibration in cryogenic conditions of deposited thin-film thermometers on quartz crystal microbalances. Sens. Actuators A Phys. 2021, 330, 112878. [Google Scholar] [CrossRef]
- Magni, M.; Scaccabarozzi, D.; Palomba, E.; Zampetti, E.; Saggin, B. Characterization of Thermal Gradient Effects on a Quartz Crystal Microbalance. Sensors 2022, 22, 7256. [Google Scholar] [CrossRef]
- Brice, J.C. Crystals for quartz resonators. Rev. Mod. Phys. 1985, 57, 105. [Google Scholar] [CrossRef]
- Niedermeier, D.; Wex, H.; Voigtländer, J.; Stratmann, F.; Brüggemann, E.; Kiselev, A.; Heintzenberg, J. LACIS-measurements and parameterization of sea-salt particle hygroscopic growth and activation. Atmos. Chem. Phys. 2008, 8, 579–590. [Google Scholar] [CrossRef]
- Tang, I.N. Chemical and size effects of hygroscopic aerosols on light scattering coefficient. J. Geophys. Res. 1996, 101, 19245–19250. [Google Scholar] [CrossRef]
- Sarangi, B.; Aggarwal, S.G.; Sinha, D.; Gupta, P.K. Aerosol effective density measurement using scanning mobility particle sizer and quartz crystal microbalance with the estimation of involved uncertainty. Atmos. Meas. Tech. 2016, 9, 859–875. [Google Scholar] [CrossRef]
- Zhang, C.; Fen, G.; Sui, S. Study on behaviour of QCM sensor in loading variation. Sens. Actuators B Chem. 1997, 40, 111–115. [Google Scholar] [CrossRef]
- Chao, H.J.; Huang, W.C.; Chen, C.L.; Chou, C.C.K.; Hung, H.M. Water Adsorption vs Phase Transition of Aerosols Monitored by a Quartz Crystal Microbalance. ACS Omega 2020, 5, 31858–31866. [Google Scholar] [CrossRef] [PubMed]
- Zampetti, E.; Mancuso, M.A.; Dirri, F.; Palomba, E.; Papa, P.; Capocecera, A.; Bearzotti, A.; Macagnano, A.; Scaccabarozzi, D. Effects of Oscillation Amplitude Variations on QCM Response to Microspheres of Different Sizes. Sensors 2023, 23, 5682. [Google Scholar] [CrossRef]
- Kovilakam, M.; Deshler, T. On the accuracy of stratospheric aerosol extinction derived from in situ size distribution measurements and surface area density derived from remote SAGE II and HALOE extinction measurements. J. Geophys. Res. Atmos. 2015, 120, 8426–8447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).