Detailed Modeling of Surface-Plasmon Resonance Spectrometer Response for Accurate Correction
Abstract
:1. Introduction
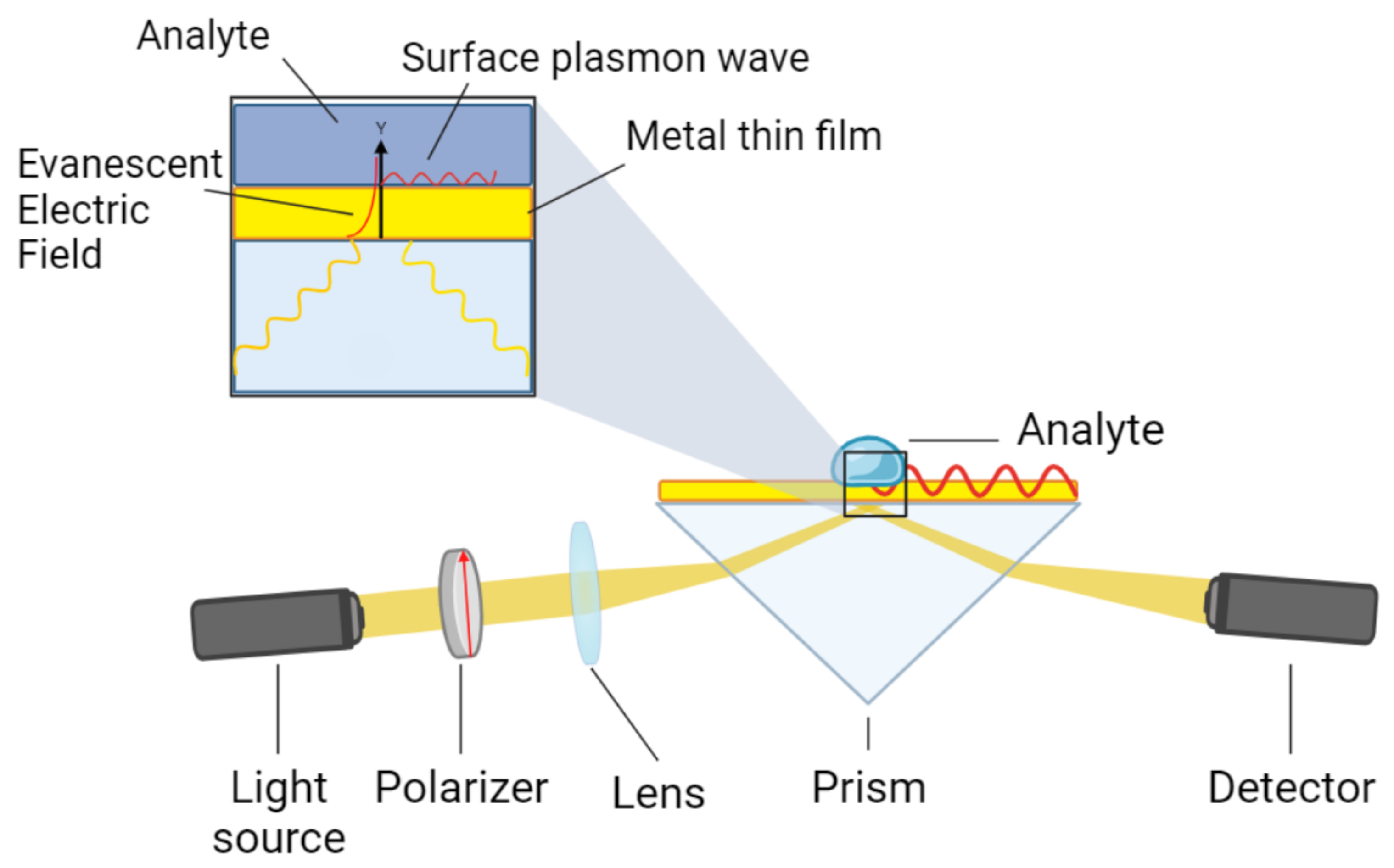
2. SPR Spectrometer System: Components and Transfer Function Determination
2.1. Spectrometer
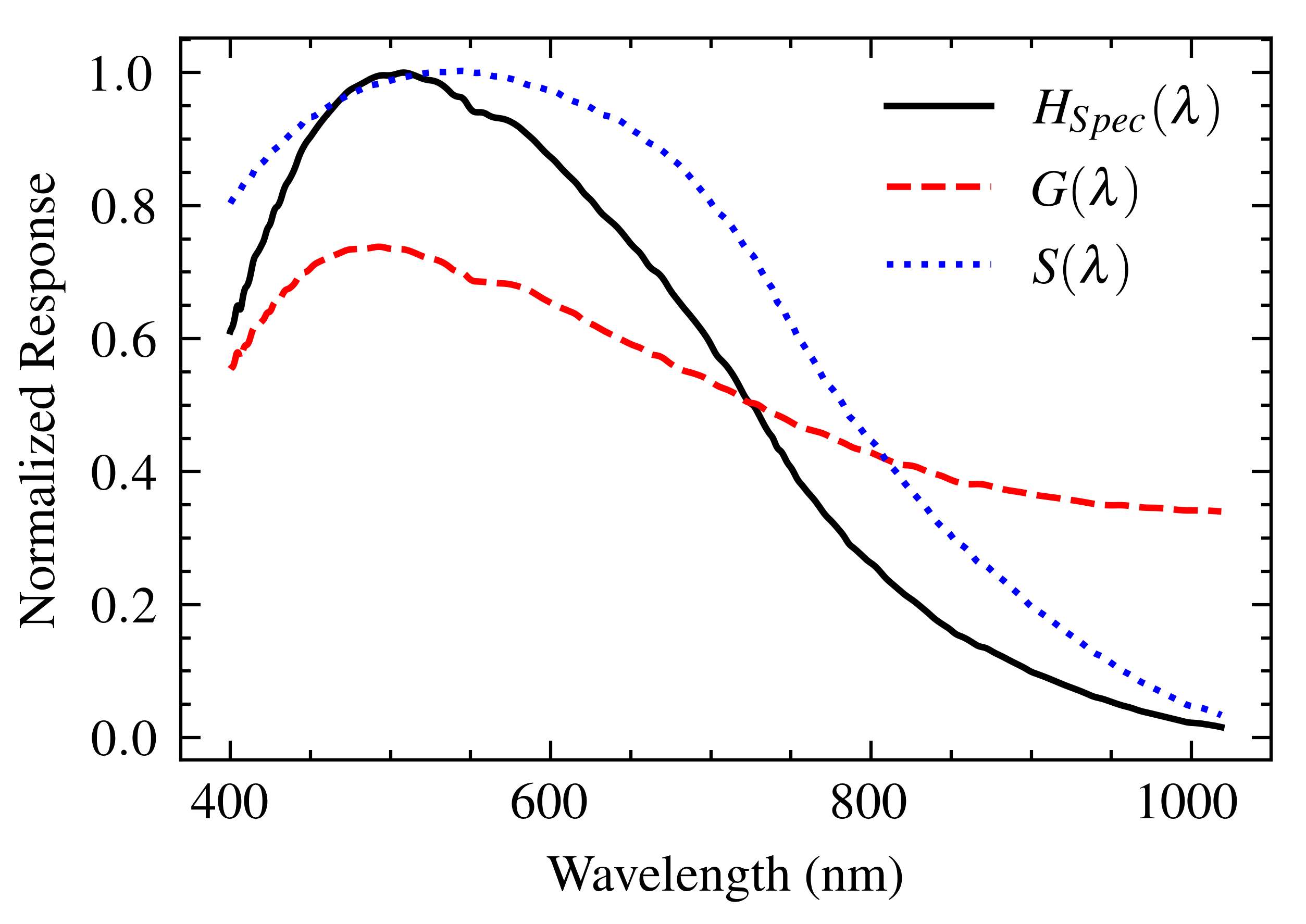
- Diffraction Grating: This device disperses incoming light according to its wavelength. The absolute efficiency, , provided by the manufacturer [18], represents its capability to diffract light toward the CCD sensor as a function of wavelength. This efficiency curve acts as the transfer function of the grating.
- CCD Sensor: The dispersed light falls onto the CCD linear sensor, which converts the incident photons into an electrical signal. Its relative responsivity curve, [19], describes the sensor’s wavelength-dependent sensitivity to light and effectively acts as its transfer function.
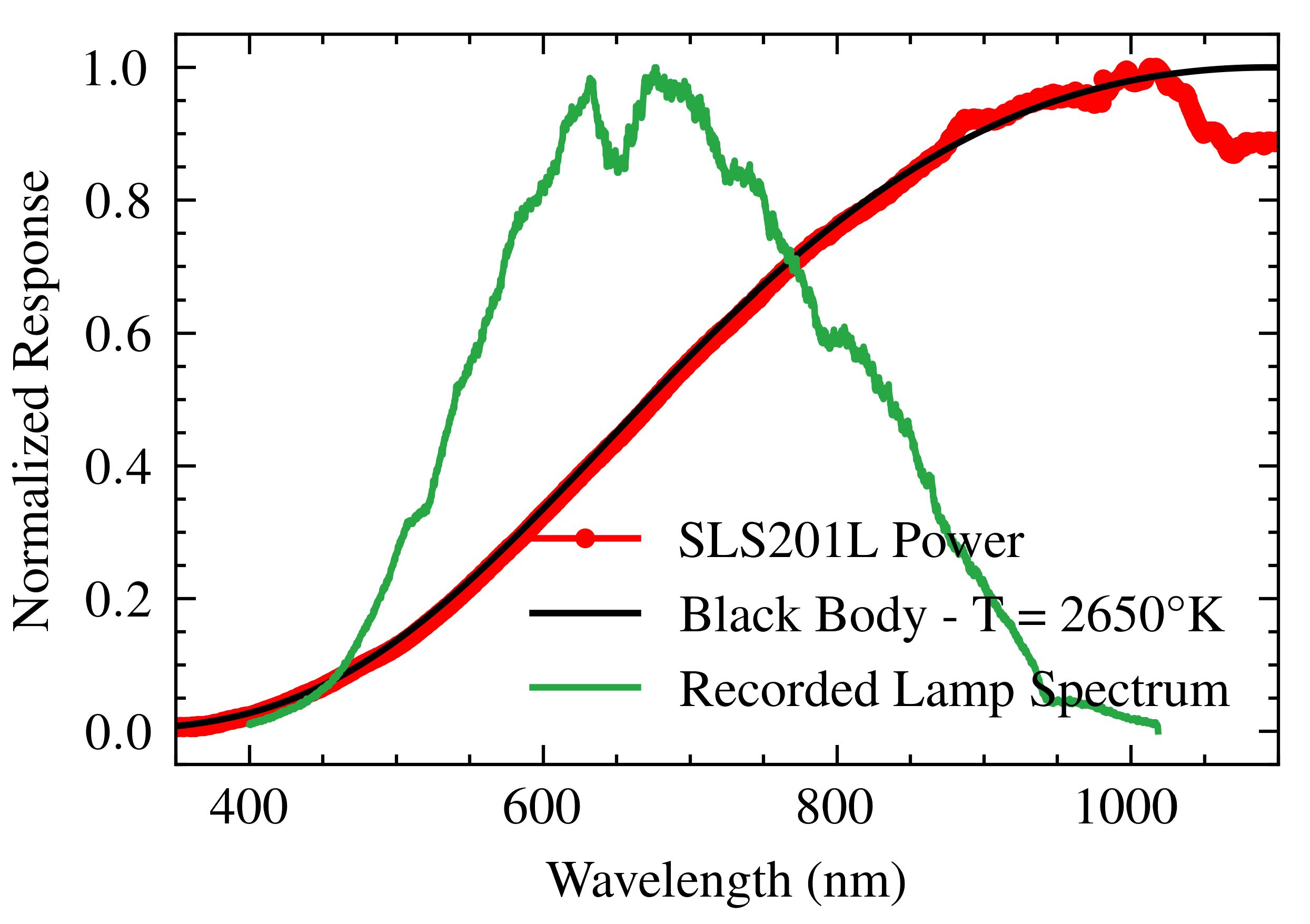
2.2. Light Source
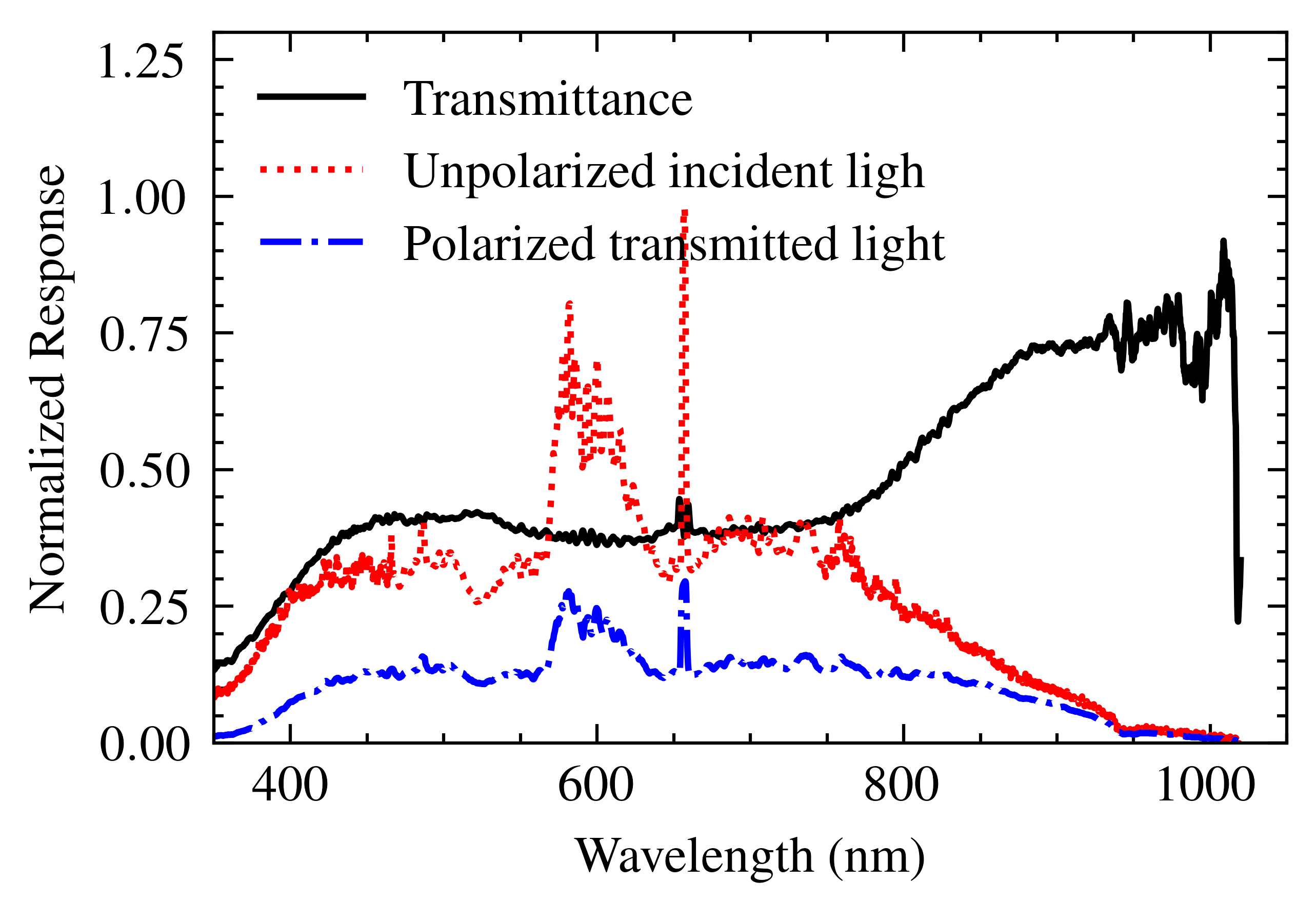
2.3. Polarizer
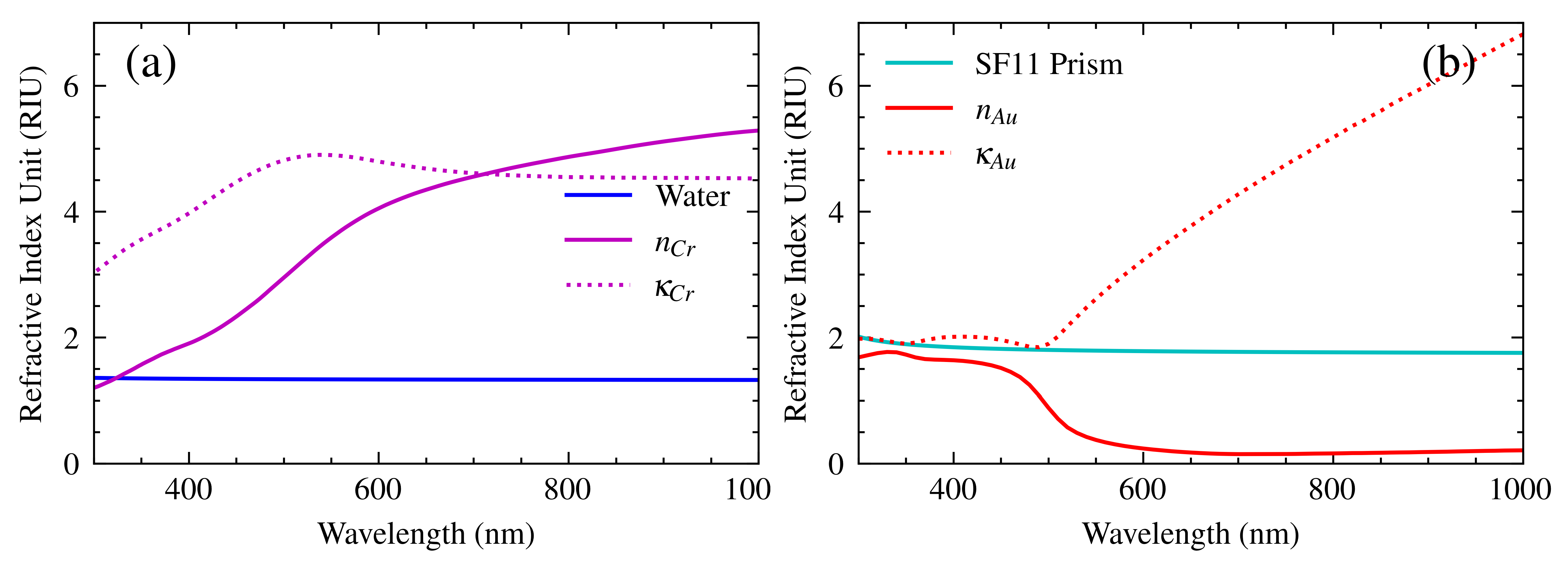
2.4. SPR Sensor
2.5. Optical Fibers
2.6. Other Optical Elements
3. Experimental Modeling Validation
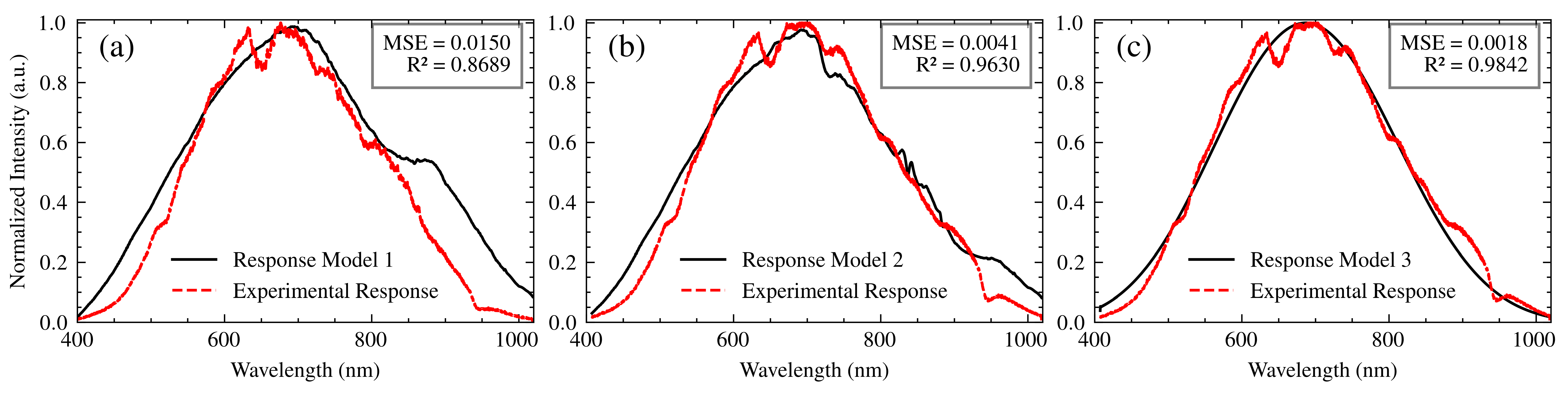
- Model 1: This model served as a baseline and assumed a negligible influence of the optical fibers employed in the setup due to their relatively short length. The overall transfer function of this model is:where is the spectral output of the light source, is the transmittance of the polarizer, and is the spectrometer’s transfer function, encompassing the effects of both the grating and the CCD sensor. This model represents a simplified scenario where the fiber’s contribution is considered minimal.
- Model 2: This model incorporates the transfer functions associated with the optical fibers, in order to quantify the effect they have on the spectral response of the system. The complete transfer function is mathematically represented as follows:In this equation, and represent the transfer functions of the illumination and collection fibers, respectively. By integrating these elements, Model 2 accounts for the wavelength-dependent attenuation characteristic of the fibers and, at the same time, allows for a more thorough examination of the system behavior under varying conditions.
- Model 3: The enhanced model is based on the core concepts outlined in Model 2 and includes a convolution operation to account for the combined effects of chromatic aberrations, spatial disturbances, and wavelength-dependent variations in the refractive index within the optical components. As described by Pérez Tudela [29], convolution is a mathematical tool that describes the modification of a signal as it passes through a system. In our case, each optical component modifies the spectrum of the incident light. These modifications can be represented as convolutions with the transfer function of each component.Chromatic aberrations, arising from the wavelength-dependent refraction of light by lenses and other optical components, cause a spatial dispersion of the spectral components. This dispersion is modeled as a convolution with a function representing the point spread function of the system for each wavelength [30].Furthermore, spatial disturbances, such as beam divergence, scattering, and imperfections in optical surfaces, contribute to the spatial diffusion of light. This diffusion is also modeled as a convolution, where the scattering function represents the spatial distribution of light after interacting with the optical component [31].Finally, the dependence of the refractive index on the wavelength within the optical components alters the speed of light propagation, modifying the spectral distribution. This modification is represented as a convolution, with a function describing the spectral phase change induced by the change in refractive index [32].Model 3 combines these contributions through successive convolutions with the transfer functions of each component, as expressed in Equation (11). The convolution function used is ‘np.convolve’ from the ‘NumPy’ library in Python. The mathematical representation of Model 3 is articulated as follows:The convolution operation provides a more realistic representation of the complex interactions between the optical elements, considering the spectral broadening and blurring effects that can occur. It accounts for how the spectral output of each component is modified and spread out by the subsequent components in the optical path. This comprehensive approach enhances the model’s ability to handle complex optical phenomena and ensures a higher accuracy in the representation of light interactions within the system.
4. Noise Sources and Mitigation Strategies
4.1. Noise-Source Characterization
- Light-Source Fluctuations: Fluctuations in the intensity and spectral distribution in the lamp, despite using a regulated power supply, can introduce noise. The manufacturer specifies power stability below 0.05% and color temperature stability of ±15 K [20]. These fluctuations can affect the baseline stability of the SPR signal and the precision of resonance wavelength determination.
- Dark Noise: With the light source off, the spectrometer measured a normalized average intensity of . This low level, which is attributed to background noise and is unaffected by the activation of the ambient light, indicates that there are negligible contributions from dark current, electronic noise, and stray light. This verifies that the system design effectively mitigates these intrinsic sources of noise through its optical and electronic configuration.
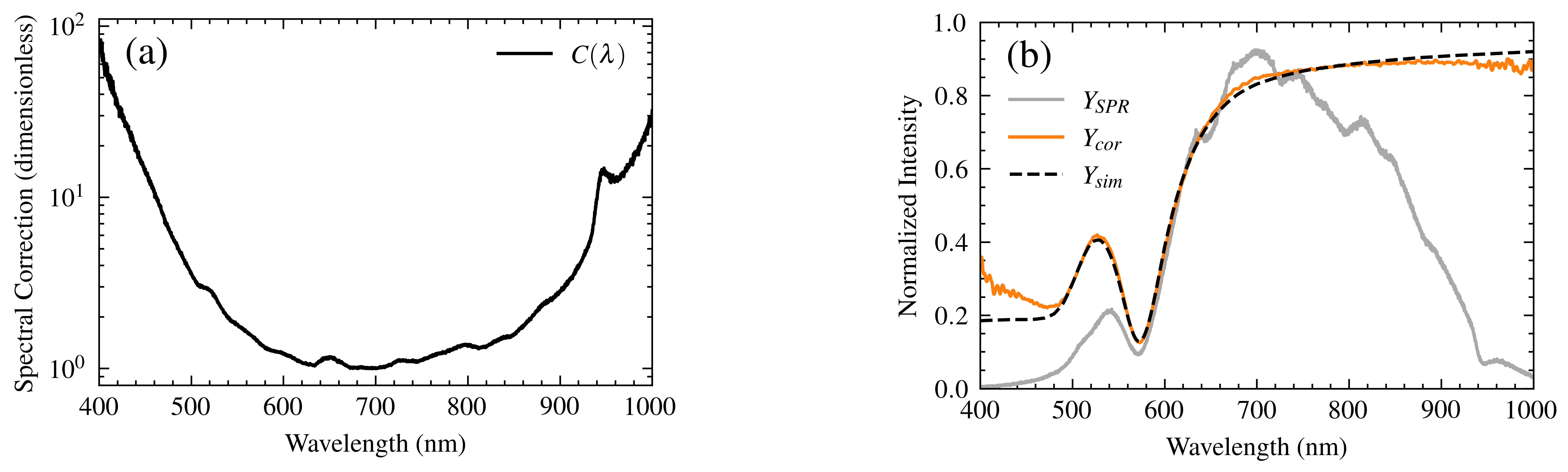
- Background Noise: With the SLS201L light source activated, the background spectrum (Figure 8, dashed red line) exhibits a maximum normalized intensity of 1 in the central spectral region, declining to approximately 3 × 10−3 at the edges. This restricts the precise identification of plasmonic resonances at the spectral extremes, despite a maximum signal-to-noise ratio (SNR) of approximately 400.
- Thermal Noise: It is important to note that temperature variations have the potential to impact the output of the light source and the performance of the spectrometer detector. Both exhibit a temperature sensitivity of 0.1%/°C, as cited in reference [18,19,20]. The laboratory temperature is maintained at 20 degrees Celsius ±2 degrees Celsius. The combined uncertainty in light output and detector response resulting from this ±2 °C variation is approximately 0.28%, calculated by adding the individual uncertainties in quadrature.
- Inherent Noise: Molecular vibrations within the sample itself introduce inherent noise, causing fluctuations in the local refractive index and affecting the interaction with the evanescent wave. This noise impacts both the intensity and spectral resolution of the SPR signal and is considered in the analysis.
4.2. Mitigation Strategies and Their Effectiveness
- Light-Source Stabilization: To mitigate light-source fluctuations, a regulated power supply was used, and the lamp was allowed to warm up for 10 min before measurements, following IES standards [33]. During this warm-up period, the spectrometer remained operational and exposed to the light source, allowing it to reach a stable thermal and electrical state. This procedure stabilized the luminous flux, ensuring a luminous intensity variation of less than 0.05% and a power variation limited to 0.01% per hour.
- Background Noise Reduction: The system design was optimized to minimize the intrusion of stray light. In this design, the elements are positioned as closely as possible. The collection optical fiber, with a numerical aperture of 0.22, was situated at a distance of 5 mm from the prism, which served to mitigate the influence of ambient light.
- Thermal Stabilization: The SPR-S system was housed in a temperature-controlled environment (18–22 °C) to minimize thermal noise and ensure stable and reliable measurements. This significantly reduced temperature-induced fluctuations in system performance.
4.3. Noise Quantification and Measurement Uncertainty
5. SPR Spectrum Correction
- Optimized Method: Adds a compensation factor based on the normalized inverse of the Si detector response:
- Routine Method: Divides the experimental spectrum by the normalized detector response:
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Zhang, P.; Chen, Y. Surface Plasmon Resonance Biosensors: A Review of Molecular Imaging with High Spatial Resolution. Biosensors 2024, 14, 84. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar, S.; Yashini, M.; Rajeshwari, S.; Mamathi, C.A.; Nirmal, T.S.; Sunil, C.K. Recent advances in Surface Plasmon Resonance (SPR) biosensors for food analysis: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1055–1077. [Google Scholar] [CrossRef] [PubMed]
- Araguillin, R.; Samaniego, E.; Sarabia, I.; Santos, V.; Cattöen, X.; Hou-Boutin, Y.; Costa-Vera, C. Interactions between Plasmonic Nanoparticles with a Kretschmann-Configuration SPR Setup. In Proceedings of the 2023 International Conference on Optical MEMS and Nanophotonics (OMN) and SBFoton International Optics and Photonics Conference (SBFoton IOPC), Campinas, Brazil, 31 July–3 August 2023; pp. 1–2. [Google Scholar]
- Zakirov, N.; Zhu, S.; Bruyant, A.; Lérondel, G.; Bachelot, R.; Zeng, S. Sensitivity enhancement of hybrid two-dimensional nanomaterials-based surface plasmon resonance biosensor. Biosensors 2022, 12, 810. [Google Scholar] [CrossRef]
- Dos Santos, P.S.; Mendes, J.P.; Dias, B.; Pérez-Juste, J.; De Almeida, J.M.; Pastoriza-Santos, I.; Coelho, L.C. Spectral analysis methods for improved resolution and sensitivity: Enhancing SPR and LSPR optical fiber sensing. Sensors 2023, 23, 1666. [Google Scholar] [CrossRef] [PubMed]
- Das, C.M.; Yang, F.; Yang, Z.; Liu, X.; Hoang, Q.T.; Xu, Z.; Neermunda, S.; Kong, K.V.; Ho, H.P.; Ju, L.A.; et al. Computational modeling for intelligent surface plasmon resonance sensor design and experimental schemes for real-time plasmonic biosensing: A Review. Adv. Theory Simul. 2023, 6, 2200886. [Google Scholar] [CrossRef]
- Liu, T.; Yuan, J.; Liang, J.; Hu, S.; Hu, X.; Chen, Y.; Chen, L.; Liu, G.S.; Chen, Z. Spectral Correction Method in Spr Measurements Based on Detector Response Efficiency Compensation. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4615068 (accessed on 1 October 2024).
- Yesudasu, V.; Pradhan, H.S.; Pandya, R.J. Recent progress in surface plasmon resonance based sensors: A comprehensive review. Heliyon 2021, 7, e06321. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, B.A.; Purwidyantri, A.; Liu, K.C. Surface plasmon resonance optical sensor: A review on light source technology. Biosensors 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Kretschmann, E.; Raether, H. Notizen: Radiative decay of non radiative surface plasmons excited by light. Z. Naturforsch. A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Wang, G.; Shi, J.; Zhang, Q.; Wang, R.; Huang, L. Resolution enhancement of angular plasmonic biochemical sensors via optimizing centroid algorithm. Chemom. Intell. Lab. Syst. 2022, 223, 104531. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, J.; Wang, X.; Cai, Z.; Shao, Y. Wavelength-scanning surface plasmon resonance microscopy: A novel tool for real time sensing of cell-substrate interactions. Biosens. Bioelectron. 2019, 145, 111717. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Liu, Z.; Cai, C.; Qi, Z. Determination of Surface Plasmon Resonance Wavelength by Combination of Radiation-Based Spectral Correction With Self-Adaptive Fitting. Spectrosc. Spectr. Anal. 2021, 41, 32–38. [Google Scholar]
- Kim, S.; Ryu, J.H.; Yang, H.; Han, K.; Kim, H.; Cho, K.; Park, S.; Hong, S.G.; Lee, K. Spectrometer-based wavelength interrogation SPR imaging via Hadamard transform. Opt. Lett. 2023, 48, 992–995. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zeng, S.; Dinh, X.Q.; Coquet, P.; Yong, K.T. Sensitivity enhancement of MoS2 nanosheet based surface plasmon resonance biosensor. Procedia Eng. 2016, 140, 134–139. [Google Scholar] [CrossRef]
- Araguillin, R.; Méndez, Á.; González, J.; Costa-Vera, Ć. Comparative evaluation of wavelength-scanning Otto and Kretschmann configurations of SPR biosensors for low analyte concentration measurement. J. Phys. Conf. Ser. 2024, 2796, 012009. [Google Scholar] [CrossRef]
- Åström, K.J.; Murray, R. Feedback Systems: An Introduction for Scientists and Engineers; Princeton University Press: Princeton, NJ, USA, 2021. [Google Scholar]
- Thorlabs. CCS Series Spectrometer Operation Manual. CCS200-Manual.pdf. Available online: https://lenlasers.ru/upload/iblock/b0a/CCS-Rukovodstvo.pdf (accessed on 2 August 2024).
- Toshiba. TCD1304DG CCD LinearSensor Specifications; Toshiba: Minato, Japan, 2004. [Google Scholar]
- Thorlabs, Inc. SLS201L Stabilized Tungsten Light Source User Guide, rev g ed.; User Guide; Thorlabs, Inc.: Newton, NJ, USA, 2024. [Google Scholar]
- Thorlabs. Linear Polarizers: Visible (420–700 nm). LPVISE050-A. Available online: https://qd-europe.com/de/en/product/visible-light-polarizers-420-700-nm/ (accessed on 2 August 2024).
- Miyazaki, C.M.; Shimizu, F.M.; Ferreira, M. Surface Plasmon Resonance (SPR) for Sensors and Biosensors. In Nanocharacterization Techniques; Elsevier: Amsterdam, The Netherlands, 2017; pp. 183–200. [Google Scholar]
- Polyanskiy, M.N. Refractiveindex. info database of optical constants. Sci. Data 2024, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Yakubovsky, D.I.; Arsenin, A.V.; Stebunov, Y.V.; Fedyanin, D.Y.; Volkov, V.S. Optical constants and structural properties of thin gold films. Opt. Express 2017, 25, 25574–25587. [Google Scholar] [CrossRef]
- Sytchkova, A.; Belosludtsev, A.; Volosevičienė, L.; Juškėnas, R.; Simniškis, R. Optical, structural and electrical properties of sputtered ultrathin chromium films. Opt. Mater. 2021, 121, 111530. [Google Scholar] [CrossRef]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Thorlabs. FG050LGA Multimode Fiber. Spec Sheet. 2024. Available online: https://www.thorlabs.com/thorproduct.cfm?partnumber=FG050LGA (accessed on 5 October 2024).
- P400-2 UV-VIS Fiber for Ocean Optics Spectrometers. 2024. Available online: https://www.laserlabsource.com/Spectrometers/shop/P400-2m-UV-VIS-Fiber-Ocean-Optics (accessed on 21 November 2024).
- Pérez Tudela, J.D. Análisis de la Influencia de las Aberraciones del Sistema Difractor en el Reconocimiento de Imágenes por Correlación óptica; Universitat de Barcelona: Barcelona, Spain, 2006. [Google Scholar]
- Goodman, J.W. Introduction to Fourier Optics, 4th ed.; W. H. Freeman and Company: New York, NY, USA, 2017. [Google Scholar]
- Hecht, E. Optics, 5th ed.; Pearson Education: New York, NY, USA, 2017. [Google Scholar]
- Born, M.; Wolf, E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light, 7th ed.; Cambridge University Press: New York, NY, USA, 2019. [Google Scholar]
- Rea, M.S. IESNA Lighting Handbook: Reference and Application, 9th ed.; Illuminating Engineering Society of North America (IESNA): New York, NY, USA, 2000. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araguillin-López, R.D.; Méndez-Cevallos, A.D.; Costa-Vera, C. Detailed Modeling of Surface-Plasmon Resonance Spectrometer Response for Accurate Correction. Sensors 2025, 25, 894. https://doi.org/10.3390/s25030894
Araguillin-López RD, Méndez-Cevallos AD, Costa-Vera C. Detailed Modeling of Surface-Plasmon Resonance Spectrometer Response for Accurate Correction. Sensors. 2025; 25(3):894. https://doi.org/10.3390/s25030894
Chicago/Turabian StyleAraguillin-López, Ricardo David, Angel Dickerson Méndez-Cevallos, and César Costa-Vera. 2025. "Detailed Modeling of Surface-Plasmon Resonance Spectrometer Response for Accurate Correction" Sensors 25, no. 3: 894. https://doi.org/10.3390/s25030894
APA StyleAraguillin-López, R. D., Méndez-Cevallos, A. D., & Costa-Vera, C. (2025). Detailed Modeling of Surface-Plasmon Resonance Spectrometer Response for Accurate Correction. Sensors, 25(3), 894. https://doi.org/10.3390/s25030894




