A Waterborne, Flexible, and Highly Conductive Silver Ink for Ultra-Rapid Fabrication of Epidermal Electronics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silver Inks
2.3. Printing of Conductive Ink
Fabrication of Flexible Conductive Devices
2.4. Instrumentations
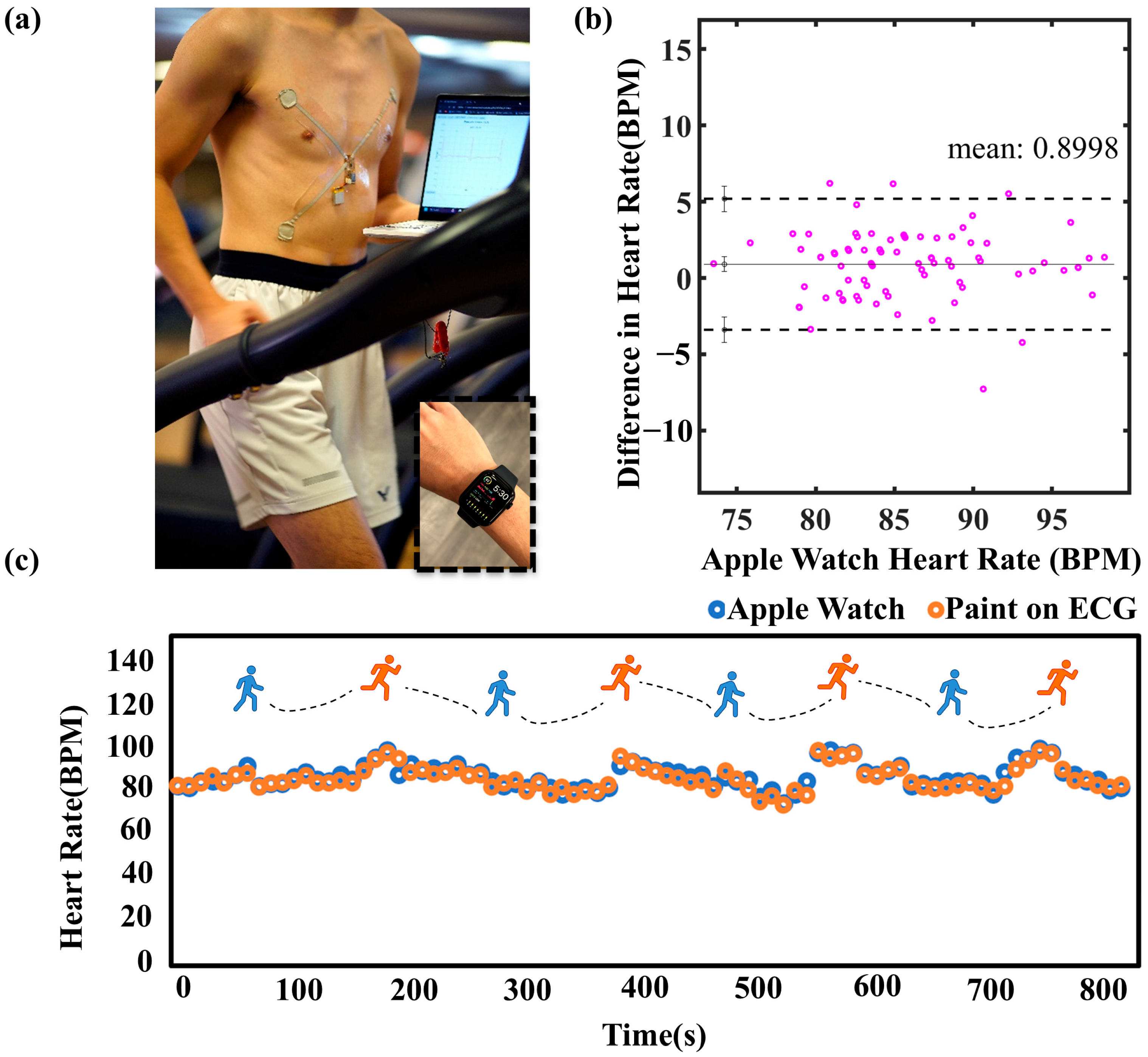
2.5. ECG Applications
3. Results and Discussion
3.1. Characterizations
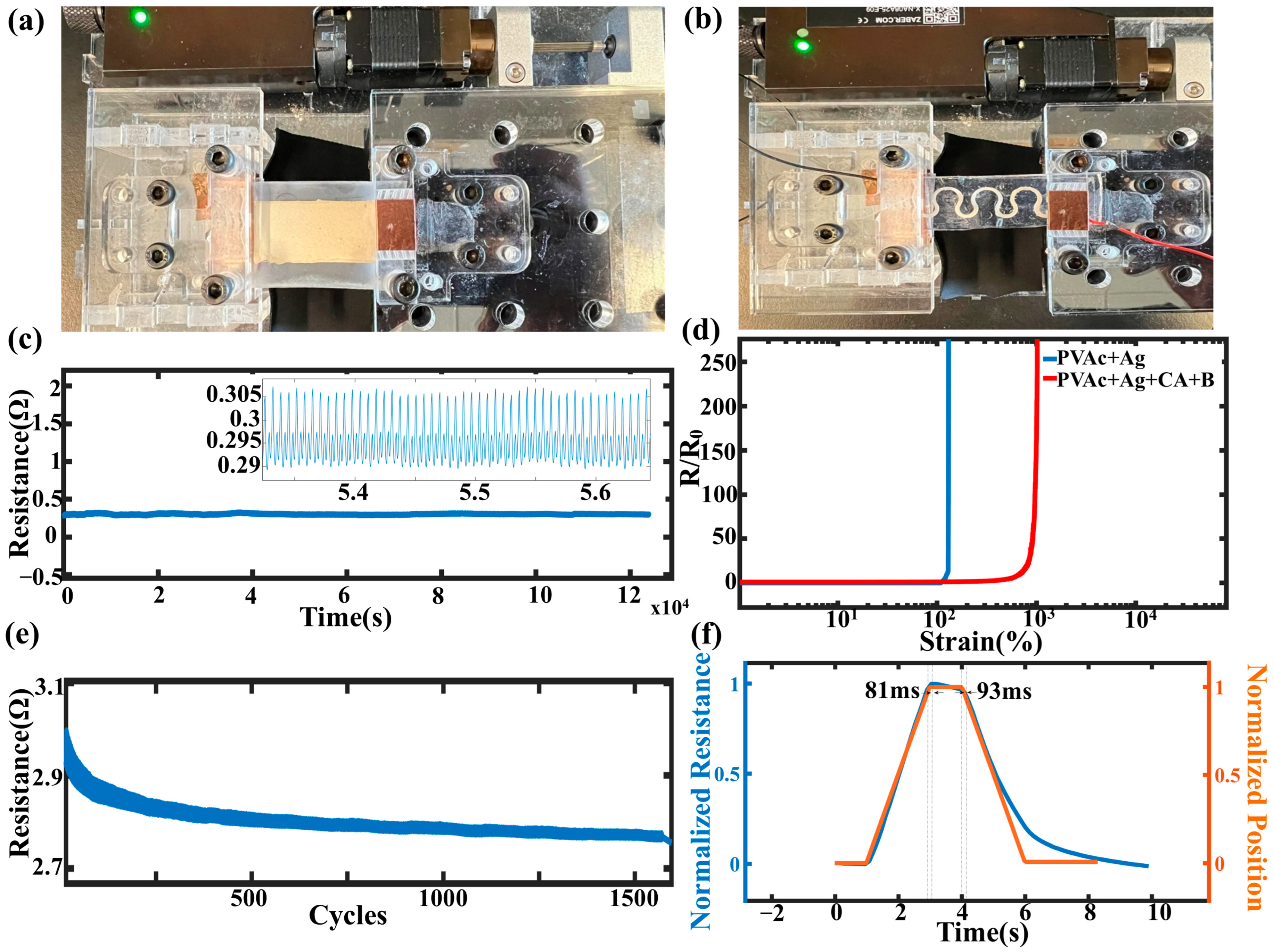
3.2. Mechanical Properties
3.3. Electrical Properties
3.4. Properties of the Flexible Conductive Devices
4. Application
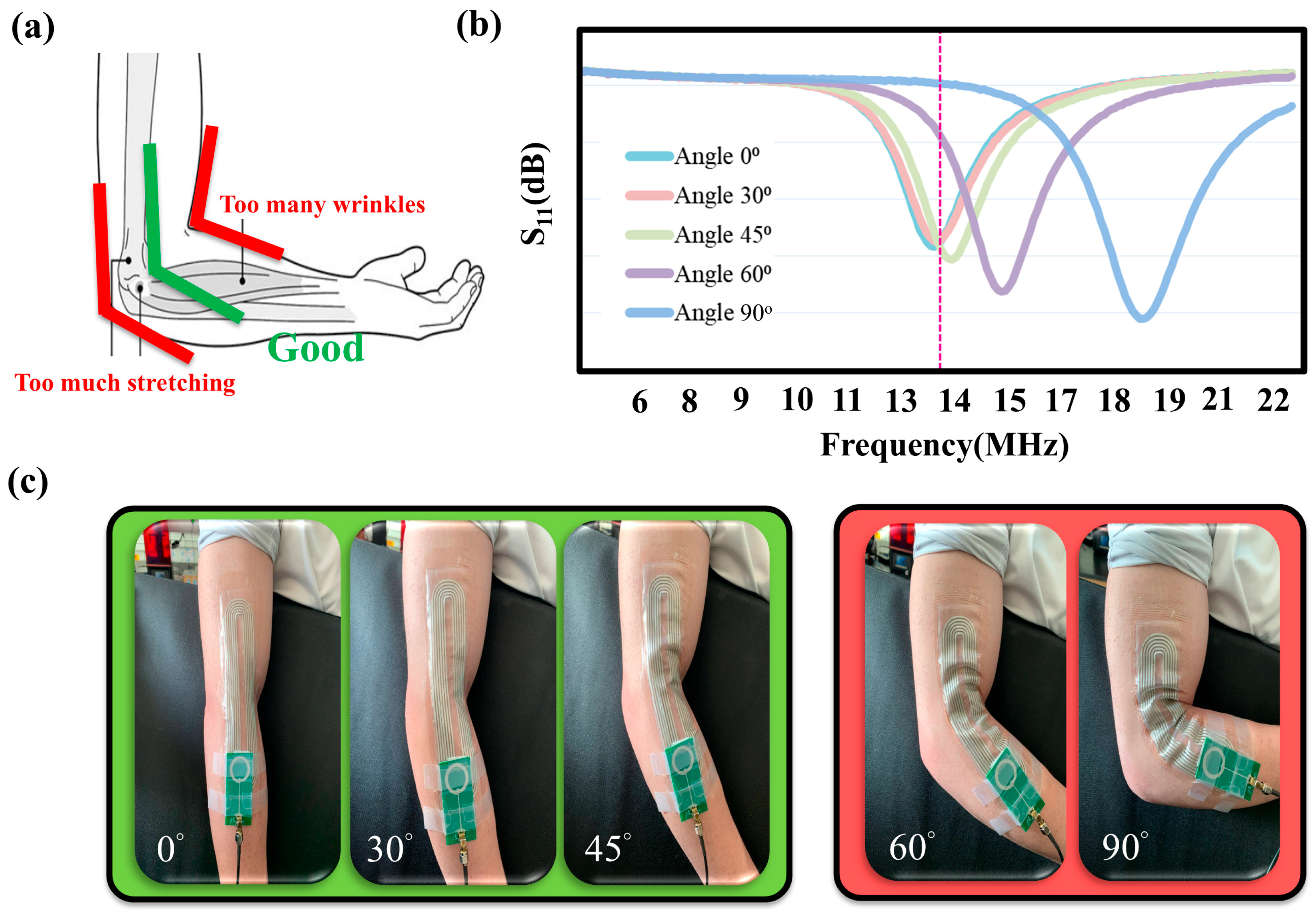
4.1. Epidermal NFC Coils Bending Test
4.2. Paint-On ECG Electrode and Ink Wires
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dankoco, M.D.; Tesfay, G.Y.; Benevent, E.; Bendahan, M. Temperature Sensor Realized by Inkjet Printing Process on Flexible Substrate. Mater. Sci. Eng. B 2016, 205, 1–5. [Google Scholar] [CrossRef]
- Dadras-Toussi, O.; Khorrami, M.; Louis Sam Titus, A.S.C.; Majd, S.; Mohan, C.; Abidian, M.R. Multiphoton Lithography of Organic Semiconductor Devices for 3D Printing of Flexible Electronic Circuits, Biosensors, and Bioelectronics. Adv. Mater. 2022, 34, e2200512. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.Y.; Song, Y.; Yao, D.R.; Gao, W. Direct-Ink-Writing 3D-Printed Bioelectronics. Mater. Today 2023, 71, 135–151. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, R.; Wang, X.; Liu, J.; Fang, Q. Flexible RFID Tag Inductor Printed by Liquid Metal Ink Printer and Its Characterization. J. Electron. Packag. 2016, 138, 031007. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A Wearable Chemical-Electrophysiological Hybrid Biosensing System for Real-Time Health and Fitness Monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Lee, S.M.; Byeon, H.J.; Lee, J.H.; Baek, D.H.; Lee, K.H.; Hong, J.S.; Lee, S.H. Self-Adhesive Epidermal Carbon Nanotube Electronics for Tether-Free Long-Term Continuous Recording of Biosignals. Sci. Rep. 2014, 4, 11650. [Google Scholar] [CrossRef]
- Kim, T.; Park, J.; Sohn, J.; Cho, D.; Jeon, S. Bioinspired, Highly Stretchable, and Conductive Dry Adhesives Based on 1D-2D Hybrid Carbon Nanocomposites for All-in-One ECG Electrodes. ACS Nano 2016, 10, 4770–4778. [Google Scholar] [CrossRef]
- Zhu, C.; Chortos, A.; Wang, Y.; Pfattner, R.; Lei, T.; Hinckley, A.C.; Pochorovski, I.; Yan, X.; To, J.W.F.; Oh, J.Y.; et al. Stretchable Temperature-Sensing Circuits with Strain Suppression Based on Carbon Nanotube Transistors. Nat. Electron. 2018, 1, 183–190. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A Soft, Wearable Microfluidic Device for the Capture, Storage, and Colorimetric Sensing of Sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. In Nature Biotechnology; Nature Publishing Group: New York, NY, USA, 2019; pp. 389–406. [Google Scholar] [CrossRef]
- Kim, J.; Chou, E.F.; Le, J.; Wong, S.; Chu, M.; Khine, M. Soft Wearable Pressure Sensors for Beat-to-Beat Blood Pressure Monitoring. Adv. Heal. Mater. 2019, 8, 1900109. [Google Scholar] [CrossRef]
- Lin, L.; Chu, M.; Park, S.J.; Zakashansky, J.A.; Khine, M. Conformal Stretch Sensors for High Resolution Motion Sensing and Control. Macromol. Mater. Eng. 2019, 304, 1800520. [Google Scholar] [CrossRef]
- Shah, D.M.; Morris, J.; Plaisted, T.A.; Amirkhizi, A.V.; Hansen, C.J. Highly Filled Resins for DLP-Based Printing of Low Density, High Modulus Materials. Addit. Manuf. 2021, 37, 101736. [Google Scholar] [CrossRef]
- Singh, R.; Singh, E.; Nalwa, H.S. Inkjet Printed Nanomaterial Based Flexible Radio Frequency Identification (RFID) Tag Sensors for the Internet of Nano Things. RSC Adv. 2017, 7, 48597–48630. [Google Scholar] [CrossRef]
- Parashkov, R.; Becker, E.; Riedl, T.; Johannes, H.H.; Kowalsky, W. Large Area Electronics Using Printing Methods. Proc. IEEE 2005, 93, 1321–1329. [Google Scholar] [CrossRef]
- Venkata Krishna Rao, R.; Venkata Abhinav, K.; Karthik, P.S.; Singh, S.P. Conductive Silver Inks and Their Applications in Printed and Flexible Electronics. In RSC Advances; Royal Society of Chemistry: London, UK, 2015; pp. 77760–77790. [Google Scholar] [CrossRef]
- Abu-Khalaf, J.; Saraireh, R.; Eisa, S.; Al-Halhouli, A. Experimental Characterization of Inkjet-Printed Stretchable Circuits for Wearable Sensor Applications. Sensors 2018, 18, 3476. [Google Scholar] [CrossRef]
- Sinha, S.K.; Noh, Y.; Reljin, N.; Treich, G.M.; Hajeb-Mohammadalipour, S.; Guo, Y.; Chon, K.H.; Sotzing, G.A. Screen-Printed PEDOT:PSS Electrodes on Commercial Finished Textiles for Electrocardiography. ACS Appl. Mater. Interfaces 2017, 9, 37524–37528. [Google Scholar] [CrossRef]
- Ma, C.; Ma, M.G.; Si, C.; Ji, X.X.; Wan, P. Flexible MXene-Based Composites for Wearable Devices. In Advanced Functional Materials; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, B.; Handschuh-Wang, S.; Zhou, X. Liquid Metal–Based Soft Microfluidics. In Small; Wiley-VCH Verlag: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef]
- Jabari, E.; Toyserkani, E. Aerosol-Jet Printing of Highly Flexible and Conductive Graphene/Silver Patterns. Mater. Lett. 2016, 174, 40–43. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Tan, S.; Novoselov, K.S.; Yeates, S.G. All Inkjet-Printed Graphene-Silver Composite Ink on Textiles for Highly Conductive Wearable Electronics Applications. Sci. Rep. 2019, 9, 8035. [Google Scholar] [CrossRef]
- Deng, D.; Feng, S.; Shi, M.; Huang, C. In Situ Preparation of Silver Nanoparticles Decorated Graphene Conductive Ink for Inkjet Printing. J. Mater. Sci. Mater. Electron. 2017, 28, 15411–15417. [Google Scholar] [CrossRef]
- Hajiaghajani, A.; Rwei, P.; Afandizadeh Zargari, A.H.; Escobar, A.R.; Kurdahi, F.; Khine, M.; Tseng, P. Amphibious Epidermal Area Networks for Uninterrupted Wireless Data and Power Transfer. Nat. Commun. 2023, 14, 7522. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, H.; Yue, X.; Yu, Y.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. A Highly Stretchable Carbon Nanotubes/Thermoplastic Polyurethane Fiber-Shaped Strain Sensor with Porous Structure for Human Motion Monitoring. Compos. Sci. Technol. 2018, 168, 126–132. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Zhang, Z.; Qian, B. Application of Ag@Cu Water-Based Nanomaterial Conductive Ink in 3D Printing. 3d Print. Addit. Manuf. 2023, 10, 552–558. [Google Scholar] [CrossRef]
- Wang, J.; Tan, H.; Xiao, D.; Navik, R.; Goto, M.; Zhao, Y. Preparation of Waterborne Graphene Paste with High Electrical Conductivity. Chem. Phys. Lett. 2020, 741, 137098. [Google Scholar] [CrossRef]
- Zhao, C.F.; Wang, J.; Zhang, Z.Q.; Sun, Z.; Maimaitimin, Z. Silver-Based Conductive Ink on Paper Electrodes Based on Micro-Pen Writing for Electroanalytical Applications. ChemElectroChem 2022, 9, e202200948. [Google Scholar] [CrossRef]
- Jia, L.C.; Zhou, C.G.; Sun, W.J.; Xu, L.; Yan, D.X.; Li, Z.M. Water-Based Conductive Ink for Highly Efficient Electromagnetic Interference Shielding Coating. Chem. Eng. J. 2020, 384, 123368. [Google Scholar] [CrossRef]
- Najafi, M.; Forestier, E.; Safarpour, M.; Ceseracciu, L.; Zych, A.; Bagheri, A.; Bertolacci, L.; Athanassiou, A.; Bayer, I. Biodegradable Polylactic Acid Emulsion Ink Based on Carbon Nanotubes and Silver for Printed Pressure Sensors. Sci. Rep. 2024, 14, 10988. [Google Scholar] [CrossRef]
- Cao, G.; Cai, S.; Zhang, H.; Tian, Y. High-Performance Conductive Adhesives Based on Water-Soluble Resins for Printed Circuits, Flexible Conductive Films, and Electromagnetic Interference Shielding Devices. Adv. Compos. Hybrid. Mater. 2022, 5, 1730–1742. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Abdullah, M.K.; Mariatti, M. Water-Based Graphene/AgNPs Hybrid Conductive Inks for Flexible Electronic Applications. J. Mater. Res. Technol. 2022, 16, 59–73. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, B.; Qing, Y.; Nan, H.; Huang, L.; Wei, W.; Peng, Y.; Yuan, F.; Dong, H.; Hou, X.; et al. Flexible and Transparent Ferroferric Oxide-Modified Silver Nanowire Film for Efficient Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2020, 12, 2826–2834. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, Q.D.; Min, B.K.; Yi, Y.; Choi, C.G. Ti3C2Tx MXene/Carbon Nanotubes/Waterborne Polyurethane Based Composite Ink for Electromagnetic Interference Shielding and Sheet Heater Applications. Chem. Eng. J. 2022, 430, 133171. [Google Scholar] [CrossRef]
- Choi, H.Y.; Shin, E.J.; Lee, S.H. Piezo-Resistive Sensor of Auxetic Structures Based on 3D-Printed TPU Coated by Castor-Oil-Based Waterborne Polyurethane/Graphene. Fibers Polym. 2023, 24, 15–28. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, Q.; Chen, Z.; Chen, K.; Xu, S.; Gao, J.; Jiang, L. Flexible Microneedle Array Electrode Using Magnetorheological Drawing Lithography for Bio-Signal Monitoring. Sens. Actuators A Phys. 2017, 268, 38–45. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, J.; Shi, G.; Sun, C.; Ingram, M.; Qian, S.; Lu, J.; Zhang, S.; Zhong, Y.L. A Focus Review on 3D Printing of Wearable Energy Storage Devices. Carbon Energy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 1242–1261. [Google Scholar] [CrossRef]
- Cheung, J.T.; Sankur, H. Growth of Thin Films by Laser-Induced Evaporation. Crit. Rev. Solid. State Mater. Sci. 1988, 15, 63–109. [Google Scholar] [CrossRef]
- Tolvanen, J.; Hannu, J.; Jantunen, H. Stretchable and Washable Strain Sensor Based on Cracking Structure for Human Motion Monitoring. Sci. Rep. 2018, 8, 13241. [Google Scholar] [CrossRef]
- Kay, R.; Desmulliez, M. A Review of Stencil Printing for Microelectronic Packaging. Solder. Surf. Mt. Technol. 2012, 24, 38–50. [Google Scholar] [CrossRef]
- Stummer, S.; Salar-Behzadi, S.; Unger, F.M.; Oelzant, S.; Penning, M.; Viernstein, H. Application of Shellac for the Development of Probiotic Formulations. Food Res. Int. 2010, 43, 1312–1320. [Google Scholar] [CrossRef]
- Arias, V.; Höglund, A.; Odelius, K.; Albertsson, A.C. Polylactides with “Green” Plasticizers: Influence of Isomer Composition. J. Appl. Polym. Sci. 2013, 130, 2962–2970. [Google Scholar] [CrossRef]
- Murariu, M.; Da Silva Ferreira, A.; Pluta, M.; Bonnaud, L.; Alexandre, M.; Dubois, P. Polylactide (PLA)-CaSO4 Composites Toughened with Low Molecular Weight and Polymeric Ester-like Plasticizers and Related Performances. Eur. Polym. J. 2008, 44, 3842–3852. [Google Scholar] [CrossRef]
- Wan, T.; Lin, Y.; Tu, Y. Plasticizing Effect of Glyceryl Tribenzoate, Dipropylene Glycol Dibenzoate, and Glyceryl Triacetate on Poly(Lactic Acid). Polym. Eng. Sci. 2016, 56, 1399–1406. [Google Scholar] [CrossRef]
- Ljungberg, N.; Andersson, T.; Wesslén, B. Film Extrusion and Film Weldability of Poly(Lactic Acid) Plasticized with Triacetine and Tributyl Citrate. J. Appl. Polym. Sci. 2003, 88, 3239–3247. [Google Scholar] [CrossRef]
- Mathew, R.; Arun, P.; Madhavarao, C.N.; Moffett, J.R.; Namboodiri, M.A.A. Progress toward Acetate Supplementation Therapy for Canavan Disease: Glyceryl Triacetate Administration Increases Acetate, but Not N-Acetylaspartate, Levels in Brain. J. Pharmacol. Exp. Ther. 2005, 315, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, S.; Xiang, J.; Lei, J.; Zhou, C. Preparation and Application of Triglyceride Plasticizers for Poly(Vinyl Chloride). J. Appl. Polym. Sci. 2013, 129, 1915–1921. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G. Plasticizing Polylactide—The Effect of Different Plasticizers on the Mechanical Properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Murariu, M.; Da Silva Ferreira, A.; Alexandre, M.; Dubois, P. Polylactide (PLA) Designed with Desired End-Use Properties: 1. PLA Compositions with Low Molecular Weight Ester-like Plasticizers and Related Performances. Polym. Adv. Technol. 2008, 19, 636–646. [Google Scholar] [CrossRef]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers Derived from Biomass Resources: A Short Review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef]
- Geng, S.; Haque, M.M.U.; Oksman, K. Crosslinked Poly(Vinyl Acetate) (PVAc) Reinforced with Cellulose Nanocrystals (CNC): Structure and Mechanical Properties. Compos. Sci. Technol. 2016, 126, 35–42. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; He, P.; Liu, K.; Ni, Y.; Chen, L.; Ouyang, X.; Huang, L.; Wang, H.; Xu, S. A Bionic Tactile Plastic Hydrogel-Based Electronic Skin Constructed by a Nerve-like Nanonetwork Combining Stretchable, Compliant, and Self-Healing Properties. Chem. Eng. J. 2020, 379, 122271. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, Z.; Yao, Y.; Pan, Y. Correlations between 11B NMR Parameters and Structural Characters in Borate and Borosilicate Minerals Investigated by High-Resolution MAS NMR and Ab Initio Calculations. Phys. Chem. Min. 2012, 39, 363–372. [Google Scholar] [CrossRef]
- Angelova, L.V.; Terech, P.; Natali, I.; Dei, L.; Carretti, E.; Weiss, R.G. Cosolvent Gel-like Materials from Partially Hydrolyzed Poly(Vinyl Acetate)s and Borax. Langmuir 2011, 27, 11671–11682. [Google Scholar] [CrossRef]
- Geng, S.; Shah, F.U.; Liu, P.; Antzutkin, O.N.; Oksman, K. Plasticizing and Crosslinking Effects of Borate Additives on the Structure and Properties of Poly(Vinyl Acetate). RSC Adv. 2017, 7, 7483–7491. [Google Scholar] [CrossRef]
- Ristić, M.D.; Rajaković, L.V. Boron Removal by Anion Exchangers Impregnated with Citric and Tartaric Acids. Sep. Sci. Technol. 1996, 31, 2805–2814. [Google Scholar] [CrossRef]
- Camargo, J.R.; Orzari, L.O.; Araújo, D.A.G.; de Oliveira, P.R.; Kalinke, C.; Rocha, D.P.; Luiz dos Santos, A.; Takeuchi, R.M.; Munoz, R.A.A.; Bonacin, J.A.; et al. Development of Conductive Inks for Electrochemical Sensors and Biosensors. Microchem. J. 2021, 164, 105998. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Sun, R.; Li, J. Low Temperature Sintering of MOD Assisted Ag Paste for Die-Attach Application. Mater. Lett. 2021, 305, 130799. [Google Scholar] [CrossRef]
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent Development in Silver-Based Ink for Flexible Electronics. J. Sci. Adv. Mater. Devices 2022, 7, 100395. [Google Scholar] [CrossRef]
- Mou, Y.; Peng, Y.; Liu, J.; Wang, Q.; Lei, Z.; Wu, F.; Chen, M. In Situ Self-Reducing Ag2O Ink for the Fabrication of Highly Flexible Printed Conductors. Mater. Today Commun. 2021, 29, 102776. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Hidayah, I.N.; Mariatti, M. Performance of Inkjet-Printed Strain Sensor Based on Graphene/Silver Nanoparticles Hybrid Conductive Inks on Polyvinyl Alcohol Substrate. J. Mater. Sci. Mater. Electron. 2020, 31, 15361–15371. [Google Scholar] [CrossRef]
- O’Grady, B.; Lambe, R.; Baldwin, M.; Acheson, T.; Doherty, C. The Validity of Apple Watch Series 9 and Ultra 2 for Serial Measurements of Heart Rate Variability and Resting Heart Rate. Sensors 2024, 24, 6220. [Google Scholar] [CrossRef]




| Group | Stress (MPa) | Elongation (%) | Resistivity (10−5 Ω·cm) | Conductivity(104 s/cm) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| Glue All | 11.46 ± 2.15 | 2.88 ± 1.10 | -- | -- | 1055.07 ± 517.92 |
| GTA | 1.77 ± 0.18 | 129.9 | -- | -- | 5.20 ± 0.43 |
| GTA + Ag | 1.85 ± 0.03 | 75.35 | 45.82 ± 4.70 | 0.22 ± 0.02 | 8.66 ± 0.40 |
| CA | 1.78 ± 0.09 | 258.36 ± 22.49 | -- | -- | 0.95 ± 0.21 |
| CA + Ag | 1.81 ± 0.15 | 187.67 ± 15.43 | 650.20 ± 42.85 | 0.02 ± 0.01 | 3.79 ± 0.15 |
| Borax1 | 2.52 ± 0.05 | 36.89 ± 1.08 | 8.52 ± 0.12 | 1.17 ± 0.02 | 167.62 ± 22.70 |
| Borax2 | 1.79 ± 0.04 | 41.62 ± 1.39 | 12.08 ± 0.45 | 0.83 ± 0.03 | 68.77 ± 22.81 |
| Borax3 | 0.68 ± 0.05 | 13.13 ± 0.17 | 84.34 ± 3.06 | 0.12 ± 0.01 | 18.81 ± 4.25 |
| Borax4 | 8.12 ± 1.19 | 46.92 ± 16.53 | 19.59 ± 1.77 | 0.54 ± 0.04 | 832.16 ± 51.05 |
| Borax5 | 2.60 ± 0.10 | 190.56 ± 24.94 | 24.88 ± 2.57 | 0.41 ± 0.04 | 3.64 ± 0.37 |
| Borax6 | 1.18 ± 0.68 | 254.33 ± 9.70 | 56.59 ± 7.18 | 4.33 ± 7.17 | 0.91 ± 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rwei, P.; Shiu, J.-W.; Senel, M.; Hajiaghajani, A.; Qian, C.; Chen, C.-W.; Tseng, P.; Khine, M. A Waterborne, Flexible, and Highly Conductive Silver Ink for Ultra-Rapid Fabrication of Epidermal Electronics. Sensors 2025, 25, 2092. https://doi.org/10.3390/s25072092
Rwei P, Shiu J-W, Senel M, Hajiaghajani A, Qian C, Chen C-W, Tseng P, Khine M. A Waterborne, Flexible, and Highly Conductive Silver Ink for Ultra-Rapid Fabrication of Epidermal Electronics. Sensors. 2025; 25(7):2092. https://doi.org/10.3390/s25072092
Chicago/Turabian StyleRwei, Patrick, Jia-Wei Shiu, Mehmet Senel, Amirhossein Hajiaghajani, Chengyang Qian, Chin-Wen Chen, Peter Tseng, and Michelle Khine. 2025. "A Waterborne, Flexible, and Highly Conductive Silver Ink for Ultra-Rapid Fabrication of Epidermal Electronics" Sensors 25, no. 7: 2092. https://doi.org/10.3390/s25072092
APA StyleRwei, P., Shiu, J.-W., Senel, M., Hajiaghajani, A., Qian, C., Chen, C.-W., Tseng, P., & Khine, M. (2025). A Waterborne, Flexible, and Highly Conductive Silver Ink for Ultra-Rapid Fabrication of Epidermal Electronics. Sensors, 25(7), 2092. https://doi.org/10.3390/s25072092








