Recent Advances in Nanomaterial-Based Self-Healing Electrodes Towards Sensing and Energy Storage Applications
Abstract
:1. Introduction
1.1. Self-Healing Electrodes Based on 0D Nanomaterials
1.1.1. 0D Nanomaterials for Health Monitoring
1.1.2. 0D Nanomaterials for Motion Monitoring
1.1.3. 0D Nanomaterials for Environmental Monitoring
1.1.4. 0D Nanomaterials for Energy Storage Applications
1.2. Self-Healing Electrodes Based on 1D Nanomaterials
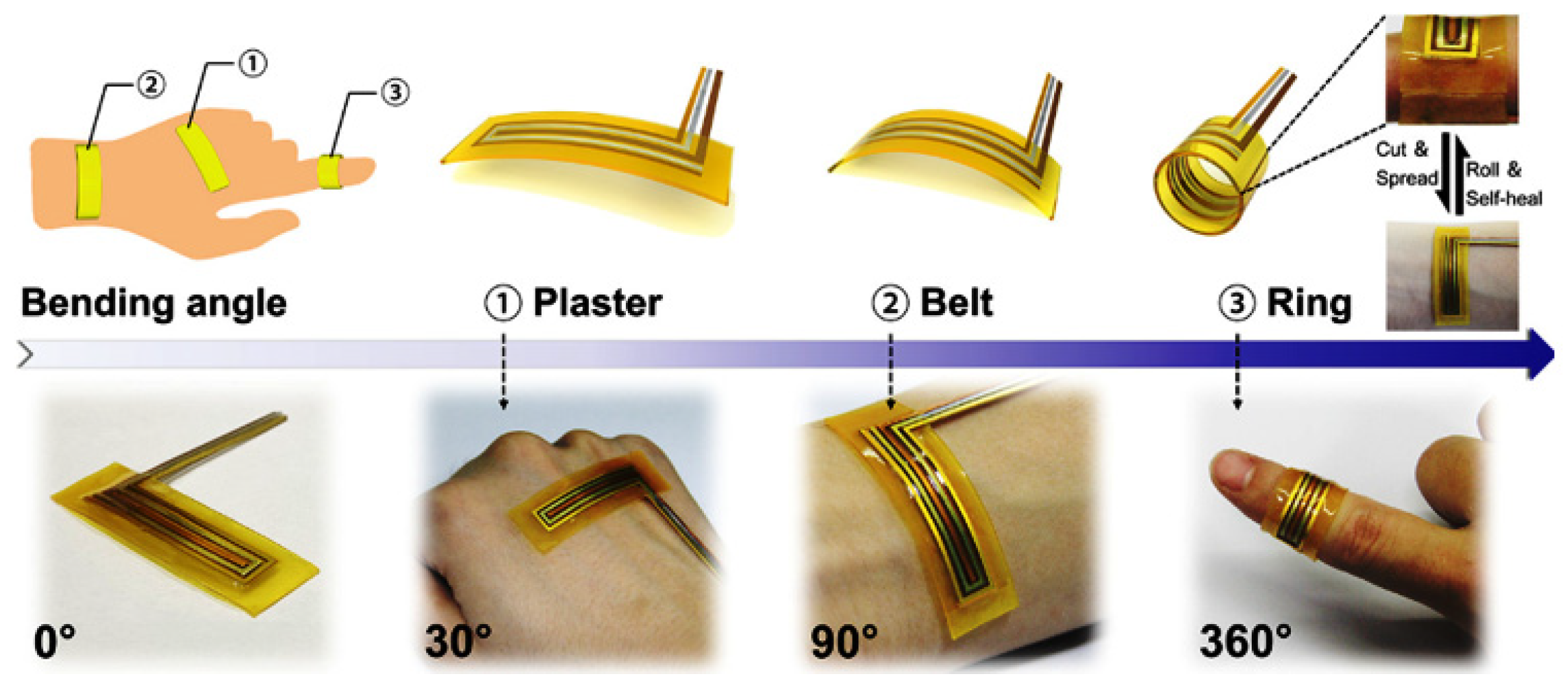
1.2.1. 1D Nanomaterials for Health Monitoring
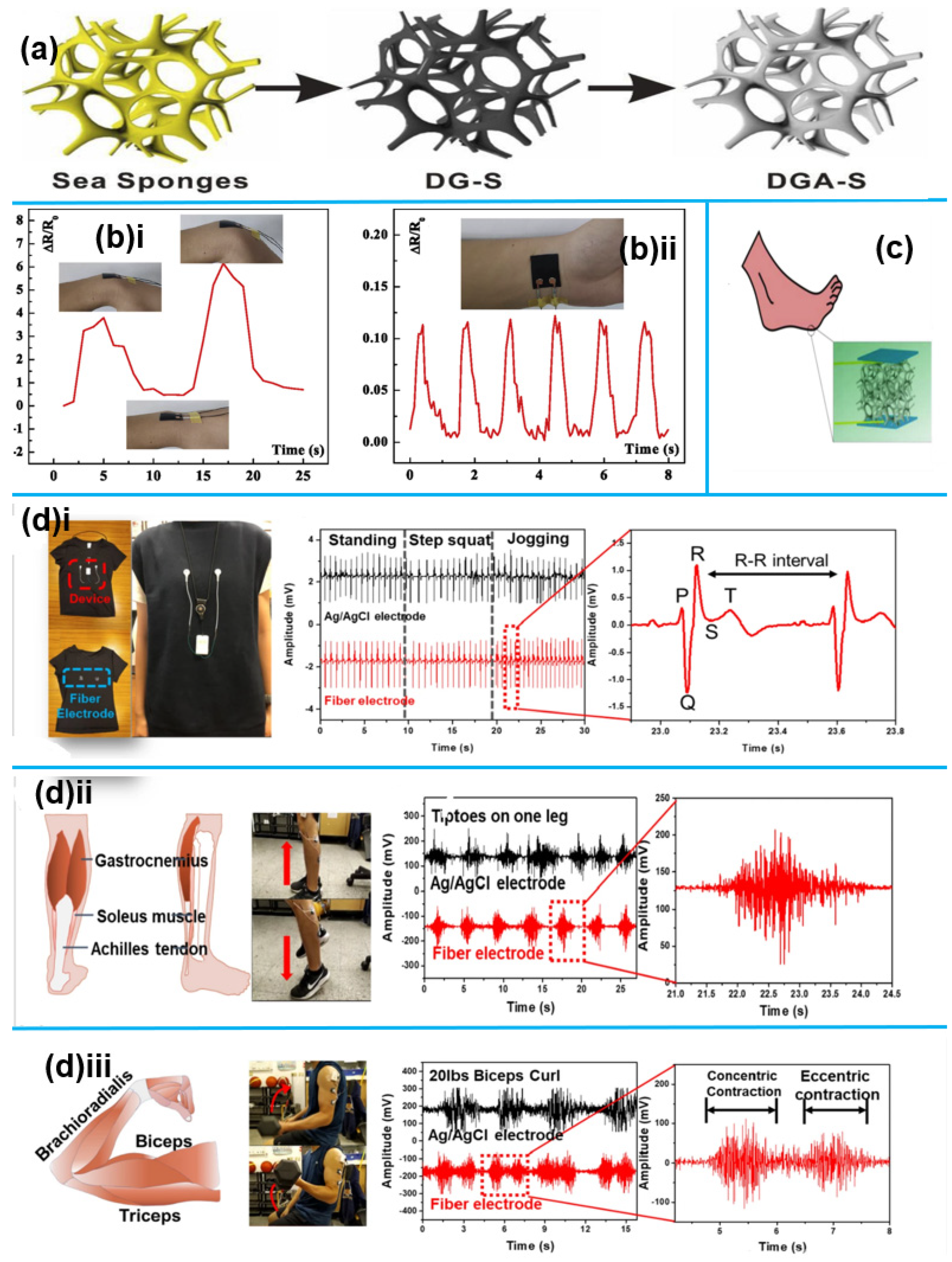
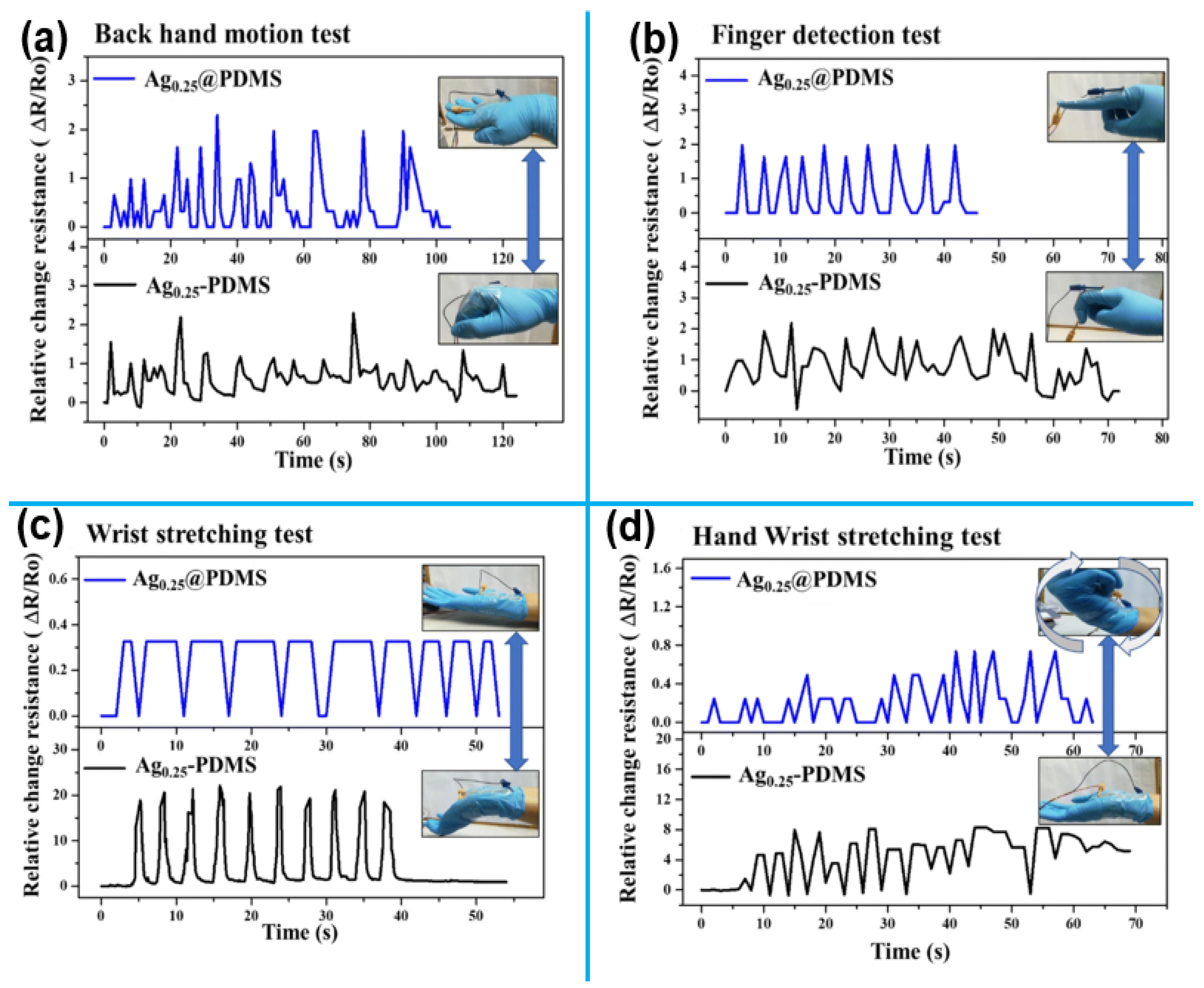
1.2.2. 1D Nanomaterials for Motion Monitoring
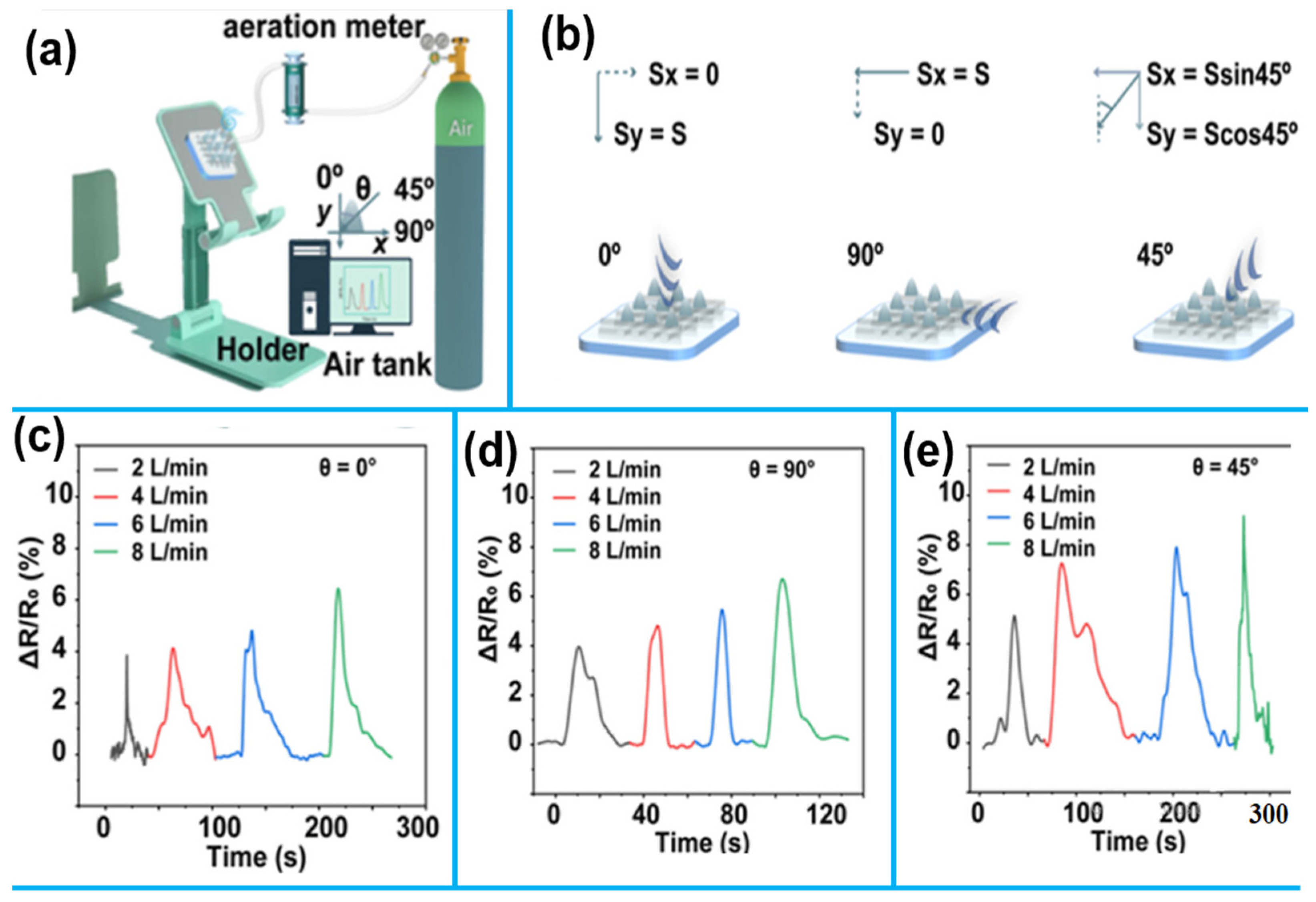
1.2.3. 1D Nanomaterials for Environmental Monitoring
| Materials | Binder | Fabrication | Conductivity S/m/Scm−1 | Healing Efficiency(%) | Stretchability After Self-Healing (%) | Sensitivity kPa−1/mPa | Response Time (ms) | Strain (%) | Gauge Factor | Stability (Cycles) | App. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cPDA@ZPD NPs | - | Copolymerization | 6.8 | 2.32 | 125 | 0.36 | - | 585 | 1.09 | - | Health | [25] |
| MWCNT | PDMS | coating | - | - | - | - | - | 5.0 | 513 | - | Health | [57] |
| AgNWs | polydopamine | Self-assembly | - | - | 0.016 | 54 | 0–60 | 1.5 | - | Health | [59] | |
| FHE nanofiber | PEDOT:PSS | Electrospinning | 1.3× 101 Ω/sq | 98.3 | - | 15 mPa | - | 200 | - | 3000 | Health | [60] |
| AgNWs | TPU | Casting | - | - | - | - | - | 372 | 6.78 | - | Health | [76] |
| AgNPs | PDMS | drop-casting | 0.11 & 0.14 | - | 50 | 2.5 | 70 | 10.08 | 10,000 | Motion | [36] | |
| AgNPs | PU | Screen printing | 1.65–2.85 | - | 20 | - | - | 15–20 | - | - | Motion | [77] |
| AuNWs | elastomeric sheet | Drop-casting | 0 | - | - | <22 | 0.01 350 | 6.9–9.9 | 5000 | Motion | [58] | |
| IrNPs & MWCNTs | P-PDMS | Atomic layer deposition | - | - | - | 34.96 | 150 | 30 | 5.12 | >10,000 | Motion | [66] |
| G/AgNWs | PAC | bubbletemplate | - | - | 200 | - | <1 | 200 | 22.90 | - | Motion | [68] |
| AgNWs | PDMS | Coating Pre-stretching Drying | - | - | 60 | - | - | 60 | 150,000 | 30,000 | Motion | [78] |
| AgNWs | PDMS | Laser cutting Drop coating | - | 88.3% | - | - | 150 | 846 | 1000 | Motion | [79] | |
| AgNW/TPU | PDMS | Electrospinning Vacuum filtration Spin coating | 50 | - | - | - | - | 50 | 12.9 | 1600 | Motion | [80] |
| AgNWs | TPU | Electrospinning Dip Coating | 3990 | - | - | - | 6 | 900 | 44.43 | 20,000 | Motion | [81] |
| AgNWs | TPU | Spray coating | - | - | - | - | - | 100 | 4.4 × 107 | 1000 | Motion | [82] |
| AgNWs | PANI/PU | Electrospinning Vacuum filtration | 32.09 | - | - | - | - | 30 | 59 | 300 | Motion | [83] |
| AgNWs | Dragon skin | Soft lithography Drop casting | - | - | - | - | - | 150 | 81 | 10,000 | Motion | [84] |
| LCGO/AgNW | PET | Ink-printing | 17,800 | 95 | 4.2 | - | <1.4 | - | - | Environmental | [74] | |
| AgNWs | PDMS | Drop casting | - | - | - | - | - | 9 | 536.98 | - | Environmental | [85] |
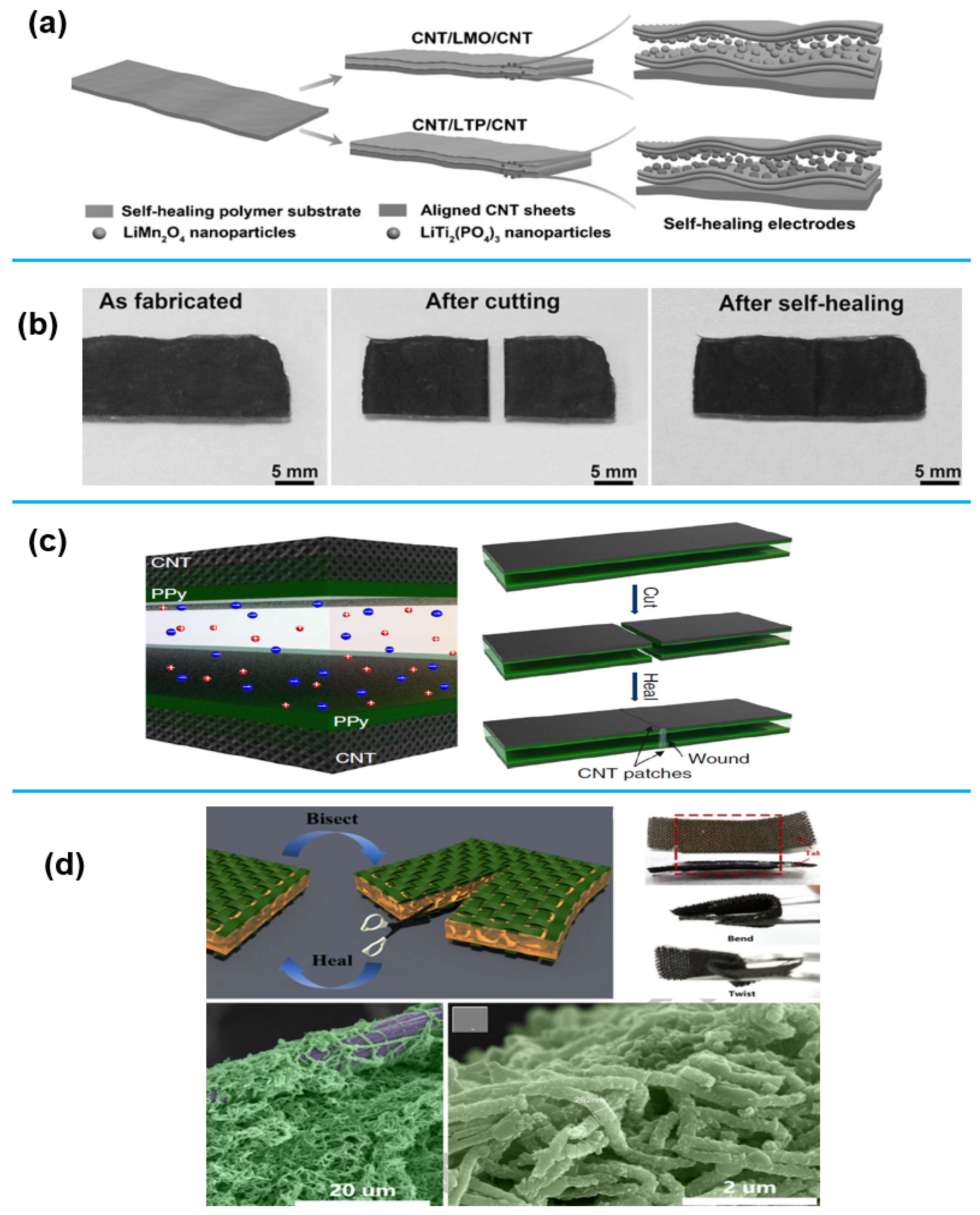
1.2.4. 1D Nanomaterials for Energy Storage Application
1.3. Self-Healing Electrodes Based on 2D Nanomaterials
1.3.1. 2D Nanomaterials for Health Monitoring

1.3.2. 2D Nanomaterials for Motion Monitoring
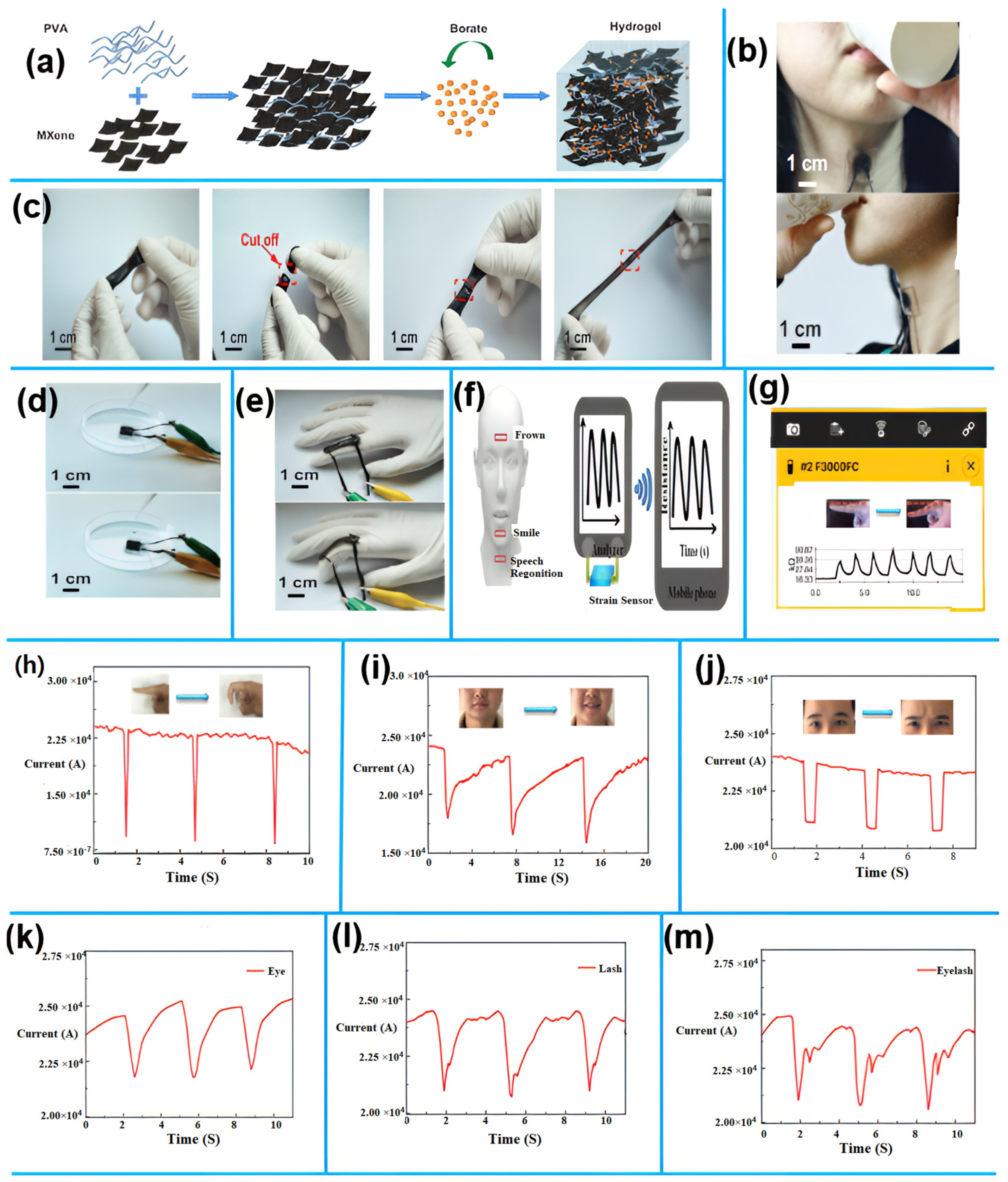
1.3.3. 2D Nanomaterials for Environmental Monitoring
1.3.4. 2D Nanomaterials for Energy Storage Application
| Materials | Binder | Fabrication Method | Sensitivity | Response Time | Gauge Factor | Strain (%) | Stability (Cycles) | Conductivity | Healing Efficiency | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rGO | silicone sealant | electrochemical exfoliation | - | 395 ms | <40,000 | 100 | 1600 | - | - | Health | [99] |
| GSCA | - | 3D printing | 26–78 kPa | 60 ms | - | 600 | 1000 | - | - | Health & Environmental | [100] |
| RGO & (CuNWs) | MF | Coating and in-situ growing | 0.088 kPa−1 | 0.3s | - | - | 5000 | - | - | Health | [101] |
| rGO | Graphite oxide | touch-sensing mechanism | - | - | - | - | - | 28 Ω/square | - | Health | [96] |
| MXene/PVA hydrogel | Mxene | Gelatinazation | 0.40 | 0.15s | - | 200 | 10,000 | - | Excellent | Motion | [102] |
| GNs | Rubber matrix | metal–ligand coordination | - | - | 45,573.1 | 50 | 1000 | - | Excellent | Motion | [111] |
| MXene | PU | Spray method | 509.8 kPa−1 | 67.3 ms | - | - | 10,000 | Excellent | Motion | [118] | |
| GO/SnO2/PANI | QCM | in-situ oxidative polymerization | 29.1 Hz/%RH | 7 s/2 s | - | 97 | - | - | - | Environmental | [119] |
1.4. Self-Healing Electrodes Based on 3D Nanomaterials
1.4.1. 3D Nanomaterials for Health Monitoring
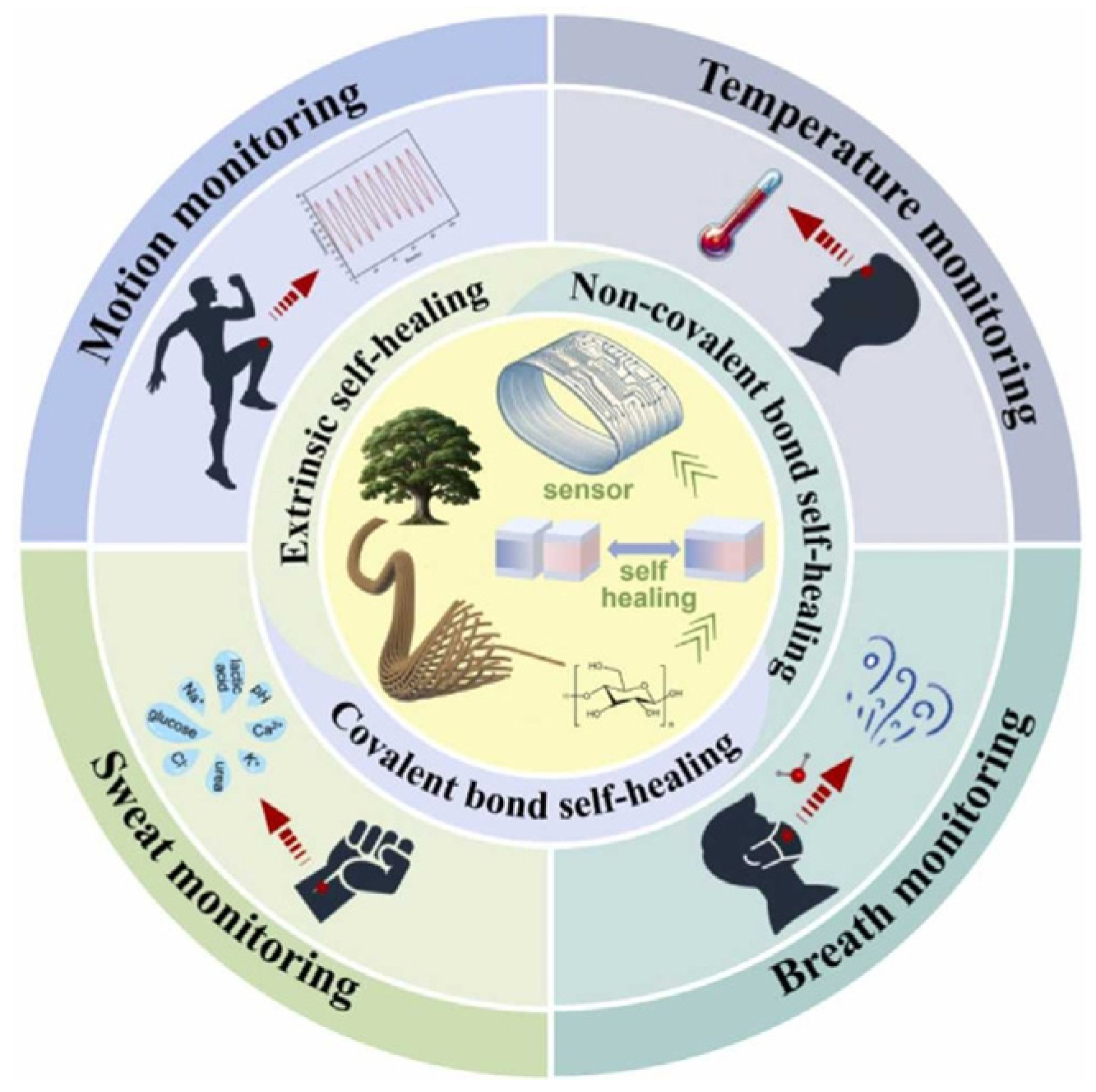

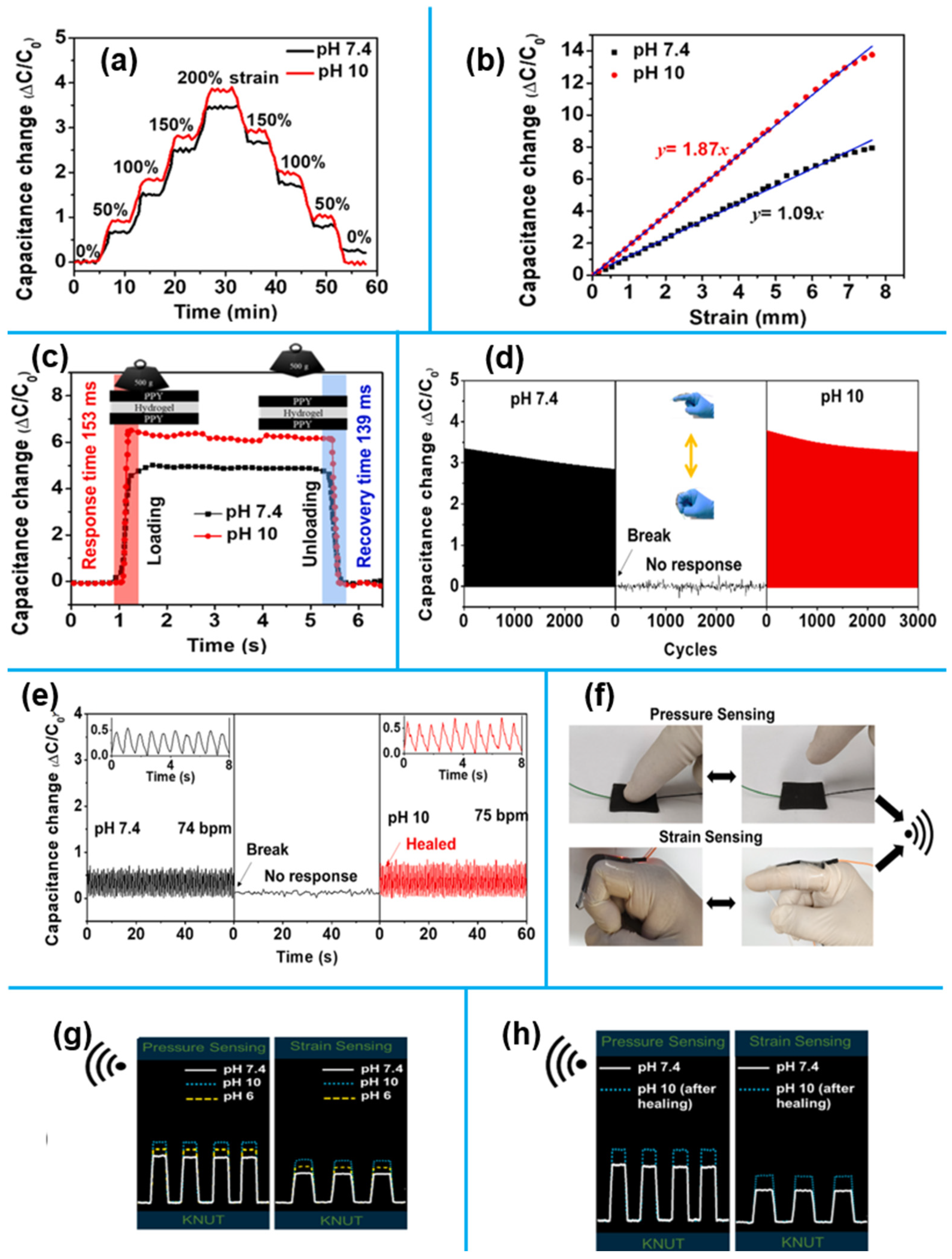
1.4.2. 3D Nanomaterials for Motion Monitoring

1.4.3. 3D Nanomaterials for Environmental Monitoring
1.4.4. 3D Nanomaterials for Energy Storage Applications
2. Comparative Analysis of Advantages and Limitations of Nano Materials Across Dimensions
3. Balancing Mechanical Robustness and Self-Healing Kinetics in Advanced Polymeric Materials
Biomimetic Approaches for Balancing Strength and Self-Healing
- i.
- 3D Interconnected Network Structures: Inspired by cartilage tissue, which integrates rigid collagen fibrils with flexible proteoglycans, synthetic polymeric materials can incorporate interwoven networks of nanofillers to maintain mechanical strength while facilitating repair. The study discusses the use of tungsten disulfide (WS2) nanosheets embedded in a polyurethane matrix, forming a hydrogen bond-driven 3D skeleton that enhances both durability and healing efficiency.
- ii.
- Hybrid Crosslinking Strategies: Combining permanent covalent crosslinks with reversible dynamic bonds allows selective breakage and reformation of specific bonds, ensuring structural stability while enabling molecular rearrangement. For example, the integration of supramolecular interactions (e.g., host-guest chemistry) within rigid frameworks improves resilience without sacrificing healability.
- iii.
- Gradient and Multiscale Structural Engineering: Inspired by fish scales and nacre, gradient architectures provide a balance between rigidity and flexibility by varying crosslinking densities across different layers. Li et al. [164] explore the role of anisotropic structural hierarchies in distributing stress efficiently while maintaining repair potential.
- iv.
- Dynamic Interfacial Bonding: Strengthening interfacial interactions between polymer chains and nanofillers reduces the risk of mechanical failure while enhancing molecular diffusion during healing. The use of tannic acid-modified nanofillers to improve hydrogen bonding interactions exemplifies a practical approach discussed in the study.
4. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tong, X.; Tian, Z.; Sun, J.; Tung, V.; Kaner, R.B.; Shao, Y. Self-healing flexible/stretchable energy storage devices. Mater. Today 2021, 44, 78–104. [Google Scholar]
- Kumar, E.K.; Patel, S.S.; Kumar, V.; Panda, S.K.; Mahmoud, S.; Balubaid, M. State of art review on applications and mechanism of self-healing materials and structure. Arch. Comput. Methods Eng. 2023, 30, 1041–1055. [Google Scholar]
- Jiang, X.; Chen, Y.; Meng, X.; Cao, W.; Liu, C.; Huang, Q.; Naik, N.; Murugadoss, V.; Huang, M.; Guo, Z. The impact of electrode with carbon materials on safety performance of lithium-ion batteries: A review. Carbon 2022, 191, 448–470. [Google Scholar]
- Ma, W.; Wan, S.; Cui, X.; Hou, G.; Xiao, Y.; Rong, J.; Chen, S. Exploration and application of self-healing strategies in lithium batteries. Adv. Funct. Mater. 2023, 33, 2212821. [Google Scholar]
- Chen, B.; Cao, Y.; Li, Q.; Yan, Z.; Liu, R.; Zhao, Y.; Zhang, X.; Wu, M.; Qin, Y.; Sun, C. Liquid metal-tailored gluten network for protein-based e-skin. Nat. Commun. 2022, 13, 1206. [Google Scholar] [PubMed]
- Wan, X.; Mu, T.; Yin, G. Intrinsic self-healing chemistry for next-generation flexible energy storage devices. Nano-Micro Lett. 2023, 15, 99. [Google Scholar]
- Ghosh, S.; Polaki, S.R.; Macrelli, A.; Casari, C.S.; Barg, S.; Jeong, S.M.; Ostrikov, K.K. Nanoparticle-enhanced multifunctional nanocarbons—Recent advances on electrochemical energy storage applications. J. Phys. D Appl. Phys. 2022, 55, 413001. [Google Scholar]
- Pu, J.; Han, M.; Wang, T.; Zhu, X.; Lu, M.; Chen, J.; Liu, W.; Dai, Y.; Tan, Y. The enhanced confinement effect of double shell hollow mesoporous spheres assembled with nitrogen-doped copper cobaltate nanoparticles for enhancing lithium–sulfur batteries. Electrochim. Acta 2022, 404, 139597. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar]
- Noor, F.; Joe, Y.H.; Nadras, O.; Abd, T.A.; Khimi, S.R. Intrinsic self-healing rubber: A review and perspective of material and reinforcement. Polym. Test. 2022, 111, 107598. [Google Scholar]
- Dong, R.; Zhao, X.; Guo, B.; Ma, P.X. Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [PubMed]
- Zhao, C.; Wang, Y.; Tang, G.; Ru, J.; Zhu, Z.; Li, B.; Guo, C.F.; Li, L.; Zhu, D. Ionic flexible sensors: Mechanisms, materials, structures, and applications. Adv. Funct. Mater. 2022, 32, 2110417. [Google Scholar]
- Ezeigwe, E.R.; Dong, L.; Manjunatha, R.; Tan, M.; Yan, W.; Zhang, J. A review of self-healing electrode and electrolyte materials and their mitigating degradation of Lithium batteries. Nano Energy 2021, 84, 105907. [Google Scholar] [CrossRef]
- Gu, J.; Li, X.; Zhou, Z.; Liu, W.; Li, K.; Gao, J.; Zhao, Y.; Wang, Q. 2D MnO 2 nanosheets generated signal transduction with 0D carbon quantum dots: Synthesis strategy, dual-mode behavior and glucose detection. Nanoscale 2019, 11, 13058–13068. [Google Scholar]
- Zarepour, A.; Ahmadi, S.; Rabiee, N.; Zarrabi, A.; Iravani, S. Self-healing MXene-and graphene-based composites: Properties and applications. Nano-Micro Lett. 2023, 15, 100. [Google Scholar]
- Liao, C.-Y.; Zhang, L.; Hu, S.-Y.; Xia, S.-J.; Li, D. Recent advances of self-healing materials for civil engineering: Models and simulations. Buildings 2024, 14, 961. [Google Scholar] [CrossRef]
- Yao, H.; Liu, T.; Jia, Y.; Du, Y.; Yao, B.; Xu, J.; Fu, J. Water-insensitive self-healing materials: From network structure design to advanced soft electronics. Adv. Funct. Mater. 2023, 33, 2307455. [Google Scholar]
- Divakaran, N.; Kale, M.B.; Wu, L. Low-dimensional carbon-based nanomaterials: Synthesis and application in polymer nanocomposites. In Design, Fabrication, and Characterization of Multifunctional Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 481–499. [Google Scholar]
- Tan, Y.J.; Wu, J.; Li, H.; Tee, B.C. Self-healing electronic materials for a smart and sustainable future. ACS Appl. Mater. Interfaces 2018, 10, 15331–15345. [Google Scholar]
- Kim, S.D.; Sarkar, A.; Ahn, J.H. Graphene-based nanomaterials for flexible and stretchable batteries. Small 2021, 17, 2006262. [Google Scholar] [CrossRef]
- Aleksandrzak, M.; Baca, M.; Pacia, M.; Wenelska, K.; Zielinska, B.; Kalenczuk, R.J.; Mijowska, E. 0D, 1D, 2D molybdenum disulfide functionalized by 2D polymeric carbon nitride for photocatalytic water splitting. Nanotechnology 2021, 32, 355703. [Google Scholar]
- Tang, M.; Wu, J.; Wang, K.; Ying, M.; Lv, H.; Li, Z. Preparation of autonomously self-healing electrode based on double network supramolecular elastomer. In Proceedings of the 2020 IEEE 70th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 3–30 June 2020. [Google Scholar]
- Zhou, Y.; Li, L.; Han, Z.; Li, Q.; He, J.; Wang, Q. Self-healing polymers for electronics and energy devices. Chem. Rev. 2022, 123, 558–612. [Google Scholar] [CrossRef] [PubMed]
- Pekkanen, A.M.; DeWitt, M.R.; Rylander, M.N. Nanoparticle enhanced optical imaging and phototherapy of cancer. J. Biomed. Nanotechnol. 2014, 10, 1677–1712. [Google Scholar] [CrossRef]
- Shit, A.; Kim, S.G.; In, I.; Park, S.Y. Self-repairable and recyclable self-powered human motion sensor with NIR/pH-responsive amplified Stretchable, Conductive, and Self-Healable hydrogel. Chem. Eng. J. 2021, 426, 131846. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Rab, S.; Singh, R.P.; Suman, R. Sensors for daily life: A review. Sens. Int. 2021, 2, 100121. [Google Scholar] [CrossRef]
- Tan, Y.J.; Susanto, G.J.; Anwar Ali, H.P.; Tee, B.C. Progress and Roadmap for Intelligent Self-Healing Materials in Autonomous Robotics. Adv. Mater. 2021, 33, 2002800. [Google Scholar] [CrossRef]
- Wen, N.; Zhang, L.; Jiang, D.; Wu, Z.; Li, B.; Sun, C.; Guo, Z. Emerging flexible sensors based on nanomaterials: Recent status and applications. J. Mater. Chem. A 2020, 8, 25499–25527. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Biesold, G.M.; Zhang, M.; Zou, H.; Ding, Y.; Wang, Z.L.; Lin, Z. Piezo-phototronic effect on photocatalysis, solar cells, photodetectors and light-emitting diodes. Chem. Soc. Rev. 2021, 50, 13646–13691. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Umapathi, R.; Park, B.; Sonwal, S.; Rani, G.M.; Cho, Y.; Huh, Y.S. Advances in optical-sensing strategies for the on-site detection of pesticides in agricultural foods. Trends Food Sci. Technol. 2022, 119, 69–89. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [PubMed]
- Sangeetha, N.M.; Decorde, N.; Viallet, B.; Viau, G.; Ressier, L. Nanoparticle-based strain gauges fabricated by convective self-assembly: Strain sensitivity and hysteresis with respect to nanoparticle sizes. J. Phys. Chem. C 2013, 117, 1935–1940. [Google Scholar] [CrossRef]
- Souri, H.; Banerjee, H.; Jusufi, A.; Radacsi, N.; Stokes, A.A.; Park, I.; Sitti, M.; Amjadi, M. Wearable and stretchable strain sensors: Materials, sensing mechanisms, and applications. Adv. Intell. Syst. 2020, 2, 2000039. [Google Scholar] [CrossRef]
- Soe, H.M.; Manaf, A.A.; Matsuda, A.; Jaafar, M. Development and fabrication of highly flexible, stretchable, and sensitive strain sensor for long durability based on silver nanoparticles–polydimethylsiloxane composite. J. Mater. Sci. Mater. Electron. 2020, 31, 11897–11910. [Google Scholar] [CrossRef]
- Justino, C.I.; Duarte, A.C.; Rocha-Santos, T.A. Recent progress in biosensors for environmental monitoring: A review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef]
- Vaseashta, A.; Vaclavikova, M.; Vaseashta, S.; Gallios, G.; Roy, P.; Pummakarnchana, O. Nanostructures in environmental pollution detection, monitoring, and remediation. Sci. Technol. Adv. Mater. 2007, 8, 47. [Google Scholar] [CrossRef]
- Yan, J.; Pan, G.; Lin, W.; Tang, Z.; Zhang, J.; Li, J.; Li, W.; Lin, X.; Luo, H.; Yi, G. Multi-responsive graphene quantum dots hybrid self-healing structural color hydrogel for information encoding and encryption. Chem. Eng. J. 2023, 451, 138922. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Wu, Q.; Xiao, H.; Han, J. Self-recovery, fatigue-resistant, and multifunctional sensor assembled by a nanocellulose/carbon nanotube nanocomplex-mediated hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 50281–50297. [Google Scholar]
- Hannan, M.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of energy storage systems for electric vehicle applications: Issues and challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar]
- Murray, C.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E= sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar]
- Shinde, V.V.; Wang, Y.; Salek, M.F.; Auad, M.L.; Beckingham, L.E.; Beckingham, B.S. Material design for enhancing properties of 3D printed polymer composites for target applications. Technologies 2022, 10, 45. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Yang, Z.; Liu, J.; Xiang, Y.; Wu, F. Self-Healing Silicon Anode via the Addition of GaInSn-Encapsulated Microcapsules. ACS Appl. Energy Mater. 2022, 5, 12945–12952. [Google Scholar]
- Wang, C.Y.; Jiao, K.; Yan, J.F.; Wan, M.C.; Wan, Q.Q.; Breschi, L.; Chen, J.H.; Tay, F.R.; Niu, L.N. Biological and synthetic template-directed syntheses of mineralized hybrid and inorganic materials. Prog. Mater. Sci. 2021, 116, 100712. [Google Scholar]
- Jian, M.; Wang, C.; Wang, Q.; Wang, H.; Xia, K.; Yin, Z.; Zhang, M.; Liang, X.; Zhang, Y. Advanced carbon materials for flexible and wearable sensors. Sci. China Mater. 2017, 60, 1026–1062. [Google Scholar]
- Lee, J.; Llerena Zambrano, B.; Woo, J.; Yoon, K.; Lee, T. Recent advances in 1D stretchable electrodes and devices for textile and wearable electronics: Materials, fabrications, and applications. Adv. Mater. 2020, 32, 1902532. [Google Scholar]
- Yao, S.; Zhu, Y. Nanomaterial-enabled stretchable conductors: Strategies, materials and devices. Adv. Mater. 2015, 27, 1480–1511. [Google Scholar]
- Wang, M.; Sun, H.; Cao, F.; Tian, W.; Li, L. Moisture-triggered self-healing flexible perovskite photodetectors with excellent mechanical stability. Adv. Mater. 2021, 33, 2100625. [Google Scholar]
- Xu, X.; Xue, P.; Gao, M.; Li, Y.; Xu, Z.; Wei, Y.; Zhang, Z.; Liu, Y.; Wang, L.; Liu, H. Assembled one-dimensional nanowires for flexible electronic devices via printing and coating: Techniques, applications, and perspectives. Adv. Colloid Interface Sci. 2023, 321, 102987. [Google Scholar]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire–elastomer nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [PubMed]
- Xiao, X.; Yuan, L.; Zhong, J.; Ding, T.; Liu, Y.; Cai, Z.; Rong, Y.; Han, H.; Zhou, J.; Wang, Z.L. High-strain sensors based on ZnO nanowire/polystyrene hybridized flexible films. Adv. Mater. 2011, 23, 5440–5444. [Google Scholar]
- Cai, L.; Song, L.; Luan, P.; Zhang, Q.; Zhang, N.; Gao, Q.; Zhao, D.; Zhang, X.; Tu, M.; Yang, F. Super-stretchable, transparent carbon nanotube-based capacitive strain sensors for human motion detection. Sci. Rep. 2013, 3, 3048. [Google Scholar]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar]
- Huang, K.; Ning, H.; Hu, N.; Liu, F.; Wu, X.; Wang, S.; Liu, Y.; Zou, R.; Yuan, W.; Wu, L. Ultrasensitive MWCNT/PDMS composite strain sensor fabricated by laser ablation process. Compos. Sci. Technol. 2020, 192, 108105. [Google Scholar]
- Gong, S.; Lai, D.T.; Su, B.; Si, K.J.; Ma, Z.; Yap, L.W.; Guo, P.; Cheng, W. Highly stretchy black gold E-skin nanopatches as highly sensitive wearable biomedical sensors. Adv. Electron. Mater. 2015, 1, 1400063. [Google Scholar]
- Dong, X.; Wei, Y.; Chen, S.; Lin, Y.; Liu, L.; Li, J. A linear and large-range pressure sensor based on a graphene/silver nanowires nanobiocomposites network and a hierarchical structural sponge. Compos. Sci. Technol. 2018, 155, 108–116. [Google Scholar]
- Li, J.-W.; Huang, B.S.; Chang, C.H.; Chiu, C.W. Advanced electrospun AgNPs/rGO/PEDOT: PSS/TPU nanofiber electrodes: Stretchable, self-healing, and perspiration-resistant wearable devices for enhanced ECG and EMG monitoring. Adv. Compos. Hybrid Mater. 2023, 6, 231. [Google Scholar]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar]
- Chinnappan, A.; Baskar, C.; Baskar, S.; Ratheesh, G.; Ramakrishna, S. An overview of electrospun nanofibers and their application in energy storage, sensors and wearable/flexible electronics. J. Mater. Chem. C 2017, 5, 12657–12673. [Google Scholar]
- He, J.; Zhang, Y.; Zhou, R.; Meng, L.; Chen, T.; Mai, W.; Pan, C. Recent advances of wearable and flexible piezoresistivity pressure sensor devices and its future prospects. J. Mater. 2020, 6, 86–101. [Google Scholar]
- Lee, P.; Ham, J.; Lee, J.; Hong, S.; Han, S.; Suh, Y.D.; Lee, S.E.; Yeo, J.; Lee, S.S.; Lee, D. Highly stretchable or transparent conductor fabrication by a hierarchical multiscale hybrid nanocomposite. Adv. Funct. Mater. 2014, 24, 5671–5678. [Google Scholar]
- Sun, D.-M.; Timmermans, M.Y.; Tian, Y.; Nasibulin, A.G.; Kauppinen, E.I.; Kishimoto, S.; Mizutani, T.; Ohno, Y. Flexible high-performance carbon nanotube integrated circuits. Nat. Nanotechnol. 2011, 6, 156–161. [Google Scholar] [PubMed]
- Lai, T.-C.; Fang, C.; Liu, C.; Zhao, X.-R.; Cao, Y.-Q.; Wu, D.; Li, A.-D. Biomimetic strain sensors based on patterned polydimethylsiloxane and Ir nanoparticles decorated multi-walled carbon nanotubes. Sens. Actuators A Phys. 2019, 289, 57–64. [Google Scholar]
- Zhou, J.; Fei, P.; Gu, Y.; Mai, W.; Gao, Y.; Yang, R.; Bao, G.; Wang, Z.L. Piezoelectric-potential-controlled polarity-reversible Schottky diodes and switches of ZnO wires. Nano Lett. 2008, 8, 3973–3977. [Google Scholar]
- Li, Q.; Ullah, Z.; Li, W.; Guo, Y.; Xu, J.; Wang, R.; Zeng, Q.; Chen, M.; Liu, C.; Liu, L. Wide-Range Strain Sensors Based on Highly Transparent and Supremely Stretchable Graphene/Ag-Nanowires Hybrid Structures. Small 2016, 12, 5058–5065. [Google Scholar]
- Hanssen, B.L.; Siraj, S.; Wong, D.K. Recent strategies to minimize fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar]
- Chakraborty, P.; Das, S.; Nandi, A.K. Conducting gels: A chronicle of technological advances. Prog. Polym. Sci. 2019, 88, 189–219. [Google Scholar]
- Choi, S.B.; Shin, H.S.; Kim, J.-W. Convolution Neural Networks for Motion Detection with Electrospun Reversibly-Cross-linkable Polymers and Encapsulated Ag Nanowires. ACS Appl. Mater. Interfaces 2023, 15, 47591–47603. [Google Scholar]
- Sun, F.; Liu, L.; Xu, J.; Fu, J. Smart healable polyurethanes: Sustainable problem solvers based on constitutional dynamic chemistry. Mater. Chem. Front. 2023, 7, 3494–3523. [Google Scholar] [CrossRef]
- Sim, H.J.; Kim, H.; Jang, Y.; Spinks, G.M.; Gambhir, S.; Officer, D.L.; Wallace, G.G.; Kim, S.J. Self-healing electrode with high electrical conductivity and mechanical strength for artificial electronic skin. ACS Appl. Mater. Interfaces 2019, 11, 46026–46033. [Google Scholar] [CrossRef] [PubMed]
- Keum, K.; Kim, J.W.; Hong, S.Y.; Son, J.G.; Lee, S.S.; Ha, J.S. Flexible/stretchable supercapacitors with novel functionality for wearable electronics. Adv. Mater. 2020, 32, 2002180. [Google Scholar] [CrossRef]
- Hashemi, P.; Mehranpour, M.; Ghasemi, I. Fabrication of a strain sensor based on polymer/silver nanowires nanocomposite for medical applications: Siloxane based versus urethane based nanocomposites. Polym. Compos. 2021, 42, 1440–1450. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, H.-K. Cost-effective stretchable Ag nanoparticles electrodes fabrication by screen printing for wearable strain sensors. Surf. Coat. Technol. 2020, 384, 125308. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, Z.; Kang, Z.; Gao, F.; Liao, Q.; Zhang, Y. Ultrasensitive and stretchable resistive strain sensors designed for wearable electronics. Mater. Horiz. 2017, 4, 502–510. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Liu, J.; Li, C. Highly stretchable, sensitive, and transparent strain sensors with a controllable in-plane mesh structure. ACS Appl. Mater. Interfaces 2018, 11, 5316–5324. [Google Scholar] [CrossRef]
- Lu, L.; Wei, X.; Zhang, Y.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. A flexible and self-formed sandwich structure strain sensor based on AgNW decorated electrospun fibrous mats with excellent sensing capability and good oxidation inhibition properties. J. Mater. Chem. C 2017, 5, 7035–7042. [Google Scholar] [CrossRef]
- Pan, W.; Wang, J.; Li, Y.-P.; Sun, X.-B.; Wang, J.-P.; Wang, X.-X.; Zhang, J.; You, H.-D.; Yu, G.-F.; Long, Y.-Z. Facile preparation of highly stretchable TPU/Ag nanowire strain sensor with spring-like configuration. Polymers 2020, 12, 339. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Xiao, Z.-C.; Yang, Y.; Deng, B.-W.; Yin, B.; Ke, K.; Yang, M.-B. Flexible TPU strain sensors with tunable sensitivity and stretchability by coupling AgNWs with rGO. J. Mater. Chem. C 2020, 8, 4040–4048. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Wang, W.; Yu, D. A wearable strain sensor based on polyurethane nanofiber membrane with silver nanowires/polyaniline electrically conductive dual-network. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127477. [Google Scholar]
- Kim, K.H.; Jang, N.S.; Ha, S.H.; Cho, J.H.; Kim, J.M. Highly sensitive and stretchable resistive strain sensors based on microstructured metal nanowire/elastomer composite films. Small 2018, 14, 1704232. [Google Scholar]
- Du, Y.; Zhang, Q.; Zhuo, K.; Ji, J.; Yuan, Z.; Ji, C.; Zhang, W.; Sang, S. Study on the performance of temperature-stabilised flexible strain sensors based on silver nanowires. Micro Nano Lett. 2019, 14, 168–172. [Google Scholar]
- Dong, L.; Xu, C.; Li, Y.; Huang, Z.-H.; Kang, F.; Yang, Q.-H.; Zhao, X. Flexible electrodes and supercapacitors for wearable energy storage: A review by category. J. Mater. Chem. A 2016, 4, 4659–4685. [Google Scholar] [CrossRef]
- Gong, S.; Cheng, W. One-dimensional nanomaterials for soft electronics. Adv. Electron. Mater. 2017, 3, 1600314. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Sun, H.; Dong, X.; Cao, J.; Wang, L.; Xu, Y.; Ren, J.; Hwang, Y.; Son, I.H. A self-healing aqueous lithium-ion battery. Angew. Chem. Int. Ed. 2016, 55, 14384–14388. [Google Scholar]
- Zhou, X.; Rajeev, A.; Subramanian, A.; Li, Y.; Rossetti, N.; Natale, G.; Lodygensky, G.A.; Cicoira, F. Self-healing, stretchable, and highly adhesive hydrogels for epidermal patch electrodes. Acta Biomater. 2022, 139, 296–306. [Google Scholar]
- Li, Y.; Li, X.; Zhang, S.; Liu, L.; Hamad, N.; Bobbara, S.R.; Pasini, D.; Cicoira, F. Autonomic self-healing of PEDOT: PSS achieved via polyethylene glycol addition. Adv. Funct. Mater. 2020, 30, 2002853. [Google Scholar]
- Huang, Y.; Zhong, M.; Huang, Y.; Zhu, M.; Pei, Z.; Wang, Z.; Xue, Q.; Xie, X.; Zhi, C. A self-healable and highly stretchable supercapacitor based on a dual crosslinked polyelectrolyte. Nat. Commun. 2015, 6, 10310. [Google Scholar] [CrossRef]
- Son, D.; Kang, J.; Vardoulis, O.; Kim, Y.; Matsuhisa, N.; Oh, J.Y.; To, J.W.; Mun, J.; Katsumata, T.; Liu, Y. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol. 2018, 13, 1057–1065. [Google Scholar] [CrossRef]
- Xu, K.; Lu, Y.; Takei, K. Multifunctional skin-inspired flexible sensor systems for wearable electronics. Adv. Mater. Technol. 2019, 4, 1800628. [Google Scholar]
- Rao, Z.; Ershad, F.; Almasri, A.; Gonzalez, L.; Wu, X.; Yu, C. Soft electronics for the skin: From health monitors to human-machine interfaces. Adv. Mater. Technol. 2020, 5, 2000233. [Google Scholar]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Das, P.S.; Hossain, M.F.; Park, J.Y. Chemically reduced graphene oxide-based dry electrodes as touch sensor for electrocardiograph measurement. Microelectron. Eng. 2017, 180, 45–51. [Google Scholar]
- Park, M.; Yang, W.; Kim, J.W.; Choi, Y.; Kim, S.; Lee, Y.; Kim, D.S.; Kim, J.; Lim, D.K.; Ha, J.S. A Fully Self-Healing Patch of Integrated Bio-Signal Monitoring Sensors with Self-Healing Microporous Foam and Au Nanosheet Electrodes. Adv. Funct. Mater. 2024, 34, 2402508. [Google Scholar]
- Son, D.I.; Kwon, B.W.; Park, D.H.; Seo, W.-S.; Yi, Y.; Angadi, B.; Lee, C.-L.; Choi, W.K. Emissive ZnO–graphene quantum dots for white-light-emitting diodes. Nat. Nanotechnol. 2012, 7, 465–471. [Google Scholar] [CrossRef]
- Verma, R.P.; Sahu, P.S.; Rathod, M.; Mohapatra, S.S.; Lee, J.; Saha, B. Ultra-Sensitive and Highly Stretchable Strain Sensors for Monitoring of Human Physiology. Macromol. Mater. Eng. 2022, 307, 2100666. [Google Scholar]
- Song, Y.; Chen, L.; Yang, Q.; Liu, G.; Yu, Q.; Xie, X.; Chen, C.; Liu, J.; Chao, G.; Chen, X. Graphene-based flexible sensors for respiratory and airflow monitoring. ACS Appl. Nano Mater. 2023, 6, 8937–8944. [Google Scholar]
- Xiong, Y.; Zhu, Y.; Liu, X.; Zhu, P.; Hu, Y.; Sun, R.; Wong, C.-P. A flexible pressure sensor based on melamine foam capped by copper nanowires and reduced graphene oxide. Mater. Today Commun. 2020, 24, 100970. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, L.; Gao, Y.; Fang, X.; Lu, T.; Pan, L.; Xuan, F. Highly stretchable and self-healable MXene/polyvinyl alcohol hydrogel electrode for wearable capacitive electronic skin. Adv. Electron. Mater. 2019, 5, 1900285. [Google Scholar]
- Novoselov, K.S.; Fal, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tan, Y.J.; Li, S.; Lee, W.W.; Guo, H.; Cai, Y.; Wang, C.; Tee, B.C.-K. Self-healing electronic skins for aquatic environments. Nat. Electron. 2019, 2, 75–82. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Hojamberdiev, M.; Kumar, M. Transition metal dichalcogenides-based flexible gas sensors. Sens. Actuators A Phys. 2020, 303, 111875. [Google Scholar] [CrossRef]
- Qiao, J.; Kong, X.; Hu, Z.-X.; Yang, F.; Ji, W. High-mobility transport anisotropy and linear dichroism in few-layer black phosphorus. Nat. Commun. 2014, 5, 4475. [Google Scholar]
- Li, X.-B.; Guo, P.; Cao, T.-F.; Liu, H.; Lau, W.-M.; Liu, L.-M. Structures, stabilities and electronic properties of defects in monolayer black phosphorus. Sci. Rep. 2015, 5, 10848. [Google Scholar]
- Wang, T.; Zhang, Y.; Liu, Q.; Cheng, W.; Wang, X.; Pan, L.; Xu, B.; Xu, H. A self-healable, highly stretchable, and solution processable conductive polymer composite for ultrasensitive strain and pressure sensing. Adv. Funct. Mater. 2018, 28, 1705551. [Google Scholar]
- Chen, F.; Xiao, H.; Peng, Z.Q.; Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Thermally conductive glass fiber reinforced epoxy composites with intrinsic self-healing capability. Adv. Compos. Hybrid Mater. 2021, 4, 1048–1058. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, J.; Wu, W. Scalably Nanomanufactured Atomically Thin Materials-Based Wearable Health Sensors. Small Struct. 2022, 3, 2100120. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, Q.; Chen, Z.; Zhang, X.; Lu, C.; Cao, J.; Zheng, Z. Scalable manufactured self-healing strain sensors based on ion-intercalated graphene nanosheets and interfacial coordination. ACS Appl. Mater. Interfaces 2019, 11, 23527–23534. [Google Scholar] [CrossRef]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monit. 2004, 6, 657–664. [Google Scholar] [CrossRef]
- He, K.; Liu, Z.; Wan, C.; Jiang, Y.; Wang, T.; Wang, M.; Zhang, F.; Liu, Y.; Pan, L.; Xiao, M. An on-skin electrode with anti-epidermal-surface-lipid function based on a zwitterionic polymer brush. Adv. Mater. 2020, 32, 2001130. [Google Scholar]
- Zhang, J.; Jin, J.; Wan, J.; Jiang, S.; Wu, Y.; Wang, W.; Gong, X.; Wang, H. Quantum dots-based hydrogels for sensing applications. Chem. Eng. J. 2021, 408, 127351. [Google Scholar]
- Kabir, M.; Demirocak, D.E. Degradation mechanisms in Li-ion batteries: A state-of-the-art review. Int. J. Energy Res. 2017, 41, 1963–1986. [Google Scholar]
- Li, P.; Kang, Z.; Zhang, Z.; Liao, Q.; Rao, F.; Lu, Y.; Zhang, Y. In situ microscopy techniques for characterizing the mechanical properties and deformation behavior of two-dimensional (2D) materials. Mater. Today 2021, 51, 247–272. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, L.; Huang, X.; Guo, X.; Liu, D.; Zheng, D.; Zhang, X.; Ren, R.; Qu, D.; Chen, J. A room-temperature liquid metal-based self-healing anode for lithium-ion batteries with an ultra-long cycle life. Energy Environ. Sci. 2017, 10, 1854–1861. [Google Scholar]
- Yang, M.; Cheng, Y.; Yue, Y.; Chen, Y.; Gao, H.; Li, L.; Cai, B.; Liu, W.; Wang, Z.; Guo, H. High-performance flexible pressure sensor with a self-healing function for tactile feedback. Adv. Sci. 2022, 9, 2200507. [Google Scholar]
- Zhang, D.; Wang, D.; Zong, X.; Dong, G.; Zhang, Y. High-performance QCM humidity sensor based on graphene oxide/tin oxide/polyaniline ternary nanocomposite prepared by in-situ oxidative polymerization method. Sens. Actuators B Chem. 2018, 262, 531–541. [Google Scholar]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. A review on flexible wearables-Recent developments in non-invasive continuous health monitoring. Sens. Actuators A Phys. 2024, 366, 114993. [Google Scholar]
- Zhang, S.; Tu, T.; Li, T.; Cai, Y.; Wang, Z.; Zhou, Y.; Wang, D.; Fang, L.; Ye, X.; Liang, B. 3D MXene/PEDOT: PSS composite aerogel with a controllable patterning property for highly sensitive wearable physical monitoring and robotic tactile sensing. ACS Appl. Mater. Interfaces 2022, 14, 23877–23887. [Google Scholar]
- Qiao, X.; Cai, Y.; Kong, Z.; Xu, Z.; Luo, X. A wearable electrochemical sensor based on anti-fouling and self-healing polypeptide complex hydrogels for sweat monitoring. ACS Sens. 2023, 8, 2834–2842. [Google Scholar]
- Abaje, I.; Bello, Y.; Ahmad, S. A review of air quality and concentrations of air pollutants in Nigeria. J. Appl. Sci. Environ. Manag. 2020, 24, 373–379. [Google Scholar]
- Liu, Y.; Wang, F.; Hu, Z.; Li, M.; Ouyang, S.; Wu, Y.; Wang, S.; Li, Z.; Qian, J.; Wang, L. Applications of cellulose-based flexible self-healing sensors for human health monitoring. Nano Energy 2024, 127, 109790. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Recent progress of nanostructured sensing materials from 0D to 3D: Overview of structure–property-application relationship for gas sensors. Small Methods 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Cao, M.; Su, J.; Fan, S.; Qiu, H.; Su, D.; Li, L. Wearable piezoresistive pressure sensors based on 3D graphene. Chem. Eng. J. 2021, 406, 126777. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, B.; Jiang, Q.; Li, Y.; Feng, Y.; Zhang, L.; Zhao, Y.; Xiong, X. Flexible and wearable sensor based on graphene nanocomposite hydrogels. Smart Mater. Struct. 2020, 29, 075027. [Google Scholar] [CrossRef]
- Nadgorny, M.; Ameli, A. Functional polymers and nanocomposites for 3D printing of smart structures and devices. ACS Appl. Mater. Interfaces 2018, 10, 17489–17507. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, X.; Yu, X.; Sun, X.; Yang, J.; Zhu, L.; Qin, G.; Dai, Y.; Chen, Q. Tough and conductive nanocomposite hydrogels for human motion monitoring. Polym. Test. 2019, 75, 38–47. [Google Scholar]
- Zheng, H.; Zhou, H.; Wang, Z.; Zhang, S.; Zhang, H. Ionically Conductive and Self-Healing Polyampholyte Hydrogels for Wearable Resistive Strain Sensors and Capacitive Pressure Sensors. ACS Appl. Polym. Mater. 2023, 5, 7581–7589. [Google Scholar]
- Xie, T.; Lv, X.; Tian, S.; Xie, Y.; Lv, A.; Lv, Z.; Jiang, L.A.; Zhao, Y.; Sun, S. Simple fabrication of silica amino sphere-reinforced ionic liquids/graphene conductive hydrogel sensors with super toughness, self-healing, and strain sensitivity properties. Macromolecules 2023, 56, 6256–6266. [Google Scholar]
- Yu, X.; Zheng, Y.; Wang, Y.; Zhang, H.; Song, H.; Li, Z.; Fan, X.; Liu, T. Facile fabrication of highly stretchable, stable, and self-healing ion-conductive sensors for monitoring human motions. Chem. Mater. 2022, 34, 1110–1120. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Chowdhury, S. Graphene-Based 3D Macrostructures for Clean Energy and Environmental Applications; Royal Society of Chemistry: London, UK, 2021; Volume 1. [Google Scholar]
- Karthik, V.; Selvakumar, P.; Kumar, P.S.; Satheeskumar, V.; Vijaysunder, M.G.; Hariharan, S.; Antony, K. Recent advances in electrochemical sensor developments for detecting emerging pollutant in water environment. Chemosphere 2022, 304, 135331. [Google Scholar] [PubMed]
- Zhang, G.-H.; Zhang, L.; Zhu, Q.-H.; Chen, H.; Yuan, W.-L.; Fu, J.; Wang, S.-L.; He, L.; Tao, G.-H. Self-healable, malleable, and flexible ionic polyimine as an environmental sensor for portable exogenous pollutant detection. ACS Mater. Lett. 2021, 4, 136–144. [Google Scholar] [CrossRef]
- Chen, D.; Wang, D.; Yang, Y.; Huang, Q.; Zhu, S.; Zheng, Z. Self-healing materials for next-generation energy harvesting and storage devices. Adv. Energy Mater. 2017, 7, 1700890. [Google Scholar] [CrossRef]
- Narayan, R.; Laberty-Robert, C.; Pelta, J.; Tarascon, J.M.; Dominko, R. Self-healing: An emerging technology for next-generation smart batteries. Adv. Energy Mater. 2022, 12, 2102652. [Google Scholar] [CrossRef]
- Chen, C.R.; Qin, H.; Cong, H.P.; Yu, S.H. A highly stretchable and real-time healable supercapacitor. Adv. Mater. 2019, 31, 1900573. [Google Scholar]
- Le, K.; Sun, X.; Chen, J.; John, J.V.; Servati, A.; Heidari, H.; Khademhosseini, A.; Ko, F.; Jiang, F.; Servati, P. Stretchable, self-healing, biocompatible, and durable ionogel for continuous wearable strain and physiological signal monitoring. Chem. Eng. J. 2023, 471, 144675. [Google Scholar]
- Choi, S.; Han, S.I.; Jung, D.; Hwang, H.J.; Lim, C.; Bae, S.; Park, O.K.; Tschabrunn, C.M.; Lee, M.; Bae, S.Y. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 2018, 13, 1048–1056. [Google Scholar]
- Qiao, H.; Qi, P.; Zhang, X.; Wang, L.; Tan, Y.; Luan, Z.; Xia, Y.; Li, Y.; Sui, K. Multiple weak H-bonds lead to highly sensitive, stretchable, self-adhesive, and self-healing ionic sensors. ACS Appl. Mater. Interfaces 2019, 11, 7755–7763. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, K.; Song, Y.; Han, J.; Yue, Y.; Biswas, S.K.; Wu, Q.; Xiao, H. A skin-inspired stretchable, self-healing and electro-conductive hydrogel with a synergistic triple network for wearable strain sensors applied in human-motion detection. Nanomaterials 2019, 9, 1737. [Google Scholar] [CrossRef]
- Lu, X.; Xie, D.; Zhu, K.; Wei, S.; Mo, Z.; Du, C.; Liang, L.; Chen, G.; Liu, Z. Swift assembly of adaptive thermocell arrays for device-level healable and energy-autonomous motion sensors. Nano-Micro Lett. 2023, 15, 196. [Google Scholar]
- Liu, C.; Li, F.; Li, G.; Li, P.; Hu, A.; Cui, Z.; Cong, Z.; Niu, J. Zwitterionic hydrogel electrolyte with tunable mechanical and electrochemical properties for a wearable motion and thermal sensor. ACS Appl. Mater. Interfaces 2022, 14, 9608–9617. [Google Scholar] [PubMed]
- Cao, J.; Zhang, X.; Lu, C.; Luo, Y.; Zhang, X. Self-healing sensors based on dual noncovalent network elastomer for human motion monitoring. Macromol. Rapid Commun. 2017, 38, 1700406. [Google Scholar]
- Wei, D.; Wang, H.; Zhu, J.; Luo, L.; Huang, H.; Li, L.; Yu, X. Highly stretchable, fast self-healing, responsive conductive hydrogels for supercapacitor electrode and motion sensor. Macromol. Mater. Eng. 2020, 305, 2000018. [Google Scholar]
- Hang, C.-Z.; Zhao, X.-F.; Xi, S.-Y.; Shang, Y.-H.; Yuan, K.-P.; Yang, F.; Wang, Q.-G.; Wang, J.-C.; Zhang, D.W.; Lu, H.-L. Highly stretchable and self-healing strain sensors for motion detection in wireless human-machine interface. Nano Energy 2020, 76, 105064. [Google Scholar]
- Huynh, T.P.; Sonar, P.; Haick, H. Advanced materials for use in soft self-healing devices. Adv. Mater. 2017, 29, 1604973. [Google Scholar]
- Mishra, R.K.; Verma, K. Defect engineering in nanomaterials: Impact, challenges, and applications. Smart Mater. Manuf. 2024, 2, 100052. [Google Scholar]
- Paras; Yadav, K.; Kumar, P.; Teja, D.R.; Chakraborty, S.; Chakraborty, M.; Mohapatra, S.S.; Sahoo, A.; Chou, M.M.C.; Liang, C.-T.; et al. A review on low-dimensional nanomaterials: Nanofabrication, characterization and applications. Nanomaterials 2022, 13, 160. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Y.; Wang, H.; Duo, Y.; Zhang, B.; Ge, Y.; Zhang, H.; Chen, W. Recent advances in 0D nanostructure-functionalized low-dimensional nanomaterials for chemiresistive gas sensors. J. Mater. Chem. C 2020, 8, 7272–7299. [Google Scholar]
- Ibrahim, O.O.; Oluwadunsin, J.D.; Antwi, M.; Mekunye, F.; Oluwatobi, M.A.; Olorunfemi, A.D.; Obanla, O.R.; Abdul-Rahman, M.I.; Babalola, K.O.; Babalola, O. The Evolution of Self-Healing Electrodes: A Critical Review of Nanomaterial Contributions. Am. J. Nanosci. 2025, 9, 8–31. [Google Scholar]
- Gong, S.; Lu, Y.; Yin, J.; Levin, A.; Cheng, W. Materials-driven soft wearable bioelectronics for connected healthcare. Chem. Rev. 2024, 124, 455–553. [Google Scholar] [CrossRef]
- Katiyar, A.K.; Hoang, A.T.; Xu, D.; Hong, J.; Kim, B.J.; Ji, S.; Ahn, J.-H. 2D materials in flexible electronics: Recent advances and future prospectives. Chem. Rev. 2023, 124, 318–419. [Google Scholar] [PubMed]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical vapor deposition growth and applications of two-dimensional materials and their heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [PubMed]
- Wang, M.; Duan, X.; Xu, Y.; Duan, X. Functional three-dimensional graphene/polymer composites. Acs Nano 2016, 10, 7231–7247. [Google Scholar] [CrossRef] [PubMed]
- Hia, I.L.; Vahedi, V.; Pasbakhsh, P. Self-healing polymer composites: Prospects, challenges, and applications. Polym. Rev. 2016, 56, 225–261. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Berdimurodov, E. Recent Trends in the Synthesis of Multifunctional Magnetic Nanoparticles. In Multifunctional Magnetic Nanoparticles in Therapy, Biology, and Pharmacy; CRC Press: Boca Raton, FL, USA, 2024; pp. 59–91. [Google Scholar]
- Han, F.; Li, M.; Ye, H.; Zhang, G. Materials, electrical performance, mechanisms, applications, and manufacturing approaches for flexible strain sensors. Nanomaterials 2021, 11, 1220. [Google Scholar] [CrossRef]
- Sun, X.; Qin, Z.; Ye, L.; Zhang, H.; Yu, Q.; Wu, X.; Li, J.; Yao, F. Carbon nanotubes reinforced hydrogel as flexible strain sensor with high stretchability and mechanically toughness. Chem. Eng. J. 2020, 382, 122832. [Google Scholar]
- Wang, H.; Lin, Y.; Yang, C.; Bai, C.; Hu, G.; Sun, Y.; Wang, M.; Lu, Y.-Q.; Kong, D. Mechanically Driven Self-Healing MXene Strain Gauges for Overstrain-Tolerant Operation. Nano Lett. 2024, 24, 13405–13413. [Google Scholar] [CrossRef]
- Pak, S.; Jang, S.; Kim, T.; Lim, J.; Hwang, J.S.; Cho, Y.; Chang, H.; Jang, A.R.; Park, K.H.; Hong, J. Electrode-Induced Self-Healed Monolayer MoS2 for High Performance Transistors and Phototransistors. Adv. Mater. 2021, 33, 2102091. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar]
- Li, X.; Wang, Y.; Zhang, X. Strong, healable materials with bio-like ordered architectures and versatile functionality. SusMat 2024, 4, e248. [Google Scholar] [CrossRef]


| Material | Fabrication | Conductivity | Healing Efficiency (%) | Response Time | Tensile Strength | Gauge Factor | Strain (%) | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PD embedded hydrogel | Copolymerizat-ion | 6.8 mS cm−1 | 2.32 | - | - | 1.09 | 585 | Health | [25] |
| AuNPs/MoS2/Pep hydrogel | - | - | - | 100 s | - | - | 55 | Health | [122] |
| Ionogel | polymerization | 0.21 mS/m | 98 | 200 ms | - | 1.05 | 0–400 | Health | [139] |
| Nanocomposite of Ag–Au nanowires and SBS elastomer | ligand exchange | 41,850 S cm−1 | - | - | - | - | 266 | Health | [140] |
| graphene nanocomposite hydrogels | facile two-step | 5 mS cm−1 | 98 | 10 s | 146.5 KPa | - | 2580 | Motion & Health | [127] |
| nanocomposite hydrogels | in situ doping | 0.04–0.09 S m−1 | 81 | - | 34 | - | 54 | Motion | [129] |
| Polyampholyte Hydrogels | free-radical polymerization | 0.015 S cm−1 | - | 250 ms | 0–7.35 kPa | 2.9 | 0–350 | Motion | [130] |
| IL-based conductive elastomers | one-pot Pickering emulsion polymerization. | - | 98 | 200 ms | - | 1.05 | 0–400 | Motion | [132] |
| Ionic hydrogel | One-pot | - | 96 | 2 h | - | 9.0 | 2100 | Motion | [141] |
| TOCNF/PAA-PPy composite hydrogel | facile combined two-step preparation | 3.9 S m−1 | 99.4 | 6 h | 0.55 MPa | 7.3 | 889 | Motion | [142] |
| MXene-boosted PAA hydrogel | Swift assembly | - | 80 | 1.5 s | - | 1.08 | 500 | Motion | [143] |
| zwitterionic hydrogel | one-step immersion | 0.16–1.65 S m−1 | 30–70 | - | 3.2–202 kPa | 9.1 | 1000–2880 | Motion | [144] |
| Dual Noncovalent Network Elastomer | - | - | 80 | 30 s | - | - | 0.2 | Motion | [145] |
| multifunctional conductive hydrogels | Uniform dispersion | - | 94 | 15 s | - | - | >1000 | Motion | [146] |
| PAAm hydrogel | - | 59.55 mS·cm−1 | 99 | 150 ms | - | 6.44 | >900 | Motion | [147] |
| PAM@SiO2-NH2/(ILs-GN) hydrogel | One-pot | 12 mS/cm | 75.01 | - | 1057 KPa | 18.94 | 1200 | Motion | [131] |
| IPIN | three-electrode system | 1.98 × 10−2 mS cm−1–4.91 × 10−3 mS cm−1 | - | 8s | 4.28 mPA | - | 0–250 | Environmental | [135] |
| Electrode | Healing Mechanism | Fabrication Methods | Damage/Healing Method | Healing Efficiency | Healing Time | Stability | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| OD &1D Nanomaterials | ||||||||
| cPDA@ZPD NPs | dynamic hydrogen bonding interactions | copolymerization | Cut/healing | 2.32% | - | 12,000 | Health monitoring | [25] |
| MWCNT | π-π stacking interactions | Laser Ablation | Crack Healing | 90% Conductivity Recovery | <5 min | 5000 cycles | Flexible Electronics | [159] |
| FHE nanofiber | hydrogen bonding and electrostatic interactions | Electrospinning | Cut/Scratch/healing | 98.3% | - | 3000 | Health Monitoring | [60] |
| AgNPs | Metallic bonding interactions | Screen Printing | Cut/Healing | 85% Conductivity Recovery | 10 min | 8000 cycles | Motion Monitoring | [36] |
| AuNWs | Surface diffusion healing | Deposition on Latex Rubber | Crack Healing | 95% Resistance Recovery | 2 min | 10,000 cycles | Wearable Biomedical Sensors | [58] |
| IrNPs | Metallic bonding interactions | Atomic layer deposition | - | - | 150 ms | >10,000 | Motion Monitoring | [66] |
| CNT/Polymer Composite | Dynamic covalent bonding | Solution Processing | Cut/Healing | 92% Conductivity Recovery | 30 min | 15,000 cycles | Wearable sensors | [160] |
| 2D Nanomaterials | ||||||||
| RGO | π-π stacking and hydrogen bonding | Electrospinning | Crack Healing | 87% Conductivity Recovery | 5 min | 10,000 cycles | Health Monitoring | [60] |
| GO | Hydrogen bonding interactions | Solution Processing | Crack Healing | 80% Conductivity Recovery | 15 min | 8000 cycles | Wearable Electronics | [78] |
| Mxene | Surface functionalization repair | Vacuum-Assisted Filtration | Cut/Healing | 94% Conductivity Recovery | 8 min | 12,000 cycles | Flexible Sensors | [161] |
| GNs | π-π interaction | Chemical Vapor Deposition | Crack Healing | 96% Conductivity Recovery | 2 min | 15,000 cycles | Wearable Electronics | [68] |
| MoS2 | Van der Waals interactions | Solution Processing | Cut/Healing | 89% Conductivity Recovery | 6 min | 10 cycles | Flexible Sensors | [162] |
| 3D Nanomaterials | ||||||||
| Hydrogel | Hydrogen bonding interactions | Copolymerization | Cut/Healing | 4.9× Conductivity Increase | - | 12,000 cycles | Health Monitoring | [25] |
| Ionogel | Ionic crosslinking | Polymerization | Crack Healing | 92% Conductivity Recovery | 5 min | 10,000 cycles | Wearable Strain Sensors | [139] |
| IL-based conductive elastomers | Reversible ion interactions | Solution Processing | Crack healing | 88% Conductivity Recovery | 7 min | 8000 cycles | Stretchable Electronics | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, O.O.; Liu, C.; Zhou, S.; Jin, B.; He, Z.; Zhao, W.; Wang, Q.; Zhang, S. Recent Advances in Nanomaterial-Based Self-Healing Electrodes Towards Sensing and Energy Storage Applications. Sensors 2025, 25, 2248. https://doi.org/10.3390/s25072248
Ibrahim OO, Liu C, Zhou S, Jin B, He Z, Zhao W, Wang Q, Zhang S. Recent Advances in Nanomaterial-Based Self-Healing Electrodes Towards Sensing and Energy Storage Applications. Sensors. 2025; 25(7):2248. https://doi.org/10.3390/s25072248
Chicago/Turabian StyleIbrahim, Oresegun Olakunle, Chen Liu, Shulan Zhou, Bo Jin, Zhaotao He, Wenjie Zhao, Qianqian Wang, and Sheng Zhang. 2025. "Recent Advances in Nanomaterial-Based Self-Healing Electrodes Towards Sensing and Energy Storage Applications" Sensors 25, no. 7: 2248. https://doi.org/10.3390/s25072248
APA StyleIbrahim, O. O., Liu, C., Zhou, S., Jin, B., He, Z., Zhao, W., Wang, Q., & Zhang, S. (2025). Recent Advances in Nanomaterial-Based Self-Healing Electrodes Towards Sensing and Energy Storage Applications. Sensors, 25(7), 2248. https://doi.org/10.3390/s25072248







