Recoverable Detection of Dichloromethane by MEMS Gas Sensor Based on Mo and Ni Co-Doped SnO2 Nanostructure
Abstract
:Highlights
- The problem of dichloromethane “poisoned” MOS materials was well solved.
- Gas sensing mechanism of dichloromethane on the MOS material was elucidate.
- The best performance of significant response and excellent recoverability by the MEMS sensor to dichloromethane is attributed to a synergetic effect from Mo and Ni co-doping into SnO2.
- Kinetically, the transportation of dichloromethane molecules onto the surface determines the response process. Thermodynamically, the output steady electronic signal of the MEMS sensor follows the equations newly derived by statistical mechanics.
Abstract
1. Introduction
2. Materials and Methods
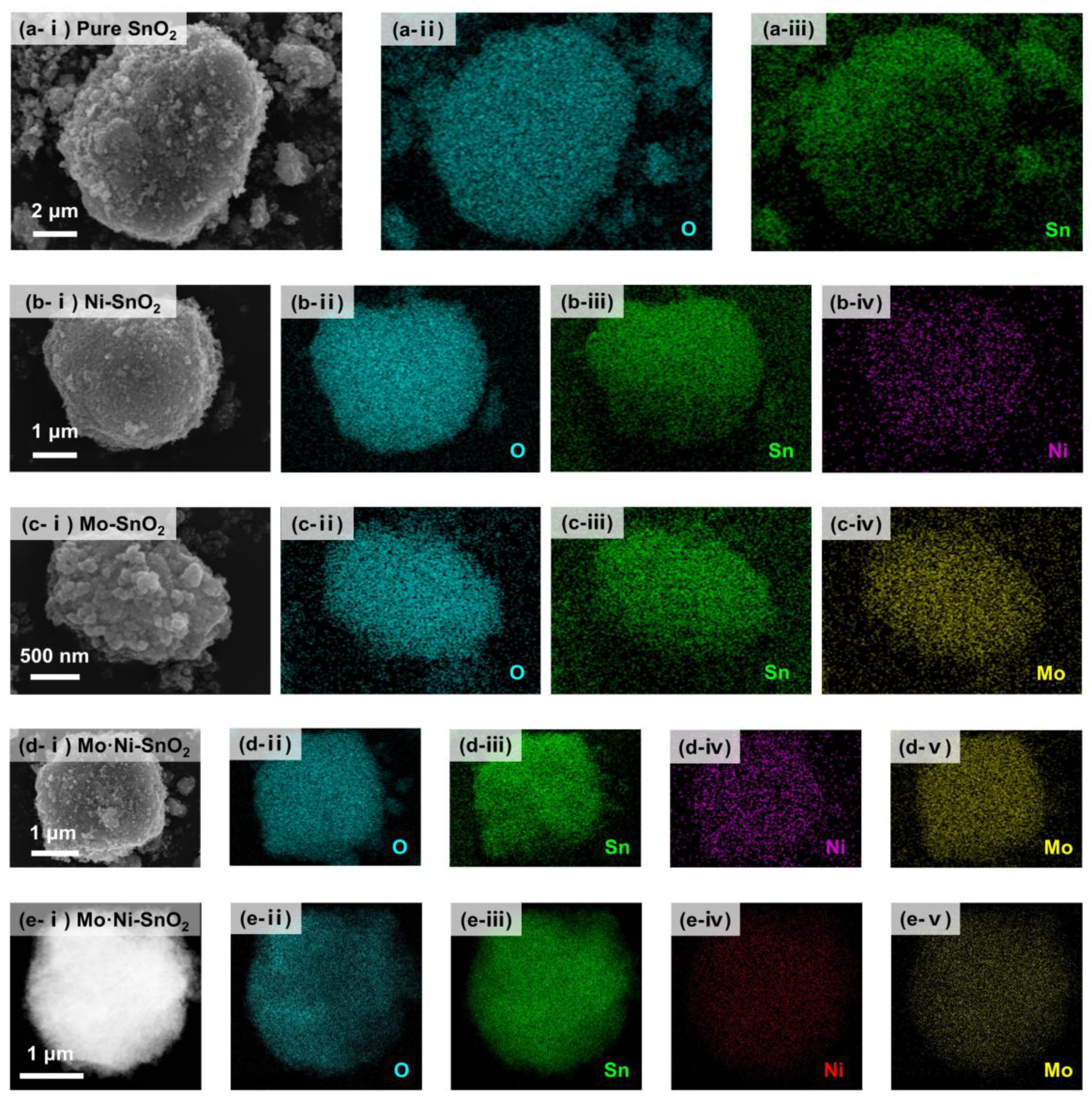
2.1. Synthesis and Characterization of SnO2-Based Materials
2.2. Fabrication and Sensing Measurement of MEMS Sensors to DCM Gas
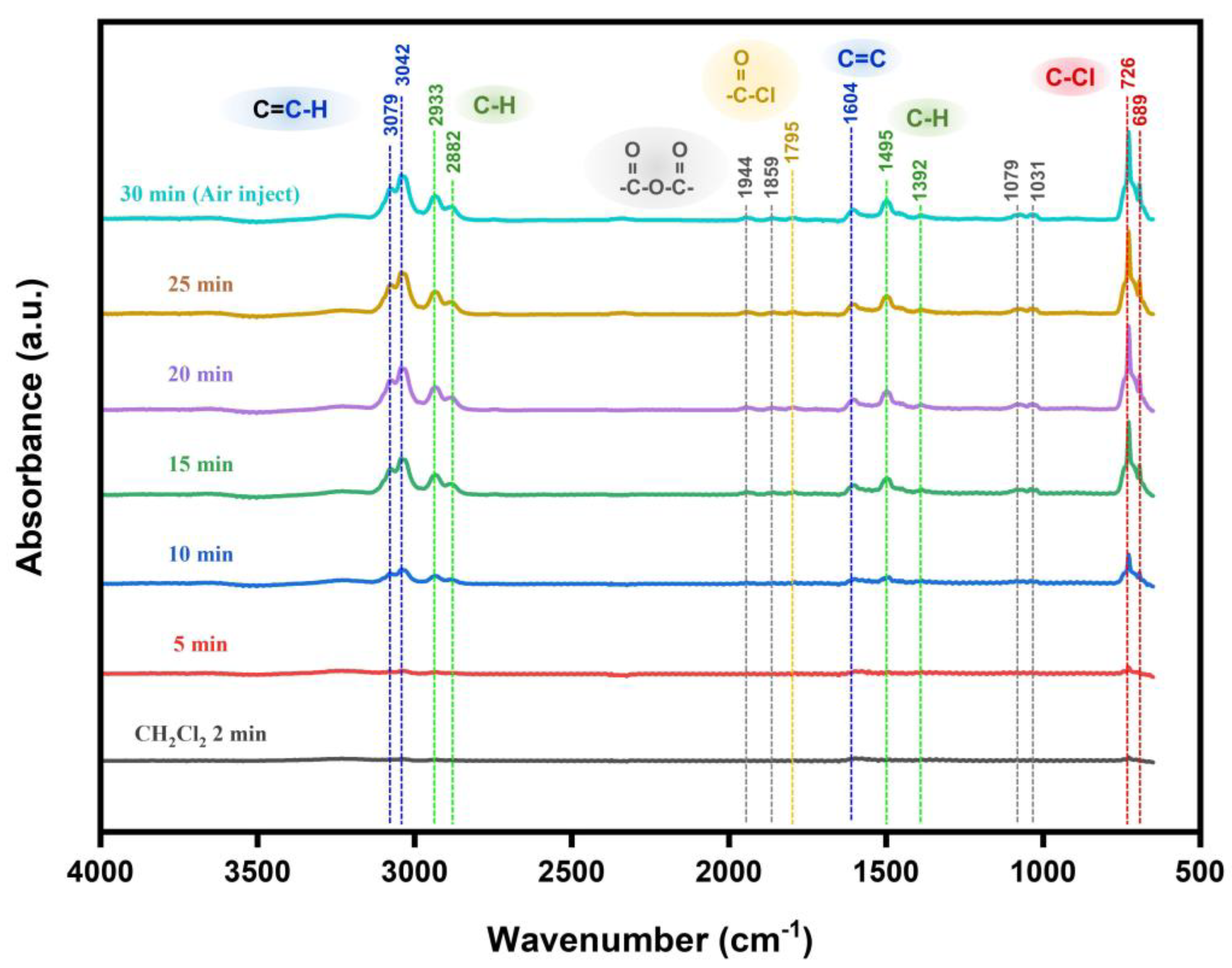
2.3. Identification of Adsorbed Species from DCM onto Sensing Materials
3. Results and Discussion
3.1. Geometric and Electronic Structures of SnO2-Based Sensing Materials
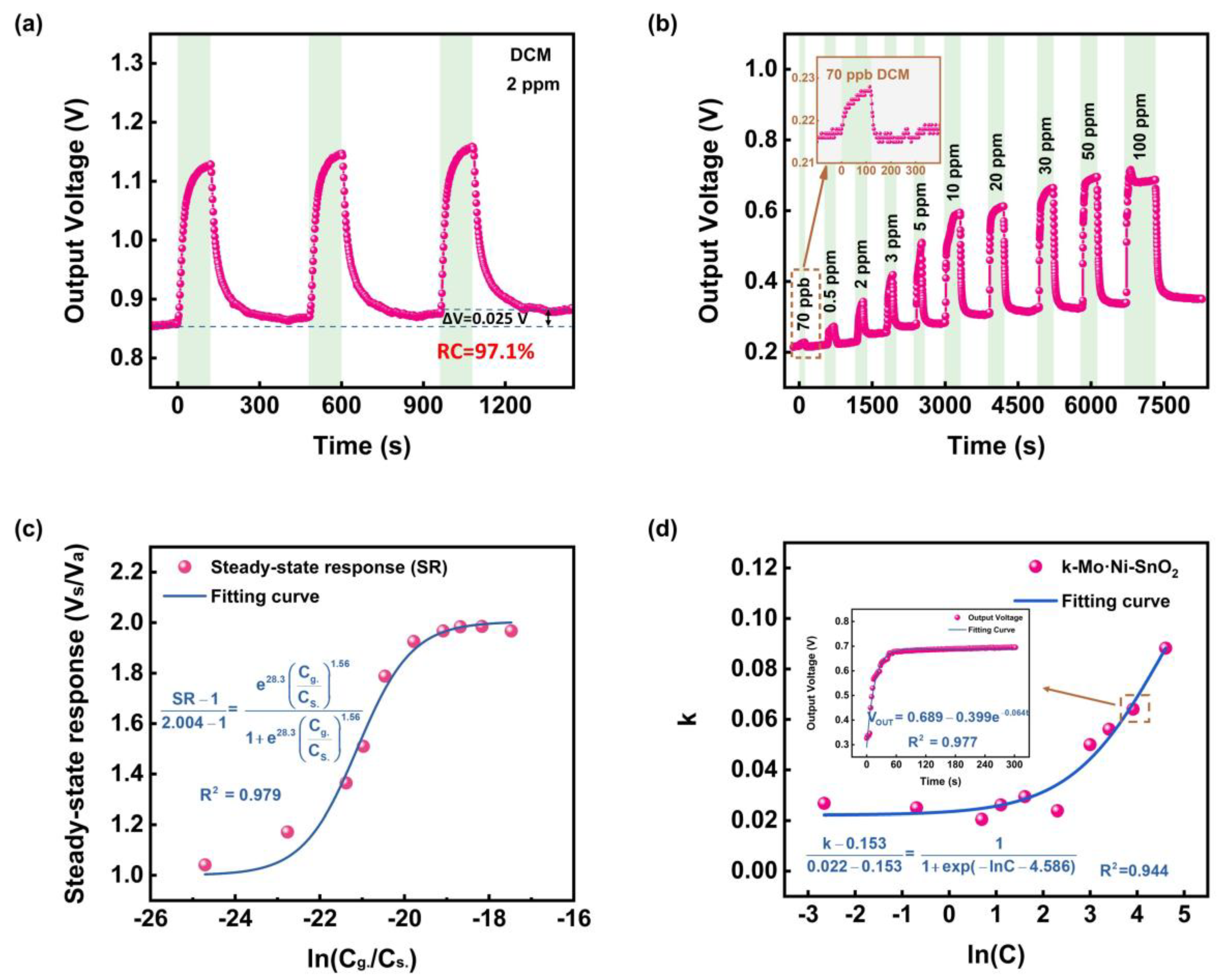
3.2. Performances of MEMS Sensors to DCM Gas
| Material | Device | T a (°C) | P b (mW) | Detection Range (ppm) | Sensing Performances | ||||
|---|---|---|---|---|---|---|---|---|---|
| CDCM c (ppm) | S (%) | tres d (s) | trec e (s) | RC f (%) | |||||
| SnO2 [24] | Alumina flat substrate | 350 | NA g | 0.02~0.8 | 0.5 | 29 | ~600 * | ~1700 * | <20 * |
| SnO2-ZnO [77] | 350 | NA | 0.5~1.5 | 1 | 60 | NA ** | NA ** | NA ** | |
| SnO2 [78] | 300 | NA | 0.5~3 | 0.5 | 20 | NA ** | NA ** | NA ** | |
| SnO2-Al2O3 [78] | 300 | NA | 0.5~3 | 0.5 | 8 | NA ** | NA ** | NA ** | |
| SnO2-In2O3 [78] | 300 | NA | 0.5~3 | 0.5 | 12 | NA ** | NA ** | NA ** | |
| SnO2-ZrO2 [78] | 300 | NA | 0.5~3 | 0.5 | 60 | NA ** | NA ** | NA ** | |
| SnO2-ZnO [78] | 300 | NA | 0.5~3 | 0.5 | 66 | NA ** | NA ** | NA ** | |
| SnO2-Pt [78] | 300 | NA | 0.5~3 | 0.5 | 25 | NA ** | NA ** | NA ** | |
| SnO2 [26] | 350 | NA | 0.1~0.8 | 0.5 | 30 | ~400 * | >2500 * | <5 * | |
| SnO2-MoO3 [26] | 350 | NA | 0.1~0.8 | 0.5 | 22 | ~150 * | ~1000 * | ~90 * | |
| SnO2-NiO-MoO3 [26] | 350 | NA | 0.1~0.8 | 0.5 | 50 | ~100 * | ~400 * | >95 * | |
| SnO2 [27] | 350 | NA | 0.1~0.8 | 0.5 | 17 | ~500 * | ~1000 * | ~50 * | |
| SnO2-Sm2O3 [25] | 250 | NA | 0.1~10 | 10 | 3470 | ~600 * | ~1200 * | NA ** | |
| Pure SnO2 (This work) | Silicon-based MEMS | ~275 | ~25 | 2 | 2 | 5 | 28 | 16 | ~100 |
| Ni-SnO2 (This work) | 210 | 16.8 | 2 | 2 | 27 | 60 | 130 | ~96 | |
| Mo-SnO2 (This work) | 330 | 38.0 | 2 | 2 | 13 | 62 | 54 | ~98 | |
| Mo·Ni-SnO2 (This work) | 310 | 34.2 | 0.07~100 | 2 | 38 | 78 | 42 | ~97 | |
| 100 | 100 | 32 | 102 | ~97 | |||||
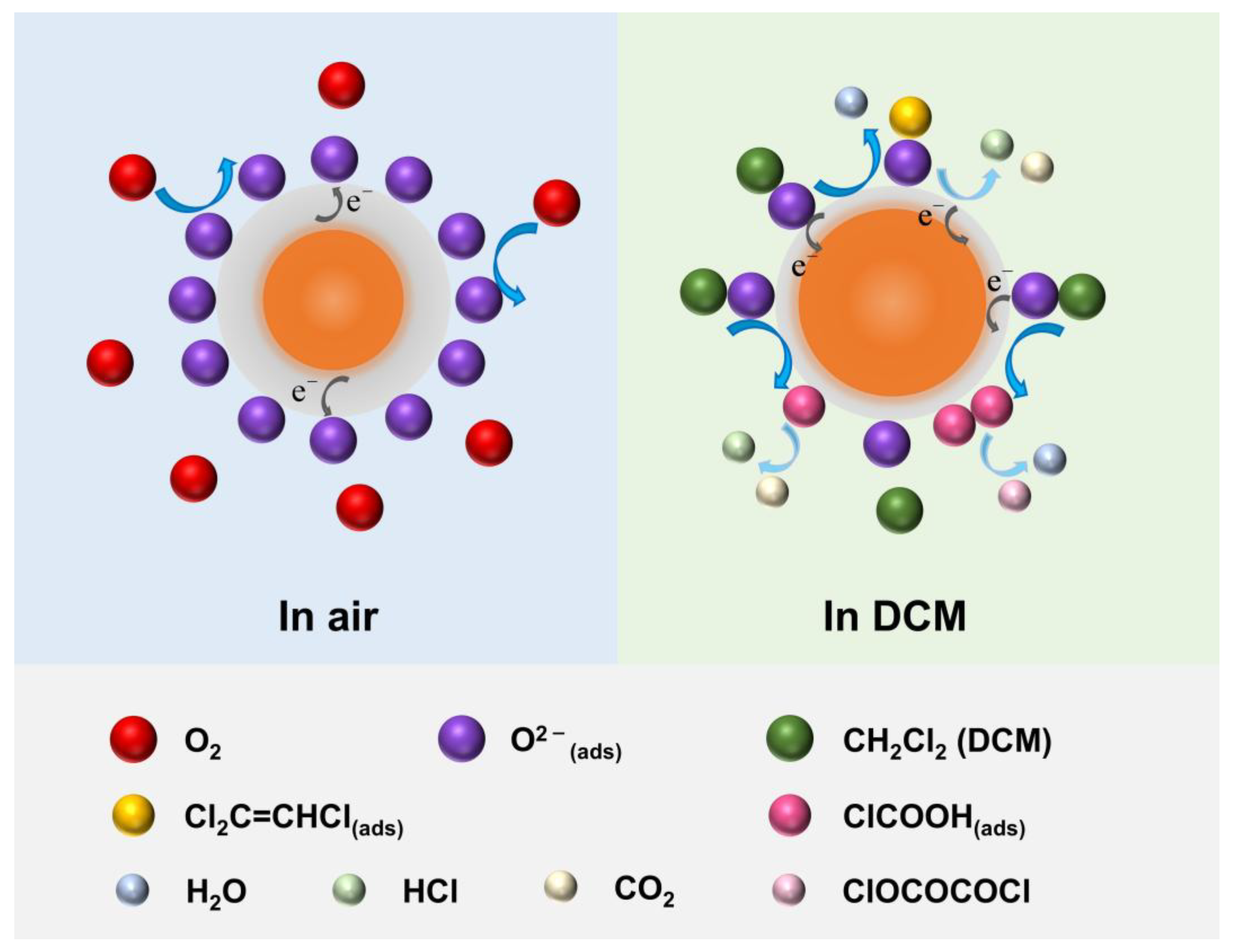
3.3. Recoverable Mechanism of MEMS Sensor to DCM Gas
3.4. Kinetics and Thermodynamics of MEMS Sensors to DCM Gas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MOS | Metal oxide semiconductor |
| CWAs | Chemical warfare agents |
| DCM | Dichloromethane |
| MHPs | Micro-hotplates |
| MEMS | Micro-Electro-Mechanical System |
| XRD | X-ray diffraction spectroscopy |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| IR | Infrared spectroscopy |
| FWHM | Full widths at half maximum |
| R | Response |
| S | Sensitivity |
| SR | Steady-state response |
| RC | Recovery capability |
References
- Kumar, S.; Pavelyev, V.; Mishra, P.; Tripathi, N.; Sharma, P.; Calle, F. A review on 2D transition metal di-chalcogenides and metal oxide nanostructures based NO2 gas sensors. Mater. Sci. Semicond. Process. 2020, 107, 104865. [Google Scholar]
- Kong, Y.; Li, Y.; Cui, X.; Su, L.; Ma, D.; Lai, T.; Yao, L.; Xiao, X.; Wang, Y. SnO2 nanostructured materials used as gas sensors for the detection of hazardous and flammable gases: A review. Nano Mater. Sci. 2022, 4, 339–350. [Google Scholar]
- Modak, M.; Rane, S.; Jagtap, S. WO3: A review of synthesis techniques, nanocomposite materials and their morphological effects for gas sensing application. Bull. Mater. Sci. 2023, 46, 28. [Google Scholar]
- Bigiani, L.; Zappa, D.; Maccato, C.; Gasparotto, A.; Sada, C.; Comini, E.; Barreca, D. Hydrogen gas sensing performances of p-type Mn3O4 nanosystems: The role of built-in Mn3O4/Ag and Mn3O4/SnO2 junctions. Nanomaterials 2020, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ionics 2021, 360, 115544. [Google Scholar]
- Masuda, Y. Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuators B Chem. 2022, 364, 131876. [Google Scholar]
- Neri, G.; Donato, N. Resistive gas sensors. In Wiley Encyclopedia of Electrical and Electronics Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–12. ISBN 978-0-471-34608-1. [Google Scholar]
- Wicker, S.; Guiltat, M.; Weimar, U.; Hémeryck, A.; Barsan, N. Ambient humidity influence on CO detection with SnO2 gas sensing materials. a combined drifts/DFT investigation. J. Phys. Chem. C 2017, 121, 25064–25073. [Google Scholar]
- Li, Q.; Zeng, W.; Li, Y. Metal oxide gas sensors for detecting NO2 in industrial exhaust gas: Recent developments. Sens. Actuators B Chem. 2022, 359, 131579. [Google Scholar]
- Zhou, S.; Wang, H.; Hu, J.; Lv, T.; Rong, Q.; Zhang, Y.; Zi, B.; Chen, M.; Zhang, D.; Wei, J.; et al. Formaldehyde gas sensor with extremely high response employing cobalt-doped SnO2 ultrafine nanoparticles. Nanoscale Adv. 2022, 4, 824–836. [Google Scholar]
- Sezigen, S.; Kenar, L. Recent sulfur mustard attacks in Middle East and experience of health professionals. Toxicol. Lett. 2020, 320, 52–57. [Google Scholar]
- Vucinic, S.; Antonijevic, B.; Tsatsakis, A.M.; Vassilopoulou, L.; Docea, A.O.; Nosyrev, A.E.; Izotov, B.N.; Thiermann, H.; Drakoulis, N.; Brkic, D. Environmental exposure to organophosphorus nerve agents. Environ. Toxicol. Pharmacol. 2017, 56, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Cheong, H.W.; Son, L.D.N.; Yoon, Y.S. Surface reaction mechanism of acetonitrile on doped SnO2 sensor element and its response behavior. Jpn. J. Appl. Phys. 2008, 47, 2119–2121. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Zhao, L.; Fei, T.; Liu, S.; Zhang, T. The synergistic effects of oxygen vacancy engineering and surface gold decoration on commercial SnO2 for ppb-level DMMP sensing. J. Colloid Interface Sci. 2022, 608, 2703–2717. [Google Scholar] [CrossRef] [PubMed]
- Patil, L.A.; Bari, A.R.; Shinde, M.D.; Deo, V.; Kaushik, M.P. Detection of dimethyl methyl phosphonate—A simulant of sarin: The highly toxic chemical warfare—Using platinum activated nanocrystalline ZnO thick films. Sens. Actuators B Chem. 2012, 161, 372–380. [Google Scholar] [CrossRef]
- Yoo, R.; Cho, S.; Song, M.J.; Lee, W. Highly sensitive gas sensor based on Al-doped ZnO nanoparticles for detection of dimethyl methylphosphonate as a chemical warfare agent simulant. Sens. Actuators B Chem. 2015, 221, 217–223. [Google Scholar] [CrossRef]
- Yoo, R.; Yoo, S.; Lee, D.; Kim, J.; Cho, S.; Lee, W. Highly selective detection of dimethyl methylphosphonate (DMMP) using CuO nanoparticles /ZnO flowers heterojunction. Sens. Actuators B Chem. 2017, 240, 1099–1105. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Moharram, D.; Liu, Q.; Yu, J.; Chen, R.; Li, R.; Wang, J. Fabrication of electrospun Co3O4/CuO p-p heterojunctions nanotubes functionalized with HFIP for detecting chemical nerve agent under visible light irradiation. Sens. Actuators B Chem. 2020, 314, 128076. [Google Scholar] [CrossRef]
- Bigiani, L.; Zappa, D.; Barreca, D.; Gasparotto, A.; Sada, C.; Tabacchi, G.; Fois, E.; Comini, E.; Maccato, C. Sensing nitrogen mustard gas simulant at the ppb scale via selective dual-site activation at Au/Mn3O4 interfaces. ACS Appl. Mater. Interfaces 2019, 11, 23692–23700. [Google Scholar] [CrossRef]
- Schwenk, M. Chemical warfare agents. Classes and targets. Toxicol. Lett. 2018, 293, 253–263. [Google Scholar] [CrossRef]
- Barreca, D.; Maccato, C.; Gasparotto, A. Metal oxide nanosystems as chemoresistive gas sensors for chemical warfare agents: A focused review. Adv. Mater. Interfaces 2022, 9, 2102525. [Google Scholar] [CrossRef]
- Sohn, J.R.; Park, H.D.; Lee, D.D. Acetonitrile sensing characteristics and infrared study of SnO2-based gas sensors. Appl. Surf. Sci. 2000, 161, 78–85. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Baratto, C.; Comini, E.; Faglia, G.; Ferroni, M.; Pardo, M.; Ponzoni, A.; Vomiero, A. Semiconducting tin oxide nanowires and thin films for chemical warfare agents detection. Thin Solid Films 2009, 517, 6156–6160. [Google Scholar] [CrossRef]
- Lee, W.S.; Lee, S.C.; Lee, S.J.; Lee, D.D.; Huh, J.S.; Jun, H.K.; Kim, J.C. The sensing behavior of SnO2 -based thick-film gas sensors at a low concentration of chemical agent simulants. Sens. Actuators B Chem. 2005, 108, 148–153. [Google Scholar] [CrossRef]
- Aliha, H.M.; Khodadadi, A.A.; Mortazavi, Y. The sensing behaviour of metal oxides (ZnO, CuO and Sm2O3) doped-SnO2 for detection of low concentrations of chlorinated volatile organic compounds. Sens. Actuators B Chem. 2013, 181, 637–643. [Google Scholar] [CrossRef]
- Lee, S.C.; Choi, H.Y.; Lee, S.J.; Lee, W.S.; Huh, J.S.; Lee, D.D.; Kim, J.C. Novel SnO2-based gas sensors promoted with metal oxides for the detection of dichloromethane. Sens. Actuators B Chem. 2009, 138, 446–452. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, S.Y.; Lee, W.S.; Jung, S.Y.; Hwang, B.W.; Ragupathy, D.; Lee, D.D.; Lee, S.Y.; Kim, J.C. Effects of textural properties on the response of a SnO2-based gas sensor for the detection of chemical warfare agents. Sensors 2011, 11, 6893–6904. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, N.; Chen, Z.; Fu, P. The effect of Ni doping concentration on the gas sensing properties of Ni doped SnO2. Sens. Actuators B Chem. 2017, 239, 501–510. [Google Scholar] [CrossRef]
- Wang, L.; Ma, S.; Li, J.; Wu, A.; Luo, D.; Yang, T.; Cao, P.; Ma, N.; Cai, Y. Mo-doped SnO2 nanotubes sensor with abundant oxygen vacancies for ethanol detection. Sens. Actuators B Chem. 2021, 347, 130642. [Google Scholar] [CrossRef]
- Guo, M.; Yang, H.; Du, Y.; Fan, J. Influence of Ni and S co-doping of SnO2 on its electrochemical performance. Int. J. Electrochem. Sci. 2021, 16, 210337. [Google Scholar] [CrossRef]
- Ji, X.; Chang, J.; Deng, Z.; Shen, C.; Li, M.; Wang, S.; You, L.; Kumar, M.; Fang, X.; Meng, G. Boosting acetone response of p-type Co3O4 sensor via Sn and Ni co-doping for diabetes diagnosis. Sens. Actuators B Chem. 2024, 410, 135705. [Google Scholar] [CrossRef]
- Guo, M.; Brewster Ii, J.T.; Zhang, H.; Zhao, Y.; Zhao, Y. Challenges and opportunities of chemiresistors based on microelectromechanical systems for chemical olfaction. ACS Nano 2022, 16, 17778–17801. [Google Scholar] [PubMed]
- Meng, L.; Bu, W.; Li, Y.; Qin, Q.; Zhou, Z.; Hu, C.; Chuai, X.; Wang, C.; Sun, P.; Lu, G. Highly selective triethylamine sensing based on SnO/SnO2 nanocomposite synthesized by one-step solvothermal process and sintering. Sens. Actuators B Chem. 2021, 342, 130018. [Google Scholar]
- Akande, A.A.; Machatine, A.G.J.; Masina, B.; Chimowa, G.; Matsoso, B.; Roro, K.; Duvenhage, M.-M.; Swart, H.; Bandyopadhyay, J.; Ray, S.S.; et al. Blue- and red-shifts of V2O5 phonons in NH3 environment by in situ Raman spectroscopy. J. Phys. D Appl. Phys. 2018, 51, 015106. [Google Scholar]
- Chu, J.; Wang, Q.; Yang, A.; Pan, J.; Yuan, H.; Wang, X.; Rong, M. MOF-doped WO3 nanofibers for the SF6 decomposition products: The effects of MOF-modification on the sensitive performance and mechanism. Appl. Surf. Sci. 2022, 606, 154889. [Google Scholar]
- Wang, X.; Yao, F.; Xu, P.; Li, M.; Yu, H.; Li, X. Quantitative structure–activity relationship of nanowire adsorption to SO2 revealed by in situ TEM technique. Nano Lett. 2021, 21, 1679–1687. [Google Scholar]
- Chu, B.; Liu, S.; Qin, Q.; Zhao, R.; Chen, K.; Hou, X.; Li, R.; Li, C.; Chen, J.; Dong, L.; et al. Surface cleaning and protection engineering via functionalization to awaken the intrinsic catalytic activity of Co3O4. Adv. Funct. Mater. 2023, 33, 2212448. [Google Scholar]
- Vuong, N.M.; Kim, D.; Kim, H. Surface gas sensing kinetics of a WO3 nanowire sensor: Part 1—Oxidizing gases. Sens. Actuators B Chem. 2015, 220, 932–941. [Google Scholar]
- Weng, X.; Yang, S.; Ding, D.; Chen, M.; Wan, H. Applications of in-situ wide spectral range infrared absorption spectroscopy for CO oxidation over Pd/SiO2 and Cu/SiO2 catalysts. Chin. J. Catal. 2022, 43, 2001–2009. [Google Scholar]
- Yang, S.; Zhang, Q.; Yang, H.; Shi, H.; Dong, A.; Wang, L.; Yu, S. Progress in infrared spectroscopy as an efficient tool for predicting protein secondary structure. Int. J. Biol. Macromol. 2022, 206, 175–187. [Google Scholar]
- Drzymała, E.; Gruzeł, G.; Depciuch, J.; Budziak, A.; Kowal, A.; Parlinska-Wojtan, M. Structural, chemical and optical properties of SnO2 NPs obtained by three different synthesis routes. J. Phys. Chem. Solids 2017, 107, 100–107. [Google Scholar] [CrossRef]
- Hu, J.; Wang, T.; Wang, Y.; Huang, D.; He, G.; Han, Y.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; et al. Enhanced formaldehyde detection based on Ni doping of SnO2 nanoparticles by one-step synthesis. Sens. Actuators B Chem. 2018, 263, 120–128. [Google Scholar]
- Azam, A.; Ahmed, A.S.; Ansari, M.S.; Shafeeq M, M.; Naqvi, A.H. Study of electrical properties of nickel doped SnO2 ceramic nanoparticles. J. Alloys Compd. 2010, 506, 237–242. [Google Scholar]
- Li, W.-T.; Zhang, X.-D.; Guo, X. Electrospun Ni-doped SnO2 nanofiber array for selective sensing of NO2. Sens. Actuators B Chem. 2017, 244, 509–521. [Google Scholar]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. ISBN 978-3-662-33517-8. [Google Scholar]
- Patterson, A.L. The scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar]
- Tirumalareddygari, S.R.; Guddeti, P.R.; Ramakrishna Reddy, K.T. A critical study of the optical and electrical properties of transparent and conductive Mo-doped ZnO films by adjustment of Mo concentration. Appl. Surf. Sci. 2018, 458, 333–343. [Google Scholar]
- Huang, X.; Chen, K.; Xie, W.; Li, Y.; Yang, F.; Deng, Y.; Li, J.; Jiang, F.; Shu, Y.; Wu, L.; et al. Chemiresistive gas sensors based on highly permeable Sn-doped bismuth subcarbonate microspheres: Facile synthesis, sensing performance, and mechanism study. Adv. Funct. Mater. 2023, 33, 2304718. [Google Scholar]
- Cheng, J.P.; Wang, B.B.; Zhao, M.G.; Liu, F.; Zhang, X.B. Nickel-doped tin oxide hollow nanofibers prepared by electrospinning for acetone sensing. Sens. Actuators B Chem. 2014, 190, 78–85. [Google Scholar]
- Wang, Q.; Zhao, H.; Li, F.; She, W.; Wang, X.; Xu, L.; Jiao, H. Mo-doped Ni2P hollow nanostructures: Highly efficient and durable bifunctional electrocatalysts for alkaline water splitting. J. Mater. Chem. A 2019, 7, 7636–7643. [Google Scholar]
- Bica De Moraes, M.A.; Trasferetti, B.C.; Rouxinol, F.P.; Landers, R.; Durrant, S.F.; Scarmínio, J.; Urbano, A. Molybdenum oxide thin films obtained by the hot-filament metal oxide deposition technique. Chem. Mater. 2004, 16, 513–520. [Google Scholar]
- Xie, F.; Choy, W.C.H.; Wang, C.; Li, X.; Zhang, S.; Hou, J. Low-temperature solution-processed hydrogen molybdenum and vanadium bronzes for an efficient hole-transport layer in organic electronics. Adv. Mater. 2013, 25, 2051–2055. [Google Scholar]
- Li, Z.; Yi, J. Enhanced ethanol sensing of Ni-doped SnO2 hollow spheres synthesized by a one-pot hydrothermal method. Sens. Actuators B Chem. 2017, 243, 96–103. [Google Scholar]
- Jian, J.; Shi, Y.; Ekeroth, S.; Keraudy, J.; Syväjärvi, M.; Yakimova, R.; Helmersson, U.; Sun, J. A nanostructured NiO/cubic SiC p–n heterojunction photoanode for enhanced solar water splitting. J. Mater. Chem. A 2019, 7, 4721–4728. [Google Scholar]
- Jin, B.; Cheng, S.; Sun, Y.; Xie, P.; Li, L. High selectivity toward electrochemical ozone production of Sb-SnO2 with Cu and Ni co-doped. Adv. Funct. Mater. 2024, 34, 2314144. [Google Scholar]
- Bozheyev, F.; Akinoglu, E.M.; Wu, L.; Lou, S.; Giersig, M. Effect of Mo-doping in SnO2 thin film photoanodes for water oxidation. Int. J. Hydrogen Energy 2020, 45, 33448–33456. [Google Scholar]
- Wang, J.; Mueller, D.N.; Crumlin, E.J. Recommended strategies for quantifying oxygen vacancies with X-ray photoelectron spectroscopy. J. Eur. Ceram. Soc. 2024, 44, 116709. [Google Scholar]
- Xiao, W.; Jin, Z.; Yang, W.; Liu, S. Construction of flower-like hierarchical Ni-doped SnO2 nanosheets and their gas sensing properties for ethanol. New J. Chem. 2023, 47, 15283–15290. [Google Scholar]
- Yu, K.; Lou, L.; Liu, S.; Zhou, W. Asymmetric oxygen vacancies: The intrinsic redox active sites in metal oxide catalysts. Adv. Sci. 2020, 7, 1901970. [Google Scholar]
- Krishna, K.G.; Umadevi, G.; Parne, S.; Pothukanuri, N. Zinc oxide based gas sensors and their derivatives: A critical review. J. Mater. Chem. C 2023, 11, 3906–3925. [Google Scholar]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar]
- Zhu, L.-Y.; Yuan, K.; Yang, J.-G.; Ma, H.-P.; Wang, T.; Ji, X.-M.; Feng, J.-J.; Devi, A.; Lu, H.-L. Fabrication of heterostructured p-CuO/n-SnO2 core-shell nanowires for enhanced sensitive and selective formaldehyde detection. Sens. Actuators B Chem. 2019, 290, 233–241. [Google Scholar]
- Zhu, L.; Miao, X.; Ou, L.; Mao, L.; Yuan, K.; Sun, S.; Devi, A.; Lu, H. Heterostructured α-Fe2O3 @ZnO@ZIF-8 core–shell nanowires for a highly selective MEMS-based ppb-level H2S gas sensor system. Small 2022, 18, 2204828. [Google Scholar]
- Fu, X.; Liu, J.; Wan, Y.; Zhang, X.; Meng, F.; Liu, J. Preparation of a leaf-like CdS micro-/nanostructure and its enhanced gas-sensing properties for detecting volatile organic compounds. J. Mater. Chem. 2012, 22, 17782. [Google Scholar]
- Holmberg, M.; Artursson, T. Drift compensation, standards, and calibration methods. In Handbook of Machine Olfaction; Pearce, T.C., Schiffman, S.S., Nagle, H.T., Gardner, J.W., Eds.; Wiley: Hoboken, NJ, USA, 2002; pp. 325–346. ISBN 978-3-527-30358-8. [Google Scholar]
- Yan, W.; Xu, H.; Ling, M.; Zhou, S.; Qiu, T.; Deng, Y.; Zhao, Z.; Zhang, E. MOF-derived porous hollow Co3 O4 @ZnO cages for high-performance MEMS trimethylamine sensors. ACS Sens. 2021, 6, 2613–2621. [Google Scholar] [PubMed]
- Liang, Z.; Zhang, L.; Tian, F.; Wang, C.; Yang, L.; Guo, T.; Xiong, L. A novel WWH problem-based semi-supervised online method for sensor drift compensation in E-nose. Sens. Actuators B Chem. 2021, 349, 130727. [Google Scholar]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar]
- Isaac, N.A.; Pikaar, I.; Biskos, G. Metal oxide semiconducting nanomaterials for air quality gas sensors: Operating principles, performance, and synthesis techniques. Microchim. Acta 2022, 189, 196. [Google Scholar]
- Chai, H.; Zheng, Z.; Liu, K.; Xu, J.; Wu, K.; Luo, Y.; Liao, H.; Debliquy, M.; Zhang, C. Stability of metal oxide semiconductor gas sensors: A review. IEEE Sens. J. 2022, 22, 5470–5481. [Google Scholar]
- Nadargi, D.Y.; Umar, A.; Nadargi, J.D.; Lokare, S.A.; Akbar, S.; Mulla, I.S.; Suryavanshi, S.S.; Bhandari, N.L.; Chaskar, M.G. Gas sensors and factors influencing sensing mechanism with a special focus on MOS sensors. J. Mater. Sci. 2023, 58, 559–582. [Google Scholar]
- Sayegh, S.; Lee, J.H.; Yang, D.H.; Weber, M.; Iatsunskyi, I.; Coy, E.; Razzouk, A.; Kim, S.S.; Bechelany, M. Humidity-resistant gas sensors based on SnO2 nanowires coated with a porous alumina nanomembrane by molecular layer deposition. Sens. Actuators B Chem. 2021, 344, 130302. [Google Scholar]
- Yan, M.; Wu, Y.; Hua, Z.; Lu, N.; Sun, W.; Zhang, J.; Fan, S. Humidity compensation based on power-law response for MOS sensors to VOCs. Sens. Actuators B Chem. 2021, 334, 129601. [Google Scholar]
- Deng, Y. Semiconducting Metal Oxides for Gas Sensing; Springer: Singapore, 2019; ISBN 9789811358524. [Google Scholar]
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Diversiform metal oxide-based hybrid nanostructures for gas sensing with versatile prospects. Coord. Chem. Rev. 2020, 413, 213272. [Google Scholar] [CrossRef]
- Hu, W.; Wan, L.; Jian, Y.; Ren, C.; Jin, K.; Su, X.; Bai, X.; Haick, H.; Yao, M.; Wu, W. Electronic noses: From advanced materials to sensors aided with data processing. Adv. Mater. Technol. 2019, 4, 1800488. [Google Scholar] [CrossRef]
- Yun, K.-H.; Yun, K.-Y.; Cha, G.-Y.; Lee, B.H.; Kim, J.C.; Lee, D.-D.; Huh, J.S. Gas sensing characteristics of ZnO-doped SnO2 sensors for simulants of the chemical agents. Mater. Sci. Forum 2005, 486–487, 9–12. [Google Scholar] [CrossRef]
- Choi, N.-J.; Kwak, J.-H.; Lim, Y.-T.; Bahn, T.-H.; Yun, K.-Y.; Kim, J.-C.; Huh, J.-S.; Lee, D.-D. Classification of chemical warfare agents using thick film gas sensor array. Sens. Actuators B Chem. 2005, 108, 298–304. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-0-470-61637-6. [Google Scholar]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in noble metal-decorated metal oxide nanomaterials for chemiresistive gas sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.M.; Zhang, H.P.; Cheng, Z.X. A new kinetic equation suitable for three different adsorption systems. Sep. Purif. Technol. 2024, 340, 126763. [Google Scholar] [CrossRef]
- Levenspiel, O. The Chemical Reactor Omnibook; OSU: Corvallis, OR, USA, 1996; ISBN 978-0-88246-160-1. [Google Scholar]
- Levenspiel, O. Tracer Technology: Modeling the Flow of Fluids; Fluid Mechanics and Its Applications; Springer: New York, NY, 2012; Volume 96, ISBN 978-1-4419-8073-1. [Google Scholar]
- Dai, X.Z.; Zhou, H.; Zhang, H.P.; Zhu, H.Y.; Wang, C.; Tang, H.M.; Cheng, Z.X. A new kinetic model on hydrolysis of sulfur mustard non-dissolved and dissolved in aqueous solution. J. Mol. Liq. 2024, 412, 125778. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Huang, Z.; Cheng, Z.; Li, T. Highly sensitive detection of sarin simulant by a functional SiNW array. Chem. Pap. 2023, 77, 5431–5440. [Google Scholar] [CrossRef]
- Tian, Z.F. Phase equilibrium theory on the interface layer model of physical interface. J. Colloid Interface Sci. 2001, 244, 282–302. [Google Scholar] [CrossRef]
- Polanyi, M. Über die adsorption vom standpunkt des dritten wärmesatzes. Verh. Dtsch. Phys. Ges. 1914, 16, 1012–1016. [Google Scholar]
- Quinn, J.J.; Yi, K.S. Solid State Physics: Principles and Modern Applications, 2nd ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zhong, Y.; Zhang, H.; Tao, Y.; Shen, Q.; Zhang, S.; Zhang, P.; Hu, X.; Liu, X.; Sun, X.; et al. Recoverable Detection of Dichloromethane by MEMS Gas Sensor Based on Mo and Ni Co-Doped SnO2 Nanostructure. Sensors 2025, 25, 2634. https://doi.org/10.3390/s25092634
Xu M, Zhong Y, Zhang H, Tao Y, Shen Q, Zhang S, Zhang P, Hu X, Liu X, Sun X, et al. Recoverable Detection of Dichloromethane by MEMS Gas Sensor Based on Mo and Ni Co-Doped SnO2 Nanostructure. Sensors. 2025; 25(9):2634. https://doi.org/10.3390/s25092634
Chicago/Turabian StyleXu, Mengxue, Yihong Zhong, Hongpeng Zhang, Yi Tao, Qingqing Shen, Shumin Zhang, Pingping Zhang, Xiaochun Hu, Xingqi Liu, Xuhui Sun, and et al. 2025. "Recoverable Detection of Dichloromethane by MEMS Gas Sensor Based on Mo and Ni Co-Doped SnO2 Nanostructure" Sensors 25, no. 9: 2634. https://doi.org/10.3390/s25092634
APA StyleXu, M., Zhong, Y., Zhang, H., Tao, Y., Shen, Q., Zhang, S., Zhang, P., Hu, X., Liu, X., Sun, X., & Cheng, Z. (2025). Recoverable Detection of Dichloromethane by MEMS Gas Sensor Based on Mo and Ni Co-Doped SnO2 Nanostructure. Sensors, 25(9), 2634. https://doi.org/10.3390/s25092634





